Nonhomologous Chromosome Interactions in Prophase I: Dynamics of Bizarre Meiotic Contacts in the Alay Mole Vole Ellobius alaicus (Mammalia, Rodentia)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
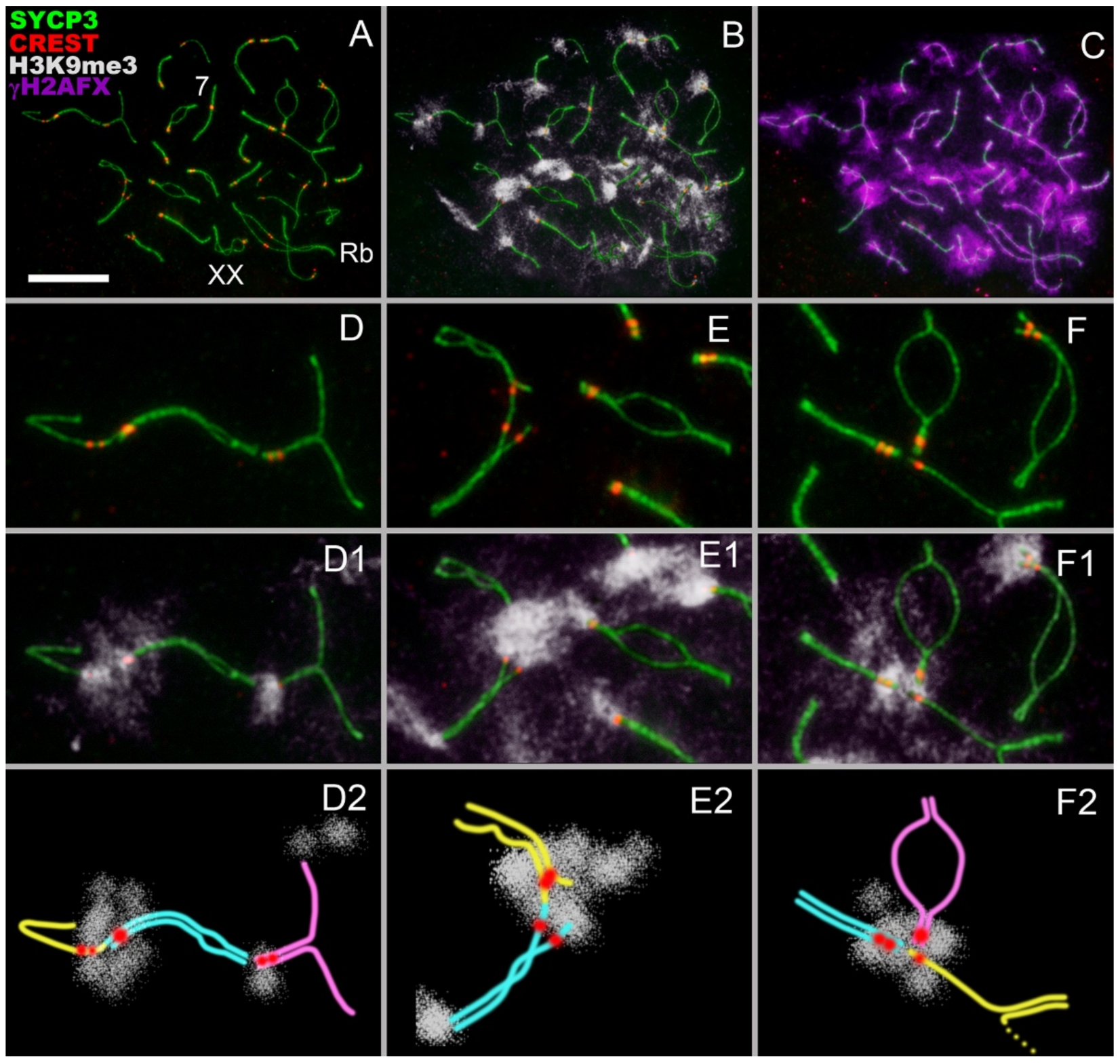
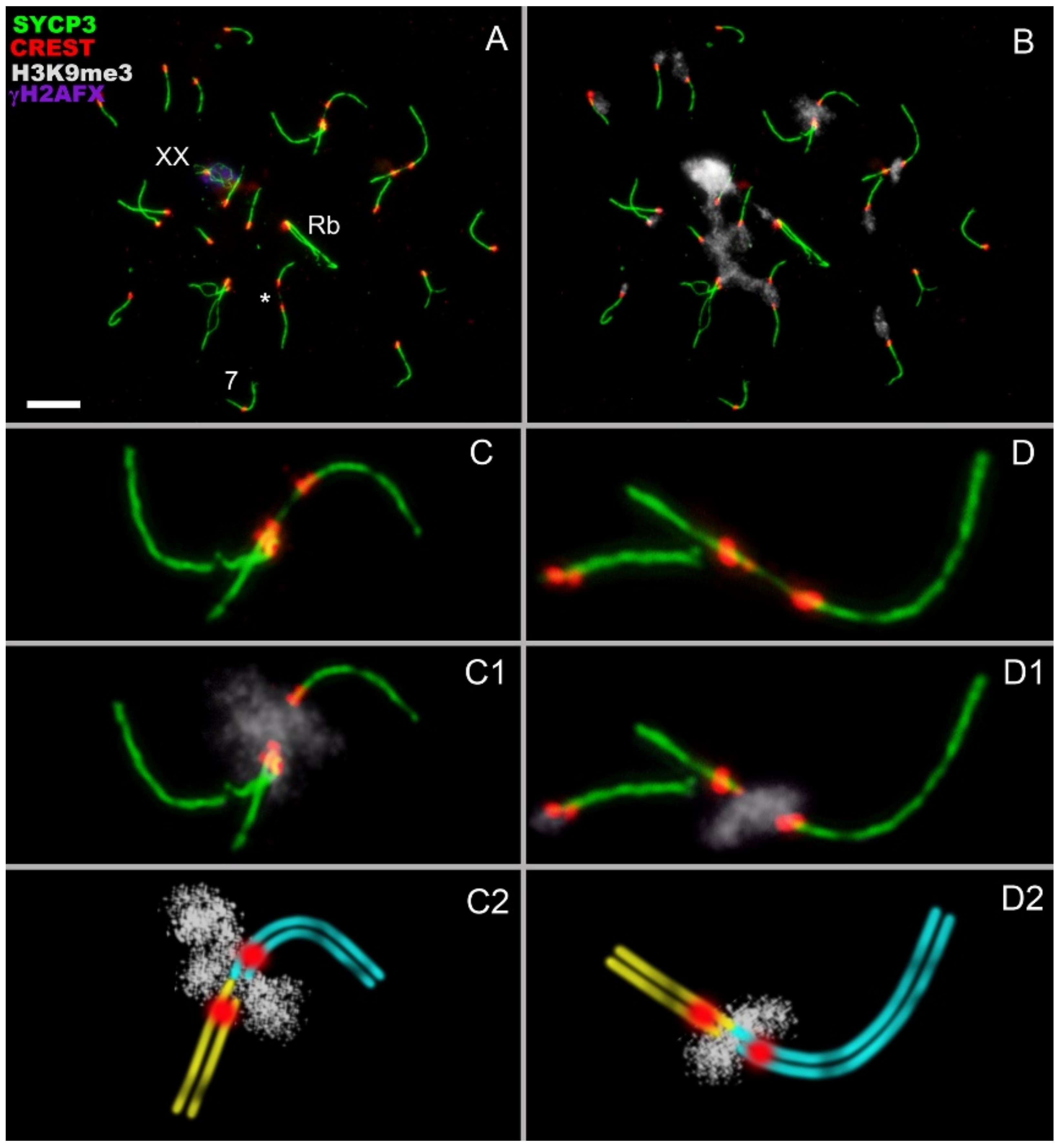
- Asynchronous synapsis of the chromosomes involved in contact takes place: synapsis of one of the bivalents is faster than that of the other (Figure 2A,I);
- Occasionally, a gap in the axial/lateral element is seen in the bivalent that manifests faster synapsis (Figure 2(A,A1,C,C1)). This feature was detected in 8 out of 75 nuclei;
- In zygotene, various chromosomes enter into meiotic contacts, as was demonstrated earlier for pachytene bivalents [20].
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zickler, D.; Kleckner, N. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a016626. [Google Scholar] [CrossRef] [Green Version]
- Zetka, M.; Paouneskou, D.; Jantsch, V. The nuclear envelope, a meiotic jack-of-all-trades. Curr. Opin. Cell Biol. 2020, 64, 34–42. [Google Scholar] [CrossRef]
- Eils, R.; Dietzel, S.; Bertin, E.; Schröck, E.; Speicher, M.R.; Ried, T.; Robert-Nicoud, M.; Cremer, C.; Cremer, T. Three-dimensional reconstruction of painted human interphase chromosomes: Active and inactive X chromosome territories have similar volumes but differ in shape and surface structure. J. Cell Biol. 1996, 135, 1427–1440. [Google Scholar] [CrossRef] [Green Version]
- Cremer, T.; Cremer, M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2010, 2, a003889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, R.; Brero, A.; Von Hase, J.; Schroeder, T.; Cremer, T.; Dietzel, S. Common themes and cell type specific variations of higher order chromatin arrangements in the mouse. BMC Cell Biol. 2005, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, H.; Müller, S.; Neusser, M.; von Hase, J.; Calcagno, E.; Cremer, M.; Solovei, I.; Cremer, C.; Cremer, T. Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates. Proc. Natl. Acad. Sci. USA 2002, 99, 4424–4429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, H.; Küpper, K.; Ishida, T.; Neusser, M.; Mizusawa, H. Inter and intra-specific gene-density-correlated radial chromosome territory arrangements are conserved in Old World monkeys. Cytogenet. Genome Res. 2005, 108, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleck, K.; Raj, R.; Erceg, J. The 3D genome landscape: Diverse chromosomal interactions and their functional implications. Front. Cell Dev. Biol. 2022, 10, 968145. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.G.; Barutcu, A.R.; Rinn, J.L. Interchromosomal interactions: A genomic love story of kissing chromosomes. J. Cell Biol. 2019, 218, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, L.; Kang, R.; Rosenberg, S.C.; Qiu, Y.; Raviram, R.; Chee, S.; Hu, R.; Ren, B.; Cole, F.; Corbett, K.D. Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nat. Struct. Mol. Biol. 2019, 26, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Chen, G.; Gao, Z.; Li, S.; Chen, Y.; Huang, C.; Chen, J.; Chen, Z.; Lei, M.; Bian, Q. Stage-resolved Hi-C analyses reveal meiotic chromosome organizational features influencing homolog alignment. Nat. Commun. 2021, 12, 5827. [Google Scholar] [CrossRef]
- Douglas, L.T. Meiosis, I: Association of non-homologous bivalents during spermatogenesis in white mice. Genetica 1966, 37, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Close, R.L.; Bell, J.N.; Dollin, A.E.; Harding, H.R. Spermatogenesis and synaptonemal complexes of hybrid Petrogale (Marsupialia). J. Hered. 1996, 87, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Moens, P.B.; Chapman, V.M. Deficiency of X and Y chromosomal pairing at meiotic prophase in spermatocytes of sterile interspecific hybrids between laboratory mice (Mus domesticus) and Mus spretus. Chromosoma 1992, 101, 483–492. [Google Scholar] [CrossRef]
- Matveevsky, S.; Bakloushinskaya, I.; Tambovtseva, V.; Romanenko, S.; Kolomiets, O. Analysis of meiotic chromosome structure and behavior in Robertsonian heterozygotes of Ellobius tancrei (Rodentia, Cricetidae): A case of monobrachial homology. Comp. Cytogenet. 2015, 9, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubouchi, T.; Roeder, G.S. A synaptonemal complex protein promotes homology-independent centromere coupling. Science 2005, 308, 870–873. [Google Scholar] [CrossRef]
- Bardhan, A. Meiotic chromosome interactions: Nonhomologous centromere (un)coupling and homologous synapsis. Int. Sch. Res. Not. 2012, 2012, 890475. [Google Scholar] [CrossRef] [Green Version]
- Takeo, S.; Lake, C.M.; Morais-de-Sa, E.; Sunkel, C.E.; Hawley, R.S. Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in drosophila oocytes. Curr. Biol. 2011, 21, 1845–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, T.; Macaisne, N.; Huynh, J.R. Mixing and matching chromosomes during female meiosis. Cells 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Alpeeva, E.; Bakloushinskaya, I. Meiotic chromosome contacts as a plausible prelude for Robertsonian translocations. Genes 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Berrios, S.; Manterola, M.; Prieto, Z.; López-Fenner, J.; Fernández-Donoso, R. Model of chromosome associations in Mus domesticus spermatocytes. Biol. Res. 2010, 43, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Ontoso, D.; Kauppi, L.; Keeney, S.; San-Segundo, P.A. Dynamics of DOT1L localization and H3K79 methylation during meiotic prophase I in mouse spermatocytes. Chromosoma 2014, 123, 147–164. [Google Scholar] [CrossRef] [Green Version]
- Eyster, C.; Chuong, H.H.; Lee, C.Y.; Pezza, R.J.; Dawson, D. The pericentromeric heterochromatin of homologous chromosomes remains associated after centromere pairing dissolves in mouse spermatocyte meiosis. Chromosoma 2019, 128, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudrimont, A.; Penkner, A.; Woglar, A.; Machacek, T.; Wegrostek, C.; Gloggnitzer, J.; Fridkin, A.; Klein, F.; Gruenbaum, Y.; Pasierbek, P.; et al. Leptotene/zygotene chromosome movement via the SUN/KASH protein bridge in Caenorhabditis elegans. PLoS Genet. 2010, 6, e1001219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikashige, Y.; Tsutsumi, C.; Yamane, M.; Okamasa, K.; Haraguchi, T.; Hiraoka, Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 2006, 125, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, J.; Benavente, R.; Hodzic, D.; Höög, C.; Stewart, C.L.; Alsheimer, M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc. Natl. Acad. Sci. USA 2007, 104, 7426–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enguita-Marruedo, A.; Martín-Ruiz, M.; García, E.; Gil-Fernández, A.; Parra, M.T.; Viera, A.; Rufas, J.S.; Page, J. Transition from a meiotic to a somatic-like DNA damage response during the pachytene stage in mouse meiosis. PLoS Genet. 2019, 15, e1007439. [Google Scholar] [CrossRef] [Green Version]
- Bakloushinskaya, I.; Lyapunova, E.A.; Saidov, A.S.; Romanenko, S.A.; O’Brien, P.C.; Serdyukova, N.A.; Ferguson-Smith, M.A.; Matveevsky, S.; Bogdanov, A.S. Rapid chromosomal evolution in enigmatic mammal with XX in both sexes, the Alay mole vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia). Comp. Cytogenet. 2019, 13, 147–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambovtseva, V.; Bakloushinskaya, I.; Matveevsky, S.; Bogdanov, A. Geographic mosaic of extensive genetic variations in subterranean mole voles Ellobius alaicus as a consequence of habitat fragmentation and hybridization. Life 2022, 12, 728. [Google Scholar] [CrossRef]
- Di Stefano, M.; Paulsen, J.; Jost, D.; Marti-Renom, M.A. 4D nucleome modeling. Curr. Opin. Genet. Dev. 2021, 67, 25–32. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, W.; Han, J.D.J. 3D chromatin structure changes during spermatogenesis and oogenesis. Comput. Struct. Biotechnol. J. 2022, 20, 2434–2441. [Google Scholar] [CrossRef]
- Peters, A.H.F.M.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germ line. Chromosome Res. 1997, 5, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Page, J.; Berríos, S.; Rufas, J.S.; Parra, M.T.; Suja, J.Á.; Heyting, C.; Fernández-Donoso, R. The pairing of X and Y chromosomes during meiotic prophase in the marsupial species Thylamys elegans is maintained by a dense plate developed from their axial elements. J. Cell Sci. 2003, 116, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveevsky, S.; Bakloushinskaya, I.; Kolomiets, O. Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation? Sci. Rep. 2016, 6, 29949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveevsky, S.; Chassovnikarova, T.; Grishaeva, T.; Atsaeva, M.; Malygin, V.; Bakloushinskaya, I.; Kolomiets, O. Kinase CDK2 in mammalian meiotic prophase I: Screening for hetero-and homomorphic sex chromosomes. Int. J. Mol. Sci. 2021, 22, 1969. [Google Scholar] [CrossRef]
- Kolomiets, O.; Matveevsky, S.; Bakloushinskaya, I. Sexual dimorphism in prophase I of meiosis in the Northern mole vole (Ellobius talpinus Pallas, 1770) with isomorphic (XX) chromosomes in males and females. Comp. Cytogenet. 2010, 4, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Moens, P.B. Synaptonemal Complex. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: New York, NY, USA, 2001; pp. 1910–1912. [Google Scholar] [CrossRef]
- Rufas, J.S. Synaptonemal Complex. In Brenner’s Encyclopedia of Genetics; Maloy, S., Hughes, K., Eds.; Academic Press: New York, NY, USA, 2013; pp. 613–616. [Google Scholar] [CrossRef]
- Page, J.; de la Fuente, R.; Manterola, M.; Parra, M.T.; Viera, A.; Berrios, S.; Fernandez-Donoso, R.; Rufas, J.S. Inactivation or non-reactivation: What accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 2012, 121, 307–326. [Google Scholar] [CrossRef]
- Sánchez, O.F.; Mendonca, A.; Min, A.; Liu, J.; Yuan, C. Monitoring histone methylation (H3K9me3) changes in live cells. ACS Omega 2019, 4, 13250–13259. [Google Scholar] [CrossRef]
- Rabl, C. Uber Zelltheilung. Morphol. Jahrb. 1885, 10, 214–330. [Google Scholar]
- Boveri, T. Die Blastomerenkerne von Ascaris megalocephala und die Theorie der Chromosomenindividualität. Arch. Zellforsch. 1909, 3, 181–268. [Google Scholar]
- Cremer, M.; Cremer, T. Nuclear compartmentalization, dynamics, and function of regulatory DNA sequences. Genes Chromosom. Cancer 2019, 58, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-González, L.; Burden, F.; Doddamani, D.; Malinverni, R.; Leach, E.; Marín-García, C.; Marín-Gual, L.; Gubern, A.; Vara, C.; Paytuví-Gallart, A.; et al. 3D chromatin remodelling in the germ line modulates genome evolutionary plasticity. Nat. Commun. 2022, 13, 2608. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; De Lange, T. Removal of shelterin reveals the telomere end-protection problem. Science 2012, 336, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruis, P.; Boulton, S.J. The end protection problem—An unexpected twist in the tail. Genes Dev. 2021, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zickler, D.; Kleckner, N. The leptotene-zygotene transition of meiosis. Annu. Rev. Genet. 1998, 32, 619. [Google Scholar] [CrossRef] [Green Version]
- Scherthan, H. A bouquet makes ends meet. Nat. Rev. Mol. Cell Biol. 2001, 2, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Golubovskaya, I.; Cande, W.Z. A bouquet of chromosomes. J. Cell Sci. 2004, 117, 4025–4032. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.M. Meiotic silencing in mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef]
- Link, J.; Jantsch, V. Meiotic chromosomes in motion: A perspective from Mus musculus and Caenorhabditis elegans. Chromosoma 2019, 128, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Richards, E.J.; Elgin, S.C. Epigenetic codes for heterochromatin formation and silencing: Rounding up the usual suspects. Cell 2002, 108, 489–500. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveevsky, S.; Bakloushinskaya, I.; Tambovtseva, V.; Atsaeva, M.; Grishaeva, T.; Bogdanov, A.; Kolomiets, O. Nonhomologous Chromosome Interactions in Prophase I: Dynamics of Bizarre Meiotic Contacts in the Alay Mole Vole Ellobius alaicus (Mammalia, Rodentia). Genes 2022, 13, 2196. https://doi.org/10.3390/genes13122196
Matveevsky S, Bakloushinskaya I, Tambovtseva V, Atsaeva M, Grishaeva T, Bogdanov A, Kolomiets O. Nonhomologous Chromosome Interactions in Prophase I: Dynamics of Bizarre Meiotic Contacts in the Alay Mole Vole Ellobius alaicus (Mammalia, Rodentia). Genes. 2022; 13(12):2196. https://doi.org/10.3390/genes13122196
Chicago/Turabian StyleMatveevsky, Sergey, Irina Bakloushinskaya, Valentina Tambovtseva, Maret Atsaeva, Tatiana Grishaeva, Aleksey Bogdanov, and Oxana Kolomiets. 2022. "Nonhomologous Chromosome Interactions in Prophase I: Dynamics of Bizarre Meiotic Contacts in the Alay Mole Vole Ellobius alaicus (Mammalia, Rodentia)" Genes 13, no. 12: 2196. https://doi.org/10.3390/genes13122196






