Efficacy of Wholistic Turmeric Supplement on Adenomatous Polyps in Patients with Familial Adenomatous Polyposis—A Randomized, Double-Blinded, Placebo-Controlled Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Protocol
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–263. [Google Scholar] [CrossRef] [PubMed]
- Moisio, A.-L.; Järvinen, H.; Peltomäki, P. Genetic and clinical characterisation of familial adenomatous polyposis: A population based study. Gut 2002, 50, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- da Luz Moreira, A.; Church, J.M.; Burke, C.A. The evolution of prophylactic colorectal surgery for familial adenomatous polyposis. Dis. Colon Rectum 2009, 52, 1481–1486. [Google Scholar] [CrossRef]
- Tudyka, V.N.; Clark, S.K. Surgical treatment in familial adenomatous polyposis. Ann. Gastroenterol. 2012, 25, 201–206. [Google Scholar]
- Gilad, O.; Gluck, N.; Brazowski, E.; Kariv, R.; Rosner, G.; Strul, H. Determinants of Pouch-Related Symptoms, a Common Outcome of Patients With Adenomatous Polyposis Undergoing Ileoanal Pouch Surgery. Clin. Transl. Gastroenterol. 2020, 11, e00245. [Google Scholar] [CrossRef]
- Saurin, J.-C.; Napoleon, B.; Gay, G.; Ponchon, T.; Arpurt, J.-P.; Boustiere, C.; Boyer, J.; Canard, J.-M.; Dalbies, P.-A.; Escourrou, J.; et al. Endoscopic management of patients with familial adenomatous polyposis (FAP) following a colectomy. Endoscopy 2005, 37, 499–501. [Google Scholar] [CrossRef]
- Arber, N.; Levin, B. Chemoprevention of colorectal neoplasia: The potential for personalized medicine. Gastroenterology 2008, 134, 1224–1237. [Google Scholar] [CrossRef]
- Kemp Bohan, P.M.; Mankaney, G.; Vreeland, T.J.; Chick, R.C.; Hale, D.F.; Cindass, J.L.; Hickerson, A.T.; Ensley, D.C.; Sohn, V.; Clifton, G.T.; et al. Chemoprevention in familial adenomatous polyposis: Past, present and future. Fam. Cancer 2021, 20, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Bishop, D.T.; Chapman, P.D.; Elliott, F.; Bertario, L.; Dunlop, M.G.; Eccles, D.; Ellis, A.; Evans, D.G.; Fodde, R.; et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev. Res. 2011, 4, 655–665. [Google Scholar] [CrossRef]
- Labayle, D.; Fischer, D.; Vielh, P.; Drouhin, F.; Pariente, A.; Bories, C.; Duhamel, O.; Trousset, M.; Attali, P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 1991, 101, 635–639. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Romans, K.E.; Booker, S.V.; Giardiello, F.M. Long-term treatment with sulindac in familial adenomatous polyposis: A prospective cohort study. Gastroenterology 2002, 122, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Yang, V.W.; Hylind, L.M.; Krush, A.J.; Petersen, G.M.; Trimbath, J.D.; Piantadosi, S.; Garrett, E.; Geiman, D.E.; Hubbard, W.; et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002, 346, 1054–1059. [Google Scholar] [CrossRef]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef]
- Lynch, P.M.; Ayers, G.D.; Hawk, E.; Richmond, E.; Eagle, C.; Woloj, M.; Church, J.; Hasson, H.; Patterson, S.; Half, E.; et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am. J. Gastroenterol. 2010, 105, 1437–1443. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.R.; Romi, M.D.; Ferreira, D.M.T.P.; Zaltman, C.; Soares-Mota, M. The Use of Curcumin as a Complementary Therapy in Ulcerative Colitis: A Systematic Review of Randomized Controlled Clinical Trials. Nutrients 2020, 12, 2296. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Prabu, S.L.; Jordan, B.C.; Purushothaman, Y.; Umamaheswari, A.; Hosseini Zare, M.S.; Thilagavathi, R. Molecular mechanisms of curcumin and its analogs in colon cancer prevention and treatment. Life Sci. 2019, 239, 117032. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomark. Prev. 2002, 11, 535–540. [Google Scholar]
- Girardi, B.; Pricci, M.; Giorgio, F.; Piazzolla, M.; Iannone, A.; Losurdo, G.; Principi, M.; Barone, M.; Ierardi, E.; Di Leo, A. Silymarin, boswellic acid and curcumin enriched dietetic formulation reduces the growth of inherited intestinal polyps in an animal model. World J. Gastroenterol. 2020, 26, 1601–1612. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.J.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Murakami, A.; Furukawa, I.; Miyamoto, S.; Tanaka, T.; Ohigashi, H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors 2013, 39, 221–232. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, K.M.; Liu, J.; Feng, Y.; Greenson, J.K.; Fearon, E.R. Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS ONE 2014, 9, e96023. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, T.; Aoki, K.; Lane, H.A.; Aoki, M.; Taketo, M.M. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc. Natl. Acad. Sci. USA 2008, 105, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Roos, V.H.; Meijer, B.J.; Kallenberg, F.G.J.; Bastiaansen, B.A.J.; Koens, L.; Bemelman, F.J.; Bossuyt, P.M.M.; Heijmans, J.; van den Brink, G.; Dekker, E. Sirolimus for the treatment of polyposis of the rectal remnant and ileal pouch in four patients with familial adenomatous polyposis: A pilot study. BMJ Open Gastroenterol. 2020, 7, e000497. [Google Scholar] [CrossRef]
- Samadder, N.J.; Neklason, D.W.; Boucher, K.M.; Byrne, K.R.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Done, M.W.; Berry, T.; et al. Effect of Sulindac and Erlotinib vs. Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 1266–1275. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef]
- Kim, K.-C.; Lee, C. Curcumin Induces Downregulation of E2F4 Expression and Apoptotic Cell Death in HCT116 Human Colon Cancer Cells; Involvement of Reactive Oxygen Species. Korean J. Physiol. Pharmacol. 2010, 14, 391–397. [Google Scholar] [CrossRef]
- Yin, T.-F.; Wang, M.; Qing, Y.; Lin, Y.-M.; Wu, D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J. Gastroenterol. 2016, 22, 7058–7068. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.-Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef]
- Park, C.H.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS Lett. 2005, 579, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Mao, Y.B.; Cai, Q.F.; Yao, L.M.; Ouyang, G.L.; Bao, S.D. Curcumin induces human HT-29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis-related protein expression. Braz. J. Med. Biol. Res. 2005, 38, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.J.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, D.H.; Jung, H.J.; Kim, J.H.; Lim, D.; Lee, S.-K.; Kim, K.-W.; Ahn, J.W.; Yoo, J.-S.; Rho, J.-R.; et al. Hydrazinocurcumin, a novel synthetic curcumin derivative, is a potent inhibitor of endothelial cell proliferation. Bioorg. Med. Chem. 2002, 10, 2987–2992. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, J.; Park, H.-J.; Park, S.-J.; Kwon, H.J. A new curcumin derivative, HBC, interferes with the cell cycle progression of colon cancer cells via antagonization of the Ca2+/calmodulin function. Chem. Biol. 2004, 11, 1455–1463. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Johnson, S.M.; Gulhati, P.; Rampy, B.A.; Han, Y.; Rychahou, P.G.; Doan, H.Q.; Weiss, H.L.; Evers, B.M. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J. Am. Coll. Surg. 2010, 210, 767–776. [Google Scholar] [CrossRef]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef]
- Ishikawa, H.; Mutoh, M.; Sato, Y.; Doyama, H.; Tajika, M.; Tanaka, S.; Horimatsu, T.; Takeuchi, Y.; Kashida, H.; Tashiro, J.; et al. Chemoprevention with low-dose aspirin, mesalazine, or both in patients with familial adenomatous polyposis without previous colectomy (J-FAPP Study IV): A multicentre, double-blind, randomised, two-by-two factorial design trial. Lancet Gastroenterol. Hepatol. 2021, 6, 474–481. [Google Scholar] [CrossRef]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Loeve, F.; Boer, R.; Zauber, A.G.; van Ballegooijen, M.; van Oortmarssen, G.J.; Winawer, S.J.; Habbema, J.D.F. National Polyp Study data: Evidence for regression of adenomas. Int. J. Cancer 2004, 111, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Knoernschild, H.E. Growth rate and malignant potential of colonic polyps: Early results. Surg. Forum 1963, 14, 137–138. [Google Scholar] [PubMed]



| Wholistic Turmeric N = 6 | Placebo N = 4 | p Value | |
|---|---|---|---|
| Age * | 45 (27–62.25) | 45 (41–48) | 0.944 |
| Gender (%male) ** | 66.67 | 75.00 | 0.635 |
| Underwent IPAA (%) ** | 1 (16.67) | 2 (50) | 0.500 |
| First endoscopy | |||
| Number of polyps * | 17 (8–23) | 25.5 (10.75–89.5) | 0.476 |
| Polyp burden (mm) * | 37.75 (29.87–78.62) | 69.75 (34.62–261.75) | 0.762 |
| Mean polyp size (mm) * | 3.70 (2.79–5.36) | 3.32 (3.2–3.9) | 0.721 |
| Maximal polyp size (mm) * | 12.5 (6.25–16.5) | 8 (7.25–8.12) | 0.352 |
| Patient Number | Treatment Group | Underwent Surgery | Polyp Number | Mean Polyp Size (mm) | Maximal Polyp Size (mm) | Polyp Burden (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline | |||
| 1 | WT | IPAA | 2 | 2 | 0.0 | 13 | 11 | −15.3 | 20 | 17.5 | −12.5 | 26 | 22 | −15.4 |
| 2 | WT | No | 24 | 20 | −16.7 | 4.07 | 3.1 | −23.9 | 15 | 8 | −46.7 | 90.5 | 62 | −31.5 |
| 3 | WT | No | 5 | 9 | 80.0 | 5.8 | 5.6 | −3.4 | 10 | 10 | 0 | 29 | 50.5 | 74.1 |
| 4 | WT | No | 17 | 11 | −35.3 | 2.3 | 5 | 111.8 | 5 | 9 | 80.0 | 32.5 | 19 | −41.5 |
| 5 | WT | No | 17 | 23 | 35.3 | 2.6 | 3.1 | 21.8 | 5 | 10 | 100 | 43 | 72.5 | 68.6 |
| 6 | WT | No | 38 | 16 | −57.9 | 3.3 | 2.2 | −33.5 | 17 | 4 | −76.5 | 110 | 36 | −67.3 |
| 7 | Placebo | IPAA | 7 | 7 | 0.0 | 3.4 | 2.1 | −38.4 | 8 | 4 | −50.0 | 23 | 19 | −17.4 |
| 8 | Placebo | No | 241 | 108 | −55.2 | 3.2 | 5.2 | 64.06 | 8 | 3.5 | −56.3 | 744.5 | 244.5 | −67.2 |
| 9 | Placebo | No | 12 | 20 | 66.7 | 3.2 | 3.8 | 19.68 | 8.5 | 15 | 76.5 | 38.5 | 98.5 | 155.8 |
| 10 | Placebo | IPAA | 39 | 29 | −25.6 | 5.2 | 3.2 | −38.09 | 5 | 6 | 20 | 101 | 50.5 | −50.0 |
| Median Change (%) from Baseline (IQR) | Wholistic Turmeric N = 6 | Placebo N = 4 | p Value |
|---|---|---|---|
| Polyp number | −10 (−31.47–26.47) | −12.8 (−33.02–16.67) | 0.896 |
| Mean polyp size (mm) | −9.41 (−21.79–15.51) | −9.20 (−38.17–30.78) | 0.610 |
| Maximal polyp size (mm) | −6.25 (−38.125–60) | −15 (−51.56–34.11) | 0.826 |
| Polyp burden (mm) | −23.43 (−39.02–47.6) | −33.69 (−54.3–25.91) | 0.886 |
| Median Change (%) from Baseline (IQR) | Wholistic Turmeric N = 6 | Placebo N = 4 | p Value |
|---|---|---|---|
| Left colon | |||
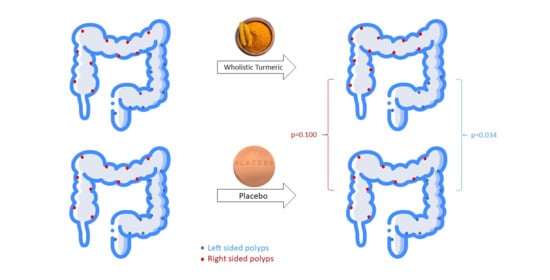
| Polyp number | −49.78 (−78.57–[−8.82]) | −12.82 (−30.1–75) | 0.257 |
| Mean polyp size (mm) | −34.61 (−43.97–[−19.87]) | −2.74 (−27.7–20.31) | 0.476 |
| Polyp burden (mm) | −57.13 (−86.51–[−35.38]) | −23.51 (−34.72–86.95) | 0.171 |
| Right colon | |||
| Polyp number | 48.61 (−5.83–93.75) | −6.24 (−30.09–19.6) | 0.533 |
| Mean polyp size (mm) | −8.07 (−19–5.31) | −64.45 (−73.58–[−55.31]) | 0.133 |
| Polyp burden (mm) | 45.2 (−20.18–111.05) | 34.92 (−18.84–88.69) | 1 |
| Right Colon | Left Colon | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyp Number | Polyp Burden (mm) | Polyp Number | Polyp Burden (mm) | |||||||||||
| Patient Number | Treatment Group | Underwent Surgery | Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline | Baseline | Follow-Up | Change (%) from Baseline |
| 1 | WT | IPAA | 2 | 2 | 0.0 | 26 | 22 | −15.4 | ||||||
| 2 | WT | No | 18 | 19 | 5.6 | 68 | 60 | −11.8 | 6 | 1 | −83.3 | 22.5 | 2 | −91.1 |
| 3 | WT | No | 4 | 7 | 75 | 23 | 46.5 | 102.2 | 1 | 2 | 100 | 6 | 4 | −33.3 |
| 4 | WT | No | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 11 | −35.3 | 32.5 | 19 | −41.5 |
| 5 | WT | No | 12 | 23 | 91.7 | 30.5 | 72.5 | 137.7 | 5 | 0 | −100 | 12.5 | 0 | −100.0 |
| 6 | WT | No | 10 | 6 | −40.0 | 22 | 12 | −45.5 | 28 | 10 | −64.3 | 88 | 24 | −72.7 |
| 7 | Placebo | IPAA | 7 | 7 | 0.0 | 23 | 19 | −17.4 | ||||||
| 8 | Placebo | No | 195 | 82 | −57.9 | 650 | 178 | −72.2 | 46 | 26 | −43.5 | 94.5 | 66.5 | −29.3 |
| 9 | Placebo | No | 11 | 16 | 45.5 | 36.5 | 88.5 | 142.5 | 1 | 4 | 300.0 | 2 | 10 | 400.0 |
| 10 | Placebo | IPAA | 39 | 29 | −25.6 | 101 | 50.5 | −50.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilad, O.; Rosner, G.; Ivancovsky-Wajcman, D.; Zur, R.; Rosin-Arbesfeld, R.; Gluck, N.; Strul, H.; Lehavi, D.; Rolfe, V.; Kariv, R. Efficacy of Wholistic Turmeric Supplement on Adenomatous Polyps in Patients with Familial Adenomatous Polyposis—A Randomized, Double-Blinded, Placebo-Controlled Study. Genes 2022, 13, 2182. https://doi.org/10.3390/genes13122182
Gilad O, Rosner G, Ivancovsky-Wajcman D, Zur R, Rosin-Arbesfeld R, Gluck N, Strul H, Lehavi D, Rolfe V, Kariv R. Efficacy of Wholistic Turmeric Supplement on Adenomatous Polyps in Patients with Familial Adenomatous Polyposis—A Randomized, Double-Blinded, Placebo-Controlled Study. Genes. 2022; 13(12):2182. https://doi.org/10.3390/genes13122182
Chicago/Turabian StyleGilad, Ophir, Guy Rosner, Dana Ivancovsky-Wajcman, Reut Zur, Rina Rosin-Arbesfeld, Nathan Gluck, Hana Strul, Dana Lehavi, Vivien Rolfe, and Revital Kariv. 2022. "Efficacy of Wholistic Turmeric Supplement on Adenomatous Polyps in Patients with Familial Adenomatous Polyposis—A Randomized, Double-Blinded, Placebo-Controlled Study" Genes 13, no. 12: 2182. https://doi.org/10.3390/genes13122182
APA StyleGilad, O., Rosner, G., Ivancovsky-Wajcman, D., Zur, R., Rosin-Arbesfeld, R., Gluck, N., Strul, H., Lehavi, D., Rolfe, V., & Kariv, R. (2022). Efficacy of Wholistic Turmeric Supplement on Adenomatous Polyps in Patients with Familial Adenomatous Polyposis—A Randomized, Double-Blinded, Placebo-Controlled Study. Genes, 13(12), 2182. https://doi.org/10.3390/genes13122182