Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers–Danlos Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Population, Criteria for Selection, and Sample Acquisition
2.2. Molecular Genetic Analysis
2.3. Post-Mortem Sample Processing and Analysis
3. Results
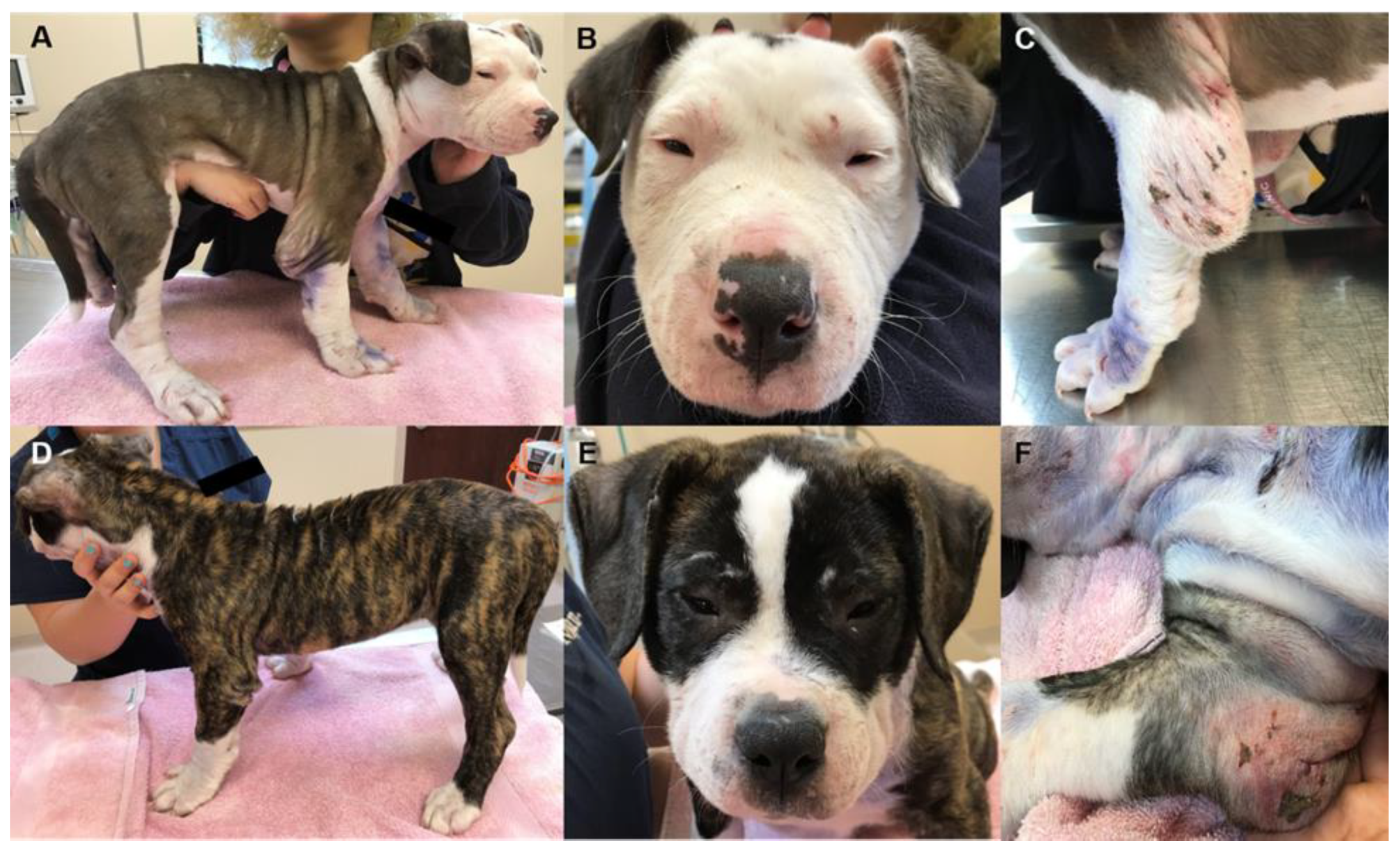
3.1. Clinical Histories and Postmortem Findings
3.1.1. Dogs 1 and 2
3.1.2. Dogs 3 and 4
3.1.3. Dogs 5 and 6
3.1.4. Dog 7
3.2. DNA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, L.; Byers, P.; Francomano, C.; Tinkle, B.; Malfait, F.; Steering Committee of the International Consortium on the Ehlers-Danlos Syndromes. The international consortium on the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Prim. 2020, 6, 64. [Google Scholar] [CrossRef]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Cortini, F.; Villa, C.; Marinelli, B.; Combi, R.; Pesatori, A.C.; Bassotti, A. Understanding the basis of Ehlers-Danlos syndrome in the era of the next-generation sequencing. Arch. Dermatol. Res. 2019, 311, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Connolly, J.J.; March, M.; Hou, C.; Chiavacci, R.; Kim, C.; Lyon, G.; Hadley, D.; Hakonarson, H. Systematic data-querying of large pediatric biorepository identifies novel Ehlers-Danlos Syndrome variant. BMC Musculoskelet. Disord. 2016, 17, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinle, J.; Hossain, W.A.; Lovell, S.; Veatch, O.J.; Butler, M.G. ADAMTSL2 gene variant in patients with features of autosomal dominant connective tissue disorders. Am. J. Med. Genet. A 2021, 185, 743–752. [Google Scholar] [CrossRef]
- Bauer, A.; Bateman, J.F.; Lamande, S.R.; Hanssen, E.; Kirejczyk, S.G.M.; Yee, M.; Ramiche, A.; Jagannathan, V.; Welle, M.; Leeb, T.; et al. Identification of Two Independent COL5A1 Variants in Dogs with Ehlers-Danlos Syndrome. Genes 2019, 10, 731. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.; de Lucia, M.; Leuthard, F.; Jagannathan, V.; Leeb, T. Compound heterozygosity for TNXB genetic variants in a mixed-breed dog with Ehlers-Danlos syndrome. Anim. Genet. 2019, 50, 546–549. [Google Scholar] [CrossRef]
- Kiener, S.; Chevallier, L.; Jagannathan, V.; Briand, A.; Cochet-Faivre, N.; Reyes-Gomez, E.; Leeb, T. A COL5A2 In-Frame Deletion in a Chihuahua with Ehlers-Danlos Syndrome. Genes 2022, 13, 934. [Google Scholar] [CrossRef]
- Anderson, J.H.; Brown, R.E. Cutaneous asthenia in a dog. J. Am. Vet. Med. Assoc. 1978, 173, 742–743. [Google Scholar]
- Arlein, M.S. Generalized acute cutaneous asthenia in a dog. J. Am. Vet. Med. Assoc. 1947, 111, 52. [Google Scholar]
- Barnett, K.; Cottrell, B.D. Ehlers-Danlos syndrome in a dog: Ocular, cutaneous and articular abnormalities. J. Small Anim. Pract. 1987, 28, 941–946. [Google Scholar] [CrossRef]
- Bellini, M.H.; Caldini, E.T.; Scapinelli, M.P.; Simoes, M.J.; Machado, D.B.; Nurmberg, R. Increased elastic microfibrilsand thickening of fibroblastic nuclear lamina in canine cutaneous asthenia. Vet. Dermatol. 2009, 20, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.I.; Jones, B.R.; Barnes, G.R.; Craig, A.S. A collagen dysplasia in a greyhound bitch. N. Z. Vet. J. 1980, 28, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.J.; Hegreberg, G.A.; Robinette, J.D. Ehlers-Danlos syndrome in dogs and cats. Semin. Vet. Med. Surg. 1987, 2, 221–227. [Google Scholar]
- Freeman, L.J.; Hegreberg, G.A.; Robinette, J.D. Cutaneous wound healing in Ehlers-Danlos syndrome. Vet. Surg. 1989, 18, 88–96. [Google Scholar] [CrossRef]
- Freeman, L.J.; Hegreberg, G.A.; Robinette, J.D.; Kimbrell, J.T. Biomechanical properties of skin and wounds in Ehlers-Danlos syndrome. Vet. Surg. 1989, 18, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gething, M.A. Suspected Ehlers-Danlos syndrome in the dog. Vet. Rec. 1971, 89, 638–641. [Google Scholar] [CrossRef]
- Hegreberg, G.A. Animal model of human disease: Ehlers-Danlos syndrome. Am. J. Pathol. 1975, 79, 383–386. [Google Scholar]
- Hegreberg, G.A.; Padgett, G.A.; Henson, J.B. Connective tissue disease of dogs and mink resembling Ehlers-Danlos syndrome of man. 3. Histopathologic changes of the skin. Arch. Pathol. 1970, 90, 159–166. [Google Scholar]
- Hegreberg, G.A.; Padgett, G.A.; Ott, R.L.; Henson, J.B. A heritable connective tissue disease of dogs and mink resembling Ehlers-Danlos syndrome of man. I. Skin tensile strength properties. J. Investig. Dermatol. 1970, 54, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, F.; Herraez, P.; de los Monteros, A.E.; Calabuig, P.; Rodriguez, J.L. Collagen dysplasia in a litter of Garafiano shepherd dogs. Zent. Vet. A 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Holbrook, K.A.; Byers, P.H. Structural abnormalities in the dermal collagen and elastic matrix from the skin of patients with inherited connective tissue disorders. J. Investig. Dermatol. 1982, 79 (Suppl. 1), 7s–16s. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Bullock, G.; Teplin, E.; Guo, J.; Villani, N.A.; Mhlanga-Mutangadura, T.; Schnabel, R.D.; Cohn, L.A.; Johnson, G.S. A homozygous ADAMTS2 nonsense mutation in a Doberman Pinscher dog with Ehlers Danlos syndrome and extreme skin fragility. Anim. Genet. 2019, 50, 543–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, B.R.; Lewis, G.T. Ehlers-Danlos syndrome in a dog. Can. Vet. J. 1990, 31, 389–390. [Google Scholar]
- Minor, R.R.; Lein, D.H.; Patterson, D.F.; Krook, L.; Porter, T.G.; Kane, A.C. Defects in collagen fibrillogenesis causing hyperextensible, fragile skin in dogs. J. Am. Vet. Med. Assoc. 1983, 182, 142–148. [Google Scholar]
- Paciello, O.; Lamagna, F.; Lamagna, B.; Papparella, S. Ehlers-Danlos-like syndrome in 2 dogs: Clinical, histologic, and ultrastructural findings. Vet. Clin. Pathol. 2003, 32, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.H.; Thomsen, M.K.; Kristensen, F. Cutaneous asthenia in the dog. A report of two cases. Nord. Vet. Med. 1985, 37, 291–297. [Google Scholar]
- Rasch, S.N. Surgical and medical treatment of ocular disease in a dog with Ehlers-Danlos syndrome. Clin. Case Rep. 2017, 5, 880–886. [Google Scholar] [CrossRef]
- Uri, M.; Verin, R.; Ressel, L.; Buckley, L.; McEwan, N. Ehlers-Danlos syndrome associated with fatal spontaneous vascular rupture in a dog. J. Comp. Pathol. 2015, 152, 211–216. [Google Scholar] [CrossRef]
- Wall, R.D. Congenital defect of the skin. N. Am. Vet. 1947, 28, 166–168. [Google Scholar]
- Ward, G.W. Cutaneous asthenia (cutis hyperelastica) of dogs. Aust. Vet. J. 1970, 46, 115. [Google Scholar] [CrossRef] [PubMed]
- Colige, A.; Sieron, A.L.; Li, S.W.; Schwarze, U.; Petty, E.; Wertelecki, W.; Wilcox, W.; Krakow, D.; Cohn, D.H.; Reardon, W.; et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am. J. Hum. Genet. 1999, 65, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, A.F.; Demirdas, S.; Fournel-Gigleux, S.; Ghali, N.; Giunta, C.; Kapferer-Seebacher, I.; Kosho, T.; Mendoza-Londono, R.; Pope, M.F.; Rohrbach, M.; et al. The Ehlers-Danlos syndromes, rare types. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 70–115. [Google Scholar] [CrossRef] [Green Version]
- Bekhouche, M.; Leduc, C.; Dupont, L.; Janssen, L.; Delolme, F.; Vadon-Le Goff, S.; Smargiasso, N.; Baiwir, D.; Mazzucchelli, G.; Zanella-Cleon, I.; et al. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-beta signaling as primary targets. FASEB J. 2016, 30, 1741–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.H.; Halper, J. Connective Tissue Disorders in Domestic Animals. Adv. Exp. Med. Biol. 2021, 1348, 325–335. [Google Scholar] [CrossRef]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem. Biophys. Res. Commun. 2005, 327, 541–547. [Google Scholar] [CrossRef]
- Zeng, R.; Coates, J.R.; Johnson, G.C.; Hansen, L.; Awano, T.; Kolicheski, A.; Ivansson, E.; Perloski, M.; Lindblad-Toh, K.; O’Brien, D.P.; et al. Breed distribution of SOD1 alleles previously associated with canine degenerative myelopathy. J. Vet. Intern. Med. 2014, 28, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Hoeppner, M.P.; Lundquist, A.; Pirun, M.; Meadows, J.R.; Zamani, N.; Johnson, J.; Sundstrom, G.; Cook, A.; FitzGerald, M.G.; Swofford, R.; et al. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS ONE 2014, 9, e91172. [Google Scholar] [CrossRef]
- Mowjoodi, A.; Paton, T.A.; Scherer, S.W. Discrimination of SNPs in GC-rich regions using a modified hydrolysis probe chemistry protocol. Biotechniques 2014, 57, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Lindeboom, R.G.; Supek, F.; Lehner, B. The rules and impact of nonsense-mediated mRNA decay in human cancers. Nat. Genet. 2016, 48, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfait, F.; De Coster, P.; Hausser, I.; van Essen, A.J.; Franck, P.; Colige, A.; Nusgens, B.; Martens, L.; De Paepe, A. The natural history, including orofacial features of three patients with Ehlers-Danlos syndrome, dermatosparaxis type (EDS type VIIC). Am. J. Med. Genet. A 2004, 131, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanset, R.; Lapiere, C.M. Inheritance of dermatosparaxis in the calf. A genetic defect of connective tissues. J. Hered. 1974, 65, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Helle, O.; Nes, N.N. A hereditary skin defect in sheep. Acta Vet. Scand. 1972, 13, 443–445. [Google Scholar] [CrossRef]
- Van Damme, T.; Colige, A.; Syx, D.; Giunta, C.; Lindert, U.; Rohrbach, M.; Aryani, O.; Alanay, Y.; Simsek-Kiper, P.O.; Kroes, H.Y.; et al. Expanding the clinical and mutational spectrum of the Ehlers-Danlos syndrome, dermatosparaxis type. Genet. Med. 2016, 18, 882–891. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, P.J.; Read, W.K.; Romane, W.M.; Bridges, C.H. A collagenous tissue dysplasia of calves. Lab. Investig. 1970, 23, 307–314. [Google Scholar]
- Hanset, R.; Ansay, M. Dermatosparaxie (peau déchirée) chez le veau: Un défaut général du tissu conjonctif, de nature héréditaire. Ann. Med. Vet. 1967, 7, 451–470. [Google Scholar]
- Ramshaw, J.A. A mild form of ovine dermatosparaxis. Collagen Relat. Res. 1984, 4, 441–451. [Google Scholar] [CrossRef]
- Bavinton, J.H.; Peters, D.E.; Ramshaw, J.A. A morphologic study of a mild form of ovine dermatosparaxis. J. Investig. Dermatol. 1985, 84, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Counts, D.F.; Byers, P.H.; Holbrook, K.A.; Hegreberg, G.A. Dermatosparaxis in a Himalayan cat: I. Biochemical studies of dermal collagen. J. Investig. Dermatol. 1980, 74, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, K.A.; Byers, P.H.; Counts, D.F.; Hegreberg, G.A. Dermatosparaxis in a Himalayan cat: II. Ultrastructural studies of dermal collagen. J. Investig. Dermatol. 1980, 74, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.; Fang, Q. A premature stop codon in the ADAMTS2 gene is likely to be responsible for dermatosparaxis in Dorper sheep. Anim. Genet. 2012, 43, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Vaatstra, B.; Halliday, W.; Waropastrakul, S. Dermatosparaxis in two White Dorper lambs. N. Z. Vet. J. 2011, 59, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Keep, J.M. Cutis hyperelastica in a dog. Aust. Vet. J. 1969, 45, 593. [Google Scholar] [PubMed]
- Wertelecki, W.; Smith, L.T.; Byers, P. Initial observations of human dermatosparaxis: Ehlers-Danlos syndrome type VIIC. J. Pediatr. 1992, 121, 558–564. [Google Scholar] [CrossRef]
- Fujimoto, A.; Wilcox, W.R.; Cohn, D.H. Clinical, morphological, and biochemical phenotype of a new case of Ehlers-Danlos syndrome type VIIC. Am. J. Med. Genet. 1997, 68, 25–28. [Google Scholar] [CrossRef]
- Fjolstad, M.; Helle, O. A hereditary dysplasia of collagen tissues in sheep. J. Pathol. 1974, 112, 183–188. [Google Scholar] [CrossRef]
- Atroshi, F.; Henriksson, K.; Lindberg, L.A.; Multia, M. A heritable disorder of collagen tissue in Finnish crossbred sheep. Zent. Vet. A 1983, 30, 233–241. [Google Scholar] [CrossRef]
- Jha, S.K.; Rauniyar, K.; Jeltsch, M. Key molecules in lymphatic development, function, and identification. Ann. Anat. 2018, 219, 25–34. [Google Scholar] [CrossRef]
- Jeltsch, M.; Jha, S.K.; Tvorogov, D.; Anisimov, A.; Leppanen, V.M.; Holopainen, T.; Kivela, R.; Ortega, S.; Karpanen, T.; Alitalo, K. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 2014, 129, 1962–1971. [Google Scholar] [CrossRef] [Green Version]
- Dupont, L.; Joannes, L.; Morfoisse, F.; Blacher, S.; Monseur, C.; Deroanne, C.F.; Noel, A.; Colige, A.C. ADAMTS2 and ADAMTS14 can substitute for ADAMTS3 in adults for pro-VEGFC activation and lymphatic homeostasis. JCI Insight 2022, 7, e151509. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Dupont, L.; Bekhouche, M.; Noel, A.; Leduc, C.; Voz, M.; Peers, B.; Cataldo, D.; Apte, S.S.; Dubail, J.; et al. ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis 2016, 19, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, K.; Kawai, T.; Senoo, H.; Shimizu, A.; Ishiko, A.; Nagata, M. Histopathological and electron microscopic study in dogs with patellar luxation and skin hyperextensibility. J. Vet. Med. Sci. 2018, 80, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusgens, B.V.; Verellen-Dumoulin, C.; Hermanns-Le, T.; De Paepe, A.; Nuytinck, L.; Pierard, G.E.; Lapiere, C.M. Evidence for a relationship between Ehlers-Danlos type VII C in humans and bovine dermatosparaxis. Nat. Genet. 1992, 1, 214–217. [Google Scholar] [CrossRef]
- Lapiere, C.M.; Nusgens, B.V. Ehlers-Danlos type VII-C, or human dermatosparaxis. The offspring of a union between basic and clinical research. Arch. Dermatol. 1993, 129, 1316–1319. [Google Scholar] [CrossRef]
- Holm, D.E.; van Wilpe, E.; Harper, C.K.; Duncan, N.M. The occurrence of dermatosparaxis in a commercial Drakensberger cattle herd in South Africa. J. S. Afr. Vet. Assoc. 2008, 79, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Giunta, C.; Chambaz, C.; Pedemonte, M.; Scapolan, S.; Steinmann, B. The arthrochalasia type of Ehlers-Danlos syndrome (EDS VIIA and VIIB): The diagnostic value of collagen fibril ultrastructure. Am. J. Med. Genet. A 2008, 146A, 1341–1346. [Google Scholar] [CrossRef]
- Byers, P.H.; Duvic, M.; Atkinson, M.; Robinow, M.; Smith, L.T.; Krane, S.M.; Greally, M.T.; Ludman, M.; Matalon, R.; Pauker, S.; et al. Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen. Am. J. Med. Genet. 1997, 72, 94–105. [Google Scholar] [CrossRef]
- Hulmes, D.J.; Kadler, K.E.; Mould, A.P.; Hojima, Y.; Holmes, D.F.; Cummings, C.; Chapman, J.A.; Prockop, D.J. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J. Mol. Biol. 1989, 210, 337–345. [Google Scholar] [CrossRef]
- Watson, R.B.; Wallis, G.A.; Holmes, D.F.; Viljoen, D.; Byers, P.H.; Kadler, K.E. Ehlers Danlos syndrome type VIIB. Incomplete cleavage of abnormal type I procollagen by N-proteinase in vitro results in the formation of copolymers of collagen and partially cleaved pNcollagen that are near circular in cross-section. J. Biol. Chem. 1992, 267, 9093–9100. [Google Scholar] [CrossRef]
- Watson, R.B.; Holmes, D.F.; Graham, H.K.; Nusgens, B.V.; Kadler, K.E. Surface located procollagen N-propeptides on dermatosparactic collagen fibrils are not cleaved by procollagen N-proteinase and do not inhibit binding of decorin to the fibril surface. J. Mol. Biol. 1998, 278, 195–204. [Google Scholar] [CrossRef]
- Eyre, D.R.; Shapiro, F.D.; Aldridge, J.F. A heterozygous collagen defect in a variant of the Ehlers-Danlos syndrome type VII. Evidence for a deleted amino-telopeptide domain in the pro-alpha 2(I) chain. J. Biol. Chem. 1985, 260, 11322–11329. [Google Scholar] [CrossRef]
- Colige, A.; Nuytinck, L.; Hausser, I.; van Essen, A.J.; Thiry, M.; Herens, C.; Ades, L.C.; Malfait, F.; Paepe, A.D.; Franck, P.; et al. Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene. J. Investig. Dermatol. 2004, 123, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobey, G. Ehlers-Danlos syndrome: How to diagnose and when to perform genetic tests. Arch. Dis. Child. 2015, 100, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Goff, C.; Somerville, R.P.; Kesteloot, F.; Powell, K.; Birk, D.E.; Colige, A.C.; Apte, S.S. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: Insights on collagen biosynthesis and dermatosparaxis. Development 2006, 133, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Meek, K.M.; Leonard, D.W.; Connon, C.J.; Dennis, S.; Khan, S. Transparency, swelling and scarring in the corneal stroma. Eye 2003, 17, 927–936. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef]






| Dog Identity | Dog 1 | Dog 2 | Dog 3 | Dog 4 | Dog 5 | Dog 6 | Dog 7 | Dog 8 |
|---|---|---|---|---|---|---|---|---|
| reed | Pit Bull Terrier | Pit Bull Terrier | Alapaha Blue Blood Bulldog | Alapaha Blue Blood Bulldog | Alapaha Blue Blood Bulldog | Alapaha Blue Blood Bulldog | Catahoula Leopard Dog | Doberman Pinscher |
| Mutation | 11:2280117delC | 11:2280117delC | 11:2280117delC | 11:2280117delC | 11:2280117delC | 11:2280117delC | 11:2491238G>A | 11:2408978C>T |
| Mutation type | Frameshift | Frameshift | Frameshift | Frameshift | Frameshift | Frameshift | Missense | Nonsense |
| Mutation zygosity | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous |
| Euthanasia | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Age at death | 8 Weeks | 8 Weeks | 12 Weeks | 12 Weeks | 8 Weeks | 8 Weeks | >9 Years | 8 Weeks |
| Fragile skin | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Atrophic scars | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hyper-extensible Skin | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Joint instability | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Swollen joints | Yes | No | Yes | Yes | Yes | Yes | Infrequent | Yes |
| Periocular lesions | Micropalpebral fissures | None | Micropalpebral fissures | Micropalpebral fissures | Micropalpebral fissures | Micropalpebral fissures | None | Ocular Chemosis |
| Ataxia | Yes | No | No | Yes | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffey, J.A.; Bullock, G.; Guo, J.; Mhlanga-Mutangadura, T.; O’Brien, D.P.; Coates, J.R.; Morrissey, R.; Hutchison, R.; Donnelly, K.S.; Cohn, L.A.; et al. Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers–Danlos Syndrome. Genes 2022, 13, 2158. https://doi.org/10.3390/genes13112158
Jaffey JA, Bullock G, Guo J, Mhlanga-Mutangadura T, O’Brien DP, Coates JR, Morrissey R, Hutchison R, Donnelly KS, Cohn LA, et al. Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers–Danlos Syndrome. Genes. 2022; 13(11):2158. https://doi.org/10.3390/genes13112158
Chicago/Turabian StyleJaffey, Jared A., Garrett Bullock, Juyuan Guo, Tendai Mhlanga-Mutangadura, Dennis P. O’Brien, Joan R. Coates, Rochelle Morrissey, Robert Hutchison, Kevin S. Donnelly, Leah A. Cohn, and et al. 2022. "Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers–Danlos Syndrome" Genes 13, no. 11: 2158. https://doi.org/10.3390/genes13112158
APA StyleJaffey, J. A., Bullock, G., Guo, J., Mhlanga-Mutangadura, T., O’Brien, D. P., Coates, J. R., Morrissey, R., Hutchison, R., Donnelly, K. S., Cohn, L. A., Katz, M. L., & Johnson, G. S. (2022). Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers–Danlos Syndrome. Genes, 13(11), 2158. https://doi.org/10.3390/genes13112158






