Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers
Abstract
:1. Introduction
2. Materials and Methods
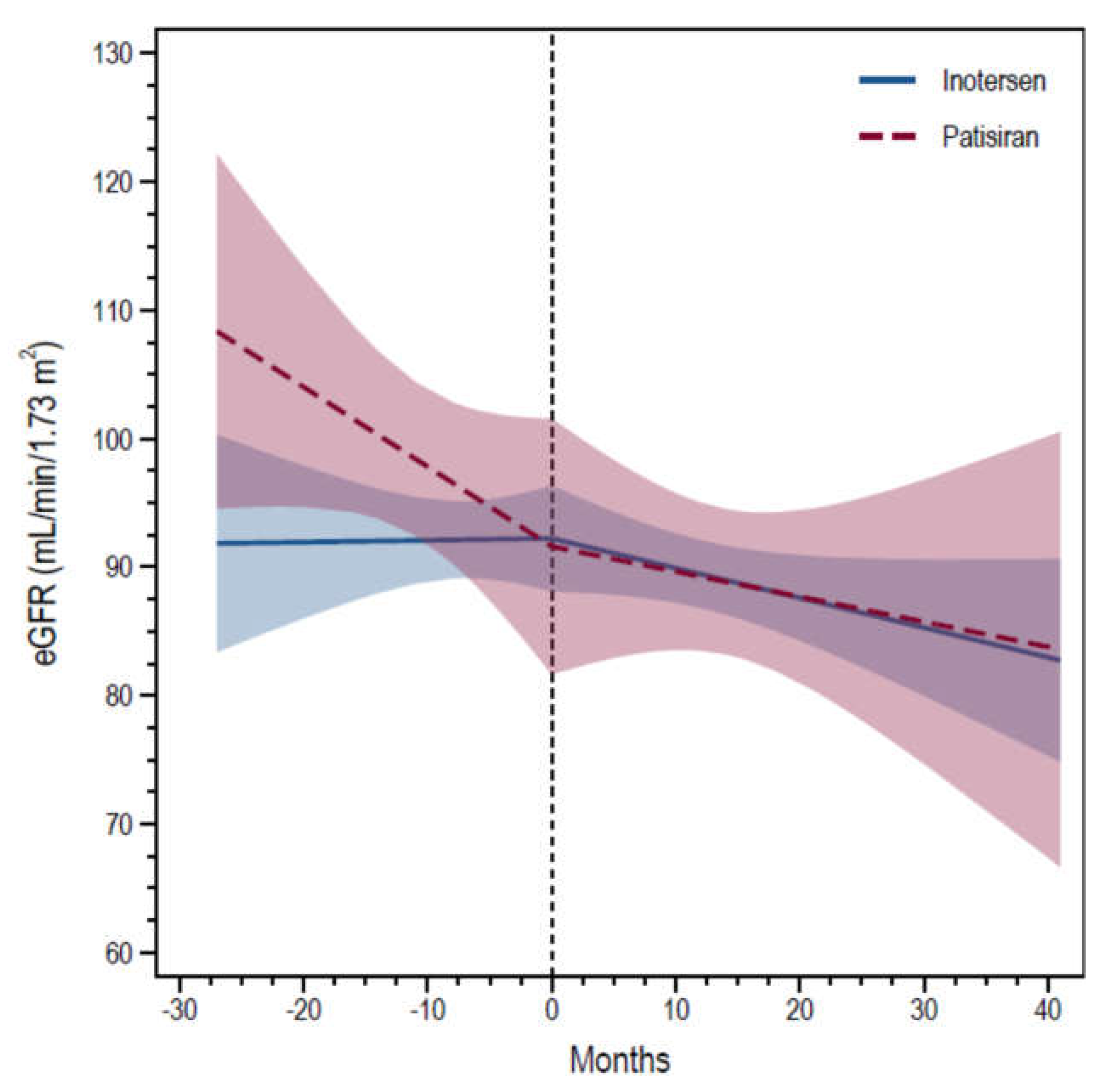
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luigetti, M.; Romano, A.; Di Paolantonio, A.; Bisogni, G.; Sabatelli, M. Diagnosis and Treatment of Hereditary Transthyretin Amyloidosis (hATTR) Polyneuropathy: Current Perspectives on Improving Patient Care. Ther. Clin. Risk Manag. 2020, 16, 109–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, M.D.; Kincaid, J.C. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 2007, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Tortora, A.; Romano, A.; Di Paolantonio, A.; Guglielmino, V.; Bisogni, G.; Gasbarrini, A.; Calabresi, P.; Sabatelli, M. Gastrointestinal Manifestations in Hereditary Transthyretin Amyloidosis: A Single-Centre Experience. J. Gastrointest. Liver Dis. 2020, 29, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Planté-Bordeneuve, V.; Said, G. Familial amyloid polyneuropathy. Lancet Neurol. 2011, 10, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Conte, A.; del Grande, A.; Bisogni, G.; Madia, F.; Monaco, M.L.; Laurenti, L.; Obici, L.; Merlini, G.; Sabatelli, M. TTR-related amyloid neuropathy: Clinical, electrophysiological and pathological findings in 15 unrelated patients. Neurol. Sci. 2013, 34, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Koike, H.; Slama, M.; Coelho, T. Hereditary transthyretin amyloidosis: A model of medical progress for a fatal disease. Nat. Rev. Neurol. 2019, 15, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Lobato, L.; Rocha, A. Transthyretin amyloidosis and the kidney. Clin. J. Am. Soc. Nephrol. CJASN 2012, 7, 1337–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, P.M.; D’Ambrosio, V.; Di Paolantonio, A.; Guglielmino, V.; Calabresi, P.; Sabatelli, M.; Luigetti, M. Renal Involvement in Hereditary Transthyretin Amyloidosis: An Italian Single-Centre Experience. Brain Sci. 2021, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, V.; Ferraro, P.M.; Guglielmino, V.; Luigetti, M. Kidney involvement in hereditary transthyretin amyloidosis: Is there a role for cystatin C? Clin. Kidney J. 2022, sfac156. [Google Scholar] [CrossRef]
- Dang, J.; Segaux, L.; Moktefi, A.; Stehlé, T.; Kharoubi, M.; El Karoui, K.; Rémy, P.; Grimbert, P.; Plante-Bordeneuve, V.; Guendouz, S.; et al. Natural course and determinants of short-term kidney function decline in hereditary transthyretin amyloidosis: A French observational study. Amyloid 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Maia, L.F.; da Silva, A.M.; Cruz, M.W.; Planté-Bordeneuve, V.; Lozeron, P.; Suhr, O.B.; Campistol, J.M.; Conceição, I.M.; Schmidt, H.H.-J.; et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology 2012, 79, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. ATTR-ACT Study Investigators. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Nadal, A.; Ripoll, T.; Uson, M.; Figuerola, A.; Andreu, H.; Losada, I.; Gonzalez, J.; Cisneros-Barroso, E.; Buades, J. Significant reduction in proteinuria after treatment with tafamidis. Amyloid 2019, 26, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 2994–3015. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Rapezzi, C.; Franzini, M.; Panichella, G.; Vergaro, G.; Gillmore, J.; Fontana, M.; Passino, C.; Emdin, M. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat. Rev. Cardiol. 2022, 19, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Silva, A.; Cardoso, M.; Beirão, I.; Alves, C.; Teles, P.; Coelho, T.; Lobato, L. Transthyretin (ATTR) amyloidosis nephropathy: Lessons from a TTR stabilizer molecule. Amyloid 2017, 24, 2481–2482. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Antonini, G.; Di Paolantonio, A.; Gentile, L.; Grandis, M.; Leonardi, L.; Lozza, A.; Manganelli, F.; Mazzeo, A.; Mussinelli, R.; et al. Real-life experience with inotersen in hereditary transthyretin amyloidosis with late-onset phenotype: Data from an early-access program in Italy. Eur. J. Neurol. 2022, 29, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
| Inotersen | Patisiran | p Value | ||
|---|---|---|---|---|
| N | 9 | 8 | ||
| Sex | Count (%) | 0.93 | ||
| Males | 8 (89%) | 7 (88%) | ||
| Females | 1 (11%) | 1 (13%) | ||
| Age at evaluation (years) | Mean (SD) | 64.4 (6.7) | 66.8 (5.8) | 0.46 |
| TTR variants | ||||
| Val30Met | Count (%) | 4 (44%) | 2 (25%) | 0.25 |
| Phe64Leu | 3 (33%) | 5 (63%) | ||
| Ile68Leu | 0 (0%) | 1 (13%) | ||
| Thr59Lys | 2 (22%) | 0 (0%) | ||
| eGFR | Mean (SD) | 93.6 (7.3) | 103.6 (21.0) | 0.20 |
| Follow-up (months) | Median (IQR) | 21.0 (14.0–24.0) | 25.5 (13.5–29.0) | 0.50 |
| Subject and Sex | TTR Variant | Age at Symptom Onset | Ongoing Treatment | Age at Gene-Silencing Therapy Initiation | Months since Treatment Initiation | Phenotype | FAP Stage | PND Score |
|---|---|---|---|---|---|---|---|---|
| M #1 | Phe64Leu | 64 | patisiran | 67 | 9 | neuropathic | I | II |
| M #2 | Phe64Leu | 72 | patisiran | 75 | 15 | mixed | I | II |
| M #3 | Phe64Leu | 69 | patisiran | 77 | 39 | mixed | II | IIIa |
| M #4 | Ile68Leu | 62 | patisiran | 65 | 25 | mixed | II | IIIb |
| M #5 | Thr59Lys | 46 | inotersen | 56 | 21 | mixed | II | IIIa |
| F #6 | Thr59Lys | 53 | inotersen | 53 | 5 | mixed | I | I |
| M #7 | Phe64Leu | 64 | inotersen | 69 | 10 | mixed | II | IIIa |
| M #8 | Phe64Leu | 57 | patisiran | 62 | 12 | mixed | III | IV |
| M #9 | Val30Met | 59 | inotersen | 64 | 40 | mixed | II | IIIb |
| M #10 | Phe64Leu | 66 | inotersen | 72 | 22 | mixed | II | IIIa |
| F #11 | Phe64Leu | 61 | patisiran | 68 | 27 | neuropathic | I | II |
| M #12 | Val30Met | 53 | patisiran | 60 | 30 | mixed | II | IIIb |
| M #13 | Val30Met | 66 | inotersen | 69 | 21 | mixed | I | II |
| M #14 | Val30Met | 56 | patisiran | 67 | 26 | mixed | I | II |
| M #15 | Val30Met | 63 | inotersen | 71 | 30 | mixed | II | IIIb |
| M #16 | Val30Met | 67 | inotersen | 70 | 14 | mixed | II | IIIb |
| M #17 | Phe64Leu | 57 | inotersen | 61 | 24 | neuropathic | I | II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luigetti, M.; Guglielmino, V.; Romano, A.; Sciarrone, M.A.; Vitali, F.; D’Ambrosio, V.; Ferraro, P.M. Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers. Genes 2022, 13, 2236. https://doi.org/10.3390/genes13122236
Luigetti M, Guglielmino V, Romano A, Sciarrone MA, Vitali F, D’Ambrosio V, Ferraro PM. Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers. Genes. 2022; 13(12):2236. https://doi.org/10.3390/genes13122236
Chicago/Turabian StyleLuigetti, Marco, Valeria Guglielmino, Angela Romano, Maria Ausilia Sciarrone, Francesca Vitali, Viola D’Ambrosio, and Pietro Manuel Ferraro. 2022. "Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers" Genes 13, no. 12: 2236. https://doi.org/10.3390/genes13122236







