Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke
Abstract
1. Introduction
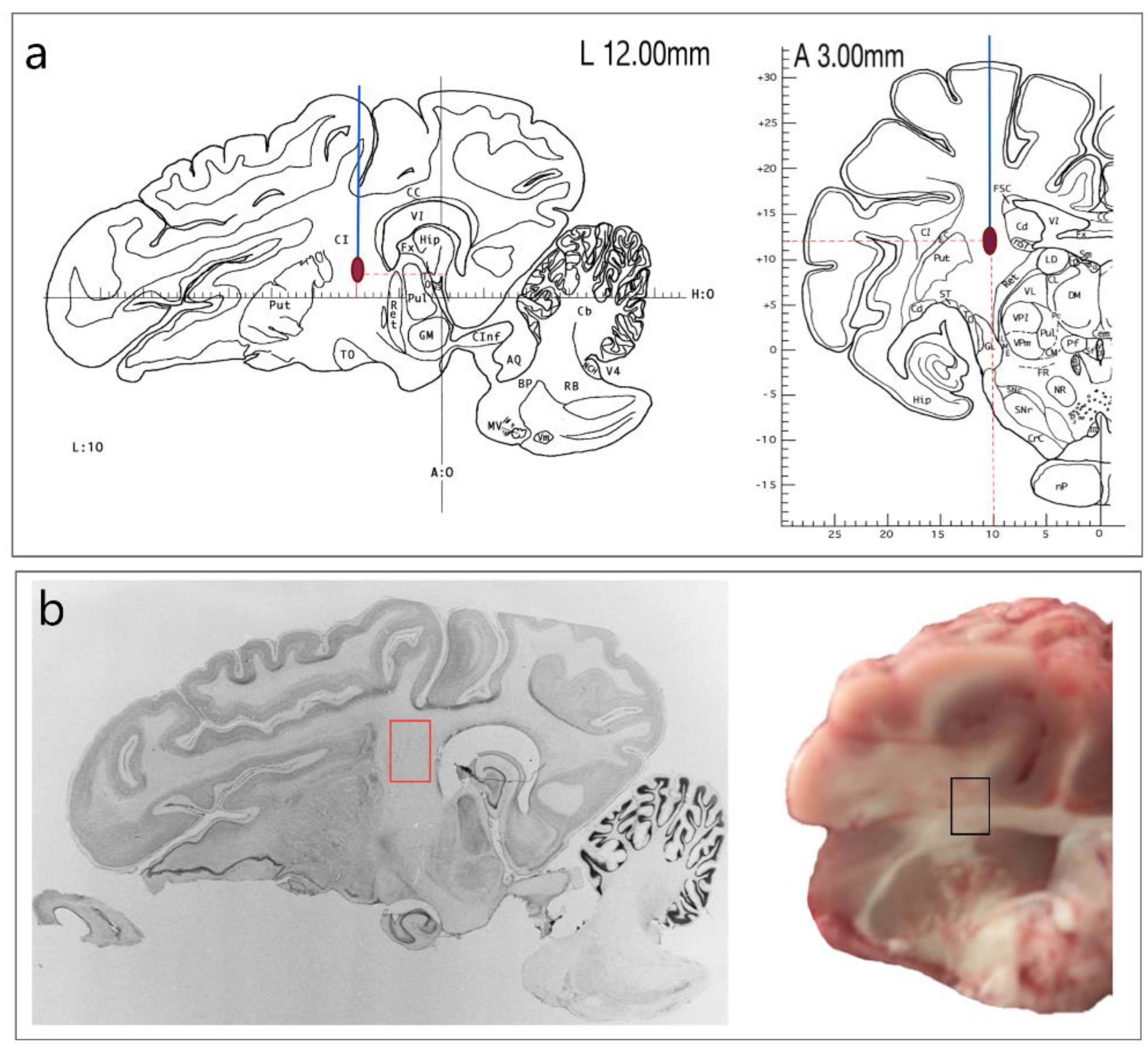
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Conforto, A.B.; Machado, A.G.; Menezes, I.; Ribeiro, N.H.V.; Luccas, R.; Pires, D.S.; Leite, C.C.; Plow, E.B.; Cohen, L.G. Treatment of Upper Limb Paresis with Repetitive Peripheral Nerve Sensory Stimulation and Motor Training: Study Protocol for a Randomized Controlled Trial. Front. Neurol. 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Rabek, J.P.; Hafer-Macko, C.E.; Amaning, J.K.; DeFord, J.H.; Dimayuga, V.L.; Madsen, M.A.; Macko, R.F.; Papaconstantinou, J. A proteomics analysis of the effects of chronic hemiparetic stroke on troponin T expression in human vastus lateralis. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Clin. Neurophysiol. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Riddell, N.; Crewther, S.G.; Faou, P.; Rajapaksha, H.; Howells, D.W.; Hankey, G.J.; Wijeratne, T.; Ma, H.; Davis, S.; et al. Longitudinal Stroke Recovery Associated with Dysregulation of Complement System-A Proteomics Pathway Analysis. Front. Neurol. 2020, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Hoffe, B.; Holahan, M.R. The Use of Pigs as a Translational Model for Studying Neurodegenerative Diseases. Front. Physiol. 2019, 10, 838. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Carey, L.M.; Giummarra, L.; Faou, P.; Cooke, I.; Howells, D.W.; Tse, T.; Macaulay, S.L.; Ma, H.; Davis, S.M.; et al. A Pathway Proteomic Profile of Ischemic Stroke Survivors Reveals Innate Immune Dysfunction in Association with Mild Symptoms of Depression—A Pilot Study. Front. Neurol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Félix, B.; Léger, M.; Albe-Fessard, E.D.; Marcilloux, J.-C.; Rampin, O.; Laplace, J.-P.; Duclos, A.; Fort, F.; Gougis, S.; Costa, M.; et al. Stereotaxic atlas of the pig brain. Brain Res. Bull. 1999, 49, 1–137. [Google Scholar] [CrossRef]
- Sidyakin, A.A.; Kaysheva, A.L.; Kopylov, A.T.; Lobanov, A.V.; Morozov, S.G. Proteomic Analysis of Cerebral Cortex Extracts from Sus scrofa with Induced Hemorrhagic Stroke. J. Mol. Neurosci. 2018, 65, 28–34. [Google Scholar] [CrossRef]
- Akhremko, A.; Fedulova, L. Comparative study of weaning pigs’ muscle proteins using two-dimensional electrophoresis. Potravinarstvo. Slovak J. Food Sci. 2021, 15, 52–57. [Google Scholar] [CrossRef]
- Zgoda, V.G.; Moshkovskii, S.A.; Ponomarenko, E.A.; Andreewski, T.V.; Kopylov, A.T.; Tikhonova, O.V.; Melnik, S.A.; Lisitsa, A.V.; Archakov, A.I. Proteomics of mouse liver microsomes: Performance of different protein separation workflows for LC-MS/MS. Proteomics 2009, 9, 4102–4105. [Google Scholar] [CrossRef]
- Naryzhny, S.N.; Maynskova, M.A.; Zgoda, V.G.; Ronzhina, N.L.; Kleyst, O.A.; Vakhrushev, I.V.; Archakov, A.I. Virtual-Experimental 2DE Approach in Chromosome-Centric Human Proteome Project. J. Proteome Res. 2016, 15, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Marin-Garcia, P.; Ping, P.; Stein, L.; D’Eustachio, P.; Hermjakob, H. Reactome diagram viewer: Data structures and strategies to boost performance. Bioinformatics 2018, 34, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, B.; Zhang, Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle 2016, 15, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Xue, M.; Yong, V.W. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: Therapeutic opportunities. Brain 2020, 143, 1297–1314. [Google Scholar] [CrossRef]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef]
- Zambusi, A.; Ninkovic, J. Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J. Stem Cells 2020, 12, 8–24. [Google Scholar] [CrossRef]
- Tittelmeier, J.; Nachman, E.; Nussbaum-Krammer, C. Molecular Chaperones: A Double-Edged Sword in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 581374. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, B.; Zheng, W.; Sun, Y.; Xu, T. eIF3k inhibits NF-κB signaling by targeting MyD88 for ATG5-mediated autophagic degradation in teleost fish. J. Biol. Chem. 2022, 298, 101730. [Google Scholar] [CrossRef] [PubMed]
- Shliapina, V.L.; Yurtaeva, S.V.; Dontsova, O.V. At the Crossroads: Mechanisms of Apoptosis and Autophagy in Cell Life and Death. Acta Nat. 2021, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kasimov, M.R.; Fatkhrakhmanova, M.R.; Mukhutdinova, K.A.; Petrov, A.M. Hydroxycholesterol enhances synaptic vesicle cycling in the mouse neuromuscular junction: Implication of glutamate NMDA receptors and nitric oxide. Neuropharmacology 2017, 117, 61–73. [Google Scholar] [CrossRef]
- Shioda, N.; Yamamoto, Y.; Watanabe, M.; Binas, B.; Owada, Y.; Fukunaga, K. Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 3146–3155. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.S.; Lee, H.J.; Park, I.S. Dendritic eIF4E-binding protein 1 (eIF4E-BP1) mRNA is upregulated by neuronal activation. J. Korean Med. Sci. 2012, 27, 1241–1247. [Google Scholar] [CrossRef][Green Version]
- Sreekumar, P.G.; Hinton, D.R.; Kannan, R. Methionine sulfoxide reductase A: Structure, function and role in ocular pathology. World J. Biol. Chem. 2011, 2, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tiago, T.; Hummel, B.; Morelli, F.F.; Basile, V.; Vinet, J.; Galli, V.; Mediani, L.; Antoniani, F.; Pomella, S.; Cassandri, M.; et al. Small heat-shock protein HSPB3 promotes myogenesis by regulating the lamin B receptor. Cell Death Dis. 2021, 12, 452. [Google Scholar] [CrossRef]
- Vendredy, L.; Adriaenssens, E.; Timmerman, V. Small heat shock proteins in neurodegenerative diseases. Cell Stress Chaperones 2020, 25, 679–699. [Google Scholar] [CrossRef] [PubMed]
- La Padula, V.; Staszewski, O.; Nestel, S.; Busch, H.; Boerries, M.; Roussa, E.; Prinz, M.; Krieglstein, K. HSPB3 protein is expressed in motoneurons and induces their survival after lesion-induced degeneration. Exp. Neurol. 2016, 286, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Brock, E.J.; Ji, K.; Mattingly, R.R. Ras and Rap1: A tale of two GTPases. Semin. Cancer Biol. 2019, 54, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cheong, A.; Archambault, D.; Degani, R.; Iverson, E.; Tremblay, K.D.; Mager, J. Nuclear-encoded mitochondrial ribosomal proteins are required to initiate gastrulation. Development 2020, 147, dev188714. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, H.; Zhang, H. Abnormal Expression of Mitochondrial Ribosomal Proteins and Their Encoding Genes with Cell Apoptosis and Diseases. Int. J. Mol. Sci. 2020, 21, 8879. [Google Scholar] [CrossRef] [PubMed]






| № | Uniprot ID | Protein | Γен | MW, kDa | Intensity |
|---|---|---|---|---|---|
| 1 | Q9BG57 | Translation initiation factor 4E binding protein 1 (Fragment) | EIF4E | 10,697 | 125,890,000 |
| 2 | H6UI30 | Heart fatty acid-binding protein | H-FABP | 14,761 | 243,990,000 |
| 3 | A0A287A271 | SHSP domain-containing protein | HSPB3 | 16,808 | 189,480,000 |
| 4 | A0A287B931 | Uncharacterized protein | RAP1B | 18,778 | 749,450,000 |
| 5 | F1S534 | Down-regulator of transcription 1 (Negative cofactor 2-beta) | DR1 | 19,245 | 15,008,000 |
| 6 | A0A286ZNK1 | Mitochondrial ribosomal protein L18 | MRPL18 | 19,919 | 194,620,000 |
| 7 | K7GP19 | 39S ribosomal protein L13, mitochondrial | MRPL13 | 2291 | 74,807,000 |
| 8 | A0A286ZJR2 | Mitochondrial ribosomal protein L58 | MRPL58 | 23,503 | 35,632,000 |
| 9 | A0A1Z1VUJ8 | Peptide-methionine (R)-S-oxide reductase (EC 1.8.4.12) | MSRB3 | 19,931 | 56,872,000 |
| 10 | A0A287B4D9 | GTPase HRas isoform 1 | HRAS | 21,223 | 281,550,000 |
| 11 | A0A287ARV8 | Mitochondrial ribosomal protein L12 | MRPL12 | 21,237 | 76,581,000 |
| 12 | A0A286ZWB1 | Transmembrane protein 11 | TMEM11 | 21,287 | 128,060,000 |
| 13 | M3V828 | Core-binding factor subunit beta isoform X2 | CBFB | 21,478 | 69,361,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedulova, L.; Vasilevskaya, E.; Tikhonova, O.; Kazieva, L.; Tolmacheva, G.; Makarenko, A. Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke. Genes 2022, 13, 2204. https://doi.org/10.3390/genes13122204
Fedulova L, Vasilevskaya E, Tikhonova O, Kazieva L, Tolmacheva G, Makarenko A. Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke. Genes. 2022; 13(12):2204. https://doi.org/10.3390/genes13122204
Chicago/Turabian StyleFedulova, Liliya, Ekaterina Vasilevskaya, Olga Tikhonova, Laura Kazieva, Galina Tolmacheva, and Alexandr Makarenko. 2022. "Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke" Genes 13, no. 12: 2204. https://doi.org/10.3390/genes13122204
APA StyleFedulova, L., Vasilevskaya, E., Tikhonova, O., Kazieva, L., Tolmacheva, G., & Makarenko, A. (2022). Proteomic Markers in the Muscles and Brain of Pigs Recovered from Hemorrhagic Stroke. Genes, 13(12), 2204. https://doi.org/10.3390/genes13122204





