Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Identification of Erythroid Differentiation
2.3. Viability
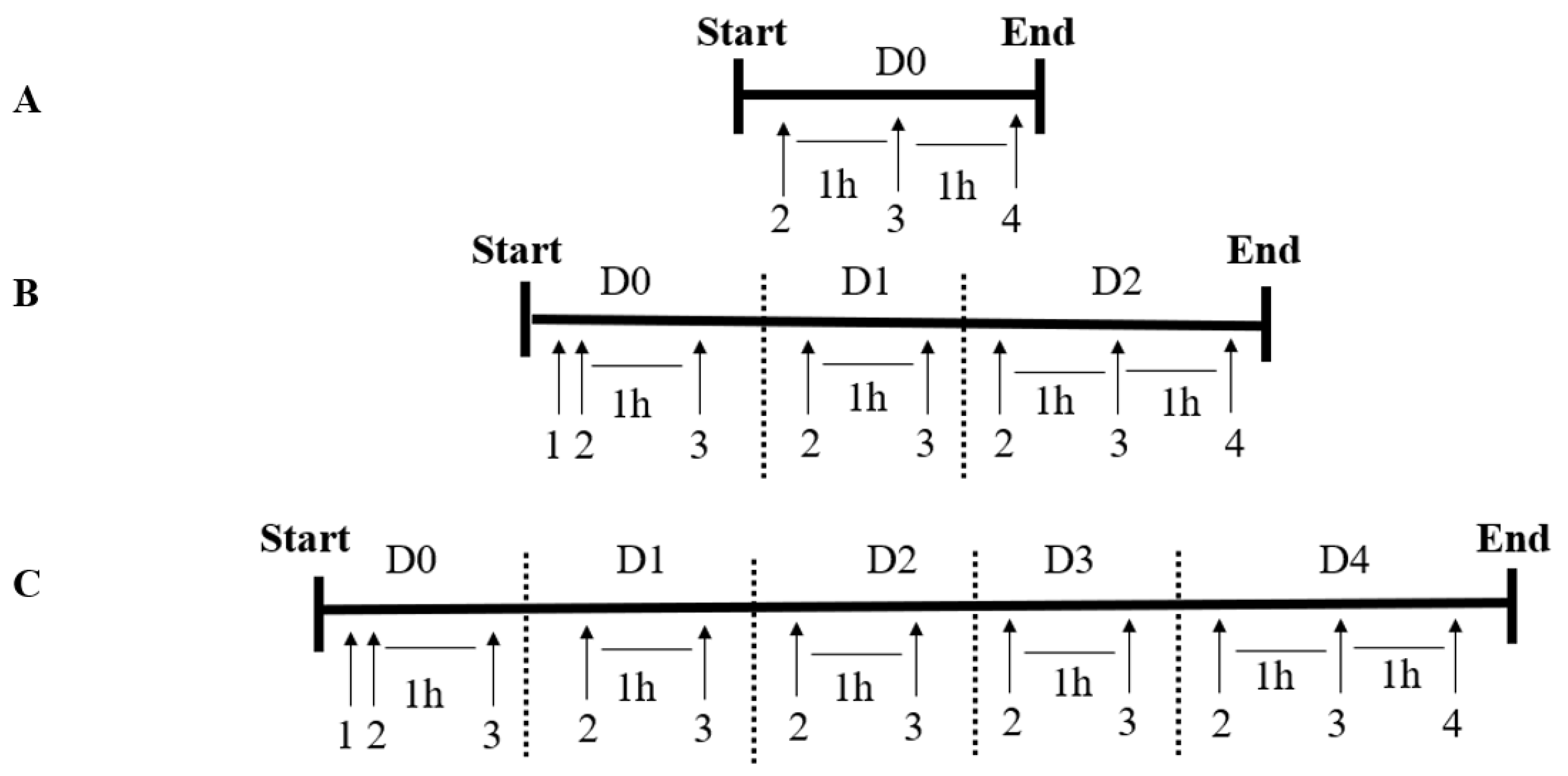
2.4. Experimental Design
2.5. Administration of Ergothioneine (Protective Agent) and Hydrogen Peroxide (Stressing Agent)
2.6. Real-Time PCR
2.7. Statistical Analyses
3. Results
3.1. Cell Viability
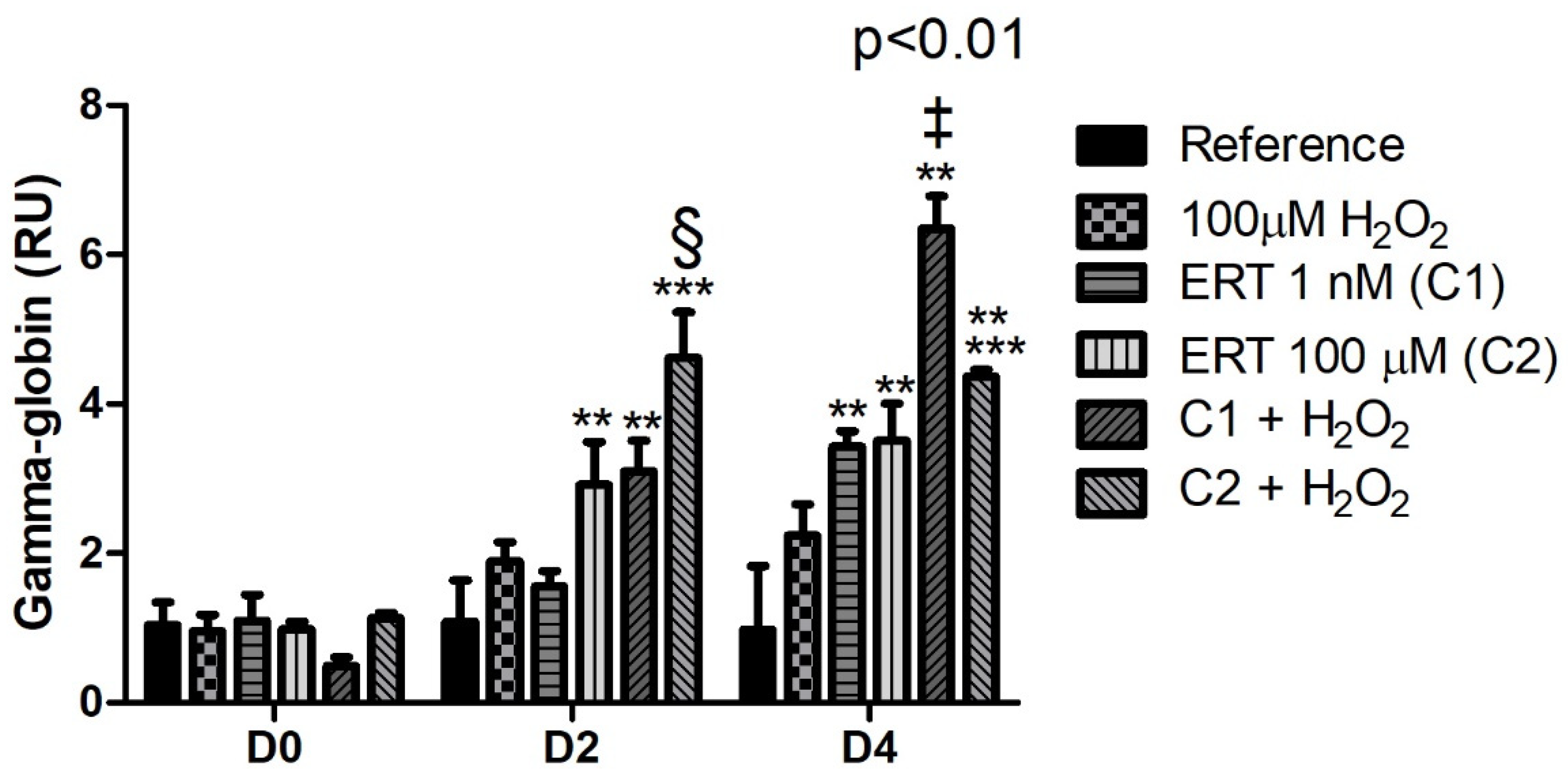
3.2. Erythroid Differentiation
3.3. Keap1/Nrf2/ARE Signaling Pathway
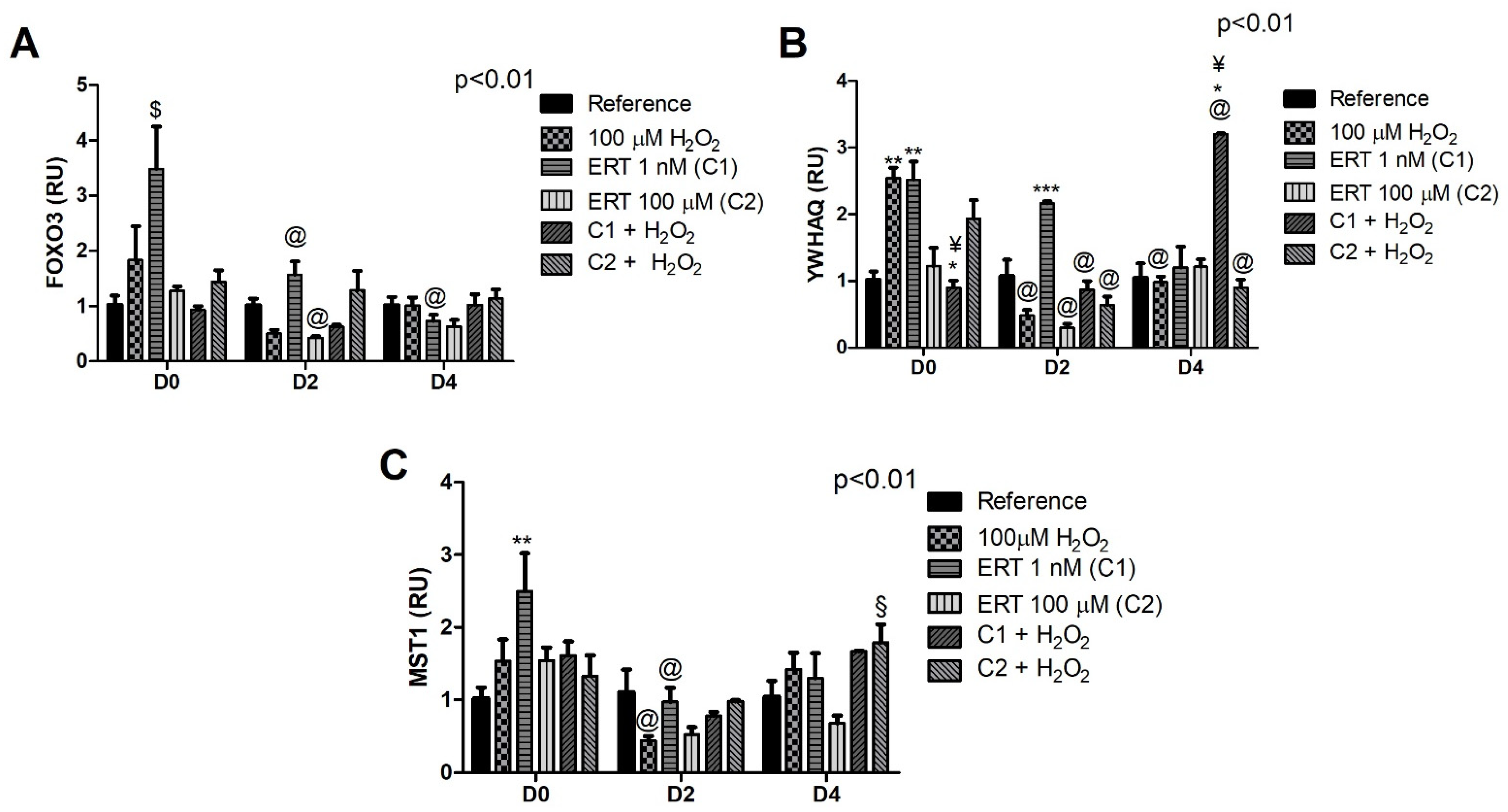
3.4. FOXO3 and Its Subcellular Location Regulators
3.5. Gene Expression of Antioxidants
3.5.1. Superoxide Dismutase (SOD1)
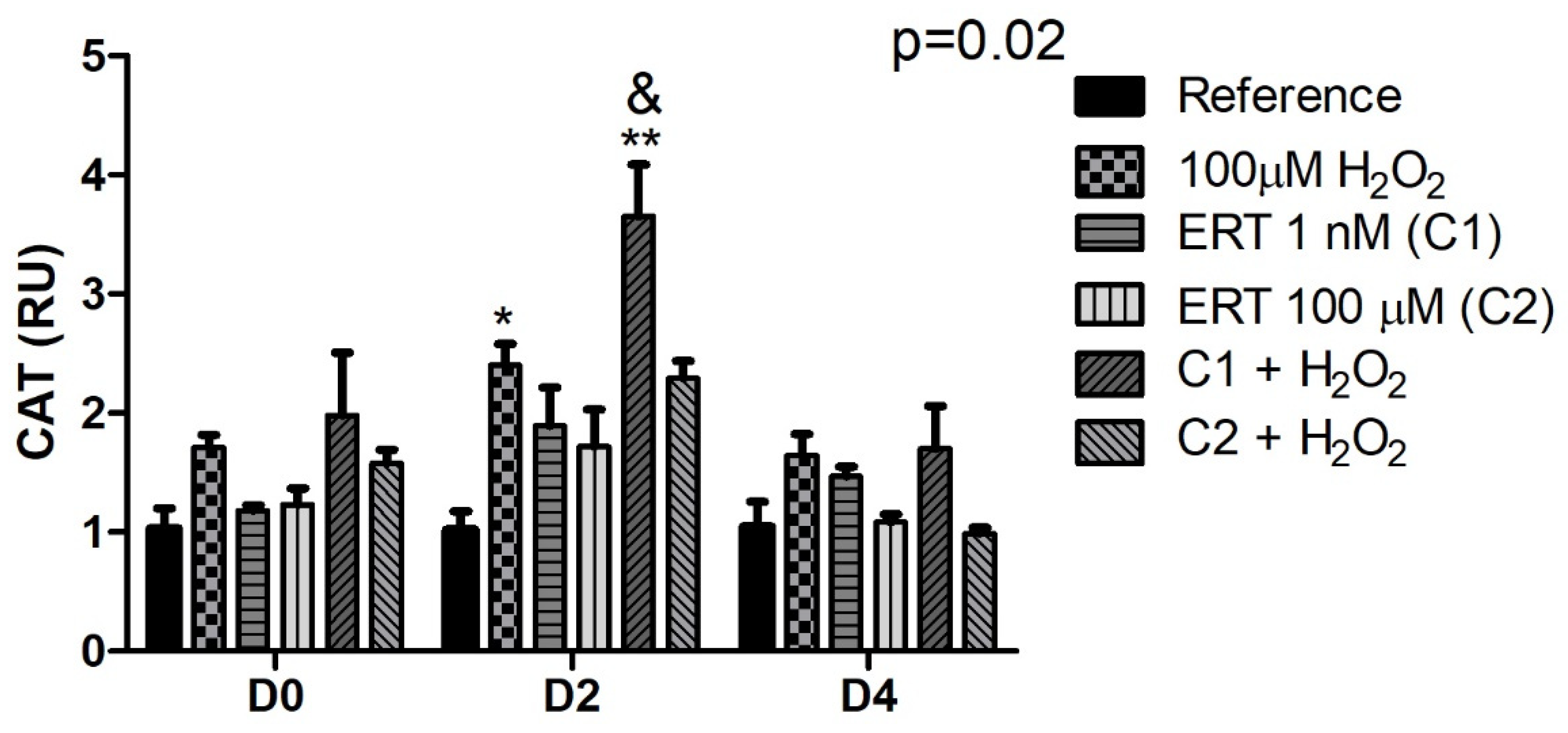
3.5.2. Catalase (CAT)
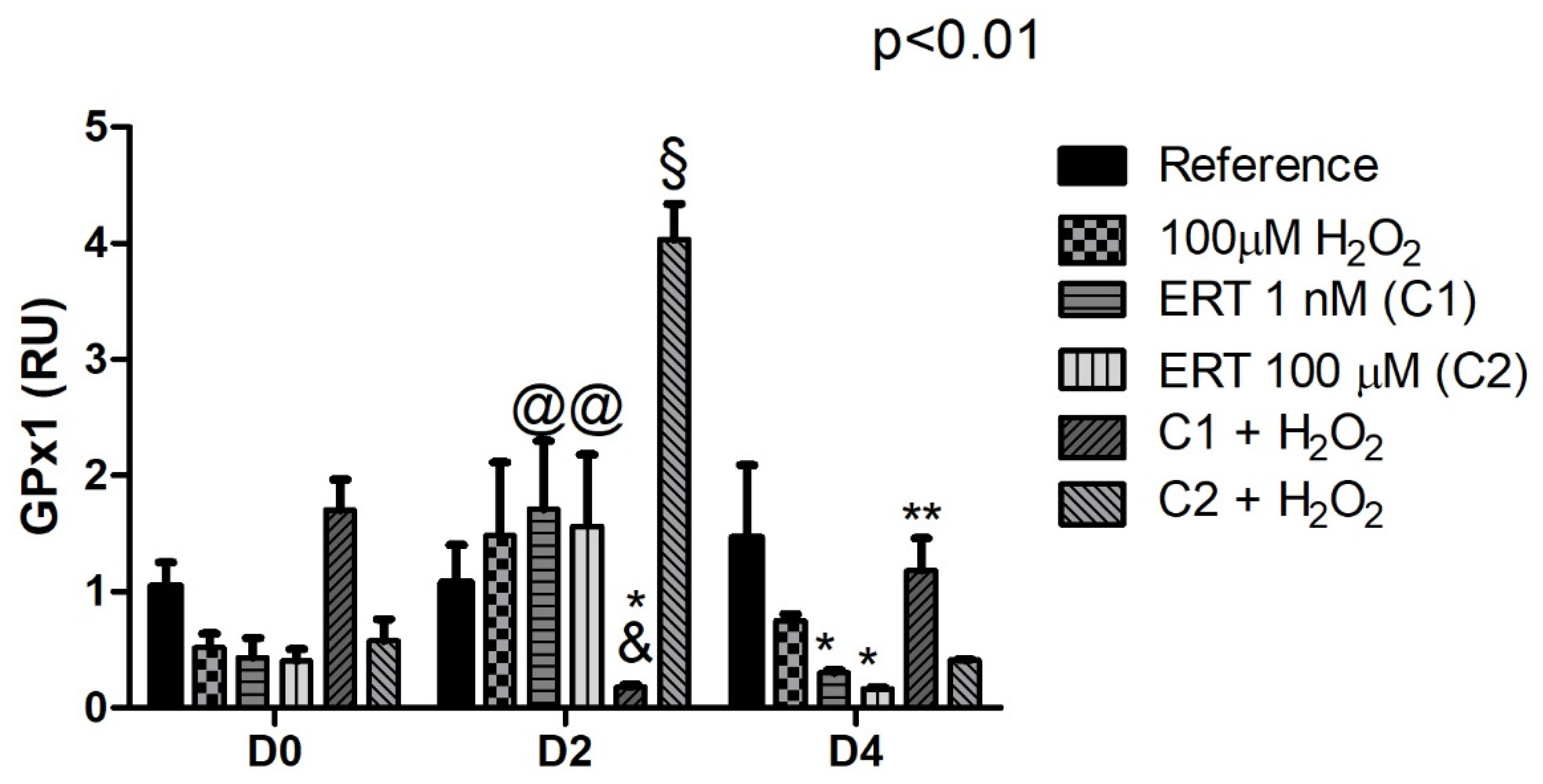
3.5.3. Glutathione Peroxidase 1 (GPX1)
3.5.4. Thioredoxin (TRX)
3.5.5. Peroxiredoxins (PRDXs)
3.6. Proteasome
3.7. Overview of the Expression Pattern of the Genes Involved in the Redox Adaptation Mechanisms in K562 Cells
3.8. Associations between Expressions of Redox Pathways Genes and Antioxidants in Erythroid Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vona, R.; Sposi, N.M.; Mattia, L.; Gambardella, L.; Straface, E.; Pietraforte, D. Sickle Cell Disease: Role of Oxidative Stress and Antioxidant Therapy. Antioxidants 2021, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A Novel and Compact Review on the Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [Green Version]
- Pala, F.S.; Gürkan, H. The Role of Free Radicals in Ethiopathogenesis of Diseases. Adv. Mol. Biol. 2008, 1, 9. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Halliwell, B. The Antioxidant Paradox: Less Paradoxical Now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2015, 96, 307–364. [Google Scholar] [CrossRef] [Green Version]
- Poljsak, B.; Milisav, I. The Neglected Significance of “Antioxidative Stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauber, S.; Steinbrenner, H.; Klotz, L.-O. FoxO Transcription Factors in the Control of Redox Homeostasis and Fuel Metabolism; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128186060. [Google Scholar]
- Chen, X.; Cao, X.; Xiao, W.; Li, B.; Xue, Q. PRDX5 as a Novel Binding Partner in Nrf2-Mediated NSCLC Progression under Oxidative Stress. Aging 2020, 12, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Macari, E.R.; Lowrey, C.H. Induction of Human Fetal Hemoglobin via the NRF2 Antioxidant Response Signaling Pathway. Blood 2011, 117, 5987–5997. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.-K.; Wakabayashi, N.; Greenlaw, J.L.; Yamamoto, M.; Kensler, T.W. Antioxidants Enhance Mammalian Proteasome Expression through the Keap1-Nrf2 Signaling Pathway. Mol. Cell. Biol. 2003, 23, 8786–8794. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.K.; Wakabayashi, N.; Itoh, K.; Motohashi, H.; Yamamoto, M.; Kensler, T.W. Modulation of Gene Expression by Cancer Chemopreventive Dithiolethiones through the Keap1-Nrf2 Pathway: Identification of Novel Gene Clusters for Cell Survival. J. Biol. Chem. 2003, 278, 8135–8145. [Google Scholar] [CrossRef] [Green Version]
- Boccitto, M.; Kalb, R. Regulation of Foxo-Dependent Transcription by Post-Translational Modifications. Curr. Drug Targets 2011, 12, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Kodani, N.; Nakae, J. Tissue-Specific Metabolic Regulation of FOXO-Binding Protein: FOXO Does Not Act Alone. Cells 2020, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and Acetylation Modifications of FOXO3a: Independently or Synergistically? Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef] [Green Version]
- Melville, D.B. Ergothioneine. Vitam. Horm. 1959, 17, 155–204. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, Recent Developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Halliwell, B. Ergothioneine; Antioxidant Potential, Physiological Function and Role in Disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 784–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A Diet-Derived Antioxidant with Therapeutic Potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [Green Version]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, R.; Spézia, F.; Papineau, D.; Sabadie, C.; Erdelmeier, I.; Moutet, M.; Yadan, J.C. Reproductive Safety Evaluation of L-Ergothioneine. Food Chem. Toxicol. 2015, 80, 85–91. [Google Scholar] [CrossRef]
- Marone, P.A.; Trampota, J.; Weisman, S. A Safety Evaluation of a Nature-Identical l-Ergothioneine in Sprague Dawley Rats. Int. J. Toxicol. 2016, 35, 568–583. [Google Scholar] [CrossRef] [Green Version]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Statement on the Safety of Synthetic L-ergothioneine as a Novel Food—Supplementary Dietary Exposure and Safety Assessment for Infants and Young Children, Pregnant and Breastfeeding Women. EFSA J. 2017, 15, 5060. [Google Scholar] [CrossRef] [Green Version]
- Song, T.Y.; Chen, C.L.; Liao, J.W.; Ou, H.C.; Tsai, M.S. Ergothioneine Protects against Neuronal Injury Induced by Cisplatin Both in Vitro and in Vivo. Food Chem. Toxicol. 2010, 48, 3492–3499. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lo, H.W.; Korivi, M.; Tsai, Y.C.; Tang, M.J.; Yang, H.L. Dermato-Protective Properties of Ergothioneine through Induction of Nrf2/ARE-Mediated Antioxidant Genes in UVA-Irradiated Human Keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the Ergothioneine Transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [PubMed]
- Grigat, S.; Harlfinger, S.; Pal, S.; Striebinger, R.; Golz, S.; Geerts, A.; Lazar, A.; Schömig, E.; Gründemann, D. Probing the Substrate Specificity of the Ergothioneine Transporter with Methimazole, Hercynine, and Organic Cations. Biochem. Pharmacol. 2007, 74, 309–316. [Google Scholar] [CrossRef]
- Jelkmann, W. Physiology and Pharmacology of Erythropoietin. Transfus. Med. Hemother. 2013, 40, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paula, C.P.D. Efeito da Melatonina na Proteção Contra Estresse Oxidativo em Células Eritrocitárias K562. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2020; pp. 1–131. [Google Scholar]
- Rowley, P.T.; Ohlsson-Wilhelm, B.M.; Farley, B.A.; LaBella, S. Inducers of Erythroid Differentiation in K562 Human Leukemia Cells. Exp. Hematol. 1981, 9, 32–37. [Google Scholar] [PubMed]
- Tennanth, J. Evaluation of the Trypan Blue Technique for Determination of Cell Viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef]
- da Silva, D.G.H.; Ricci, O.; de Almeida, E.A.; Bonini-Domingos, C.R. Potential Utility of Melatonin as an Antioxidant Therapy in the Management of Sickle Cell Anemia. J. Pineal Res. 2015, 58, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Feng, L.; Tang, R.M.Y.; Lim, K.H.C.; Halliwell, B. Ergothioneine Levels in an Elderly Population Decrease with Age and Incidence of Cognitive Decline; a Risk Factor for Neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Chaves, N.A.; Alegria, T.G.P.; Dantas, L.S.; Netto, L.E.S.; Miyamoto, S.; Bonini Domingos, C.R.; da Silva, D.G.H. Impaired Antioxidant Capacity Causes a Disruption of Metabolic Homeostasis in Sickle Erythrocytes. Free Radic. Biol. Med. 2019, 141, 34–46. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: New York, NY, USA, 2002; Volume 4, ISBN 9780521811286. [Google Scholar]
- McDonald, J.H. Handbook of Biolological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Tschirka, J.; Kreisor, M.; Betz, J.; Gründemann, D. Substrate Selectivity Check of the Ergothioneine Transporter. Drug Metab. Dispos. 2018, 46, 779–785. [Google Scholar] [CrossRef]
- Suh, J.H.; Kim, R.; Yavuz, B.; Lee, D.; Lal, A.; Ames, B.N.; Mark, K. Clinical Assay of Four Thiol Amino Acid Redox Couples by LC-MS/MS: Utility in Thalassemia. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A. Hemoglobin S Polymerization and Red Cell Membrane Changes. Hematol. Oncol. Clin. N. Am. 2014, 28, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an Adaptive Antioxidant for the Protection of Injured Tissues? A Hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Tang, R.; Ye, P.; Yew, T.S.Z.; Lim, K.H.S.; Halliwell, B. Liver Ergothioneine Accumulation in a Guinea Pig Model of Non-Alcoholic Fatty Liver Disease. A Possible Mechanism of Defence? Free Radic. Res. 2016, 50, 14–25. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.M.; Yew, T.S.K.; Halliwell, B. Distribution and Accumulation of Dietary Ergothioneine and Its Metabolites in Mouse Tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef] [Green Version]
- Cheah, I.K.; Halliwell, B. Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef]
- Schröder, E.; Eaton, P. Hydrogen Peroxide as an Endogenous Mediator and Exogenous Tool in Cardiovascular Research: Issues and Considerations. Curr. Opin. Pharmacol. 2008, 8, 153–159. [Google Scholar] [CrossRef]
- Benfeitas, R.; Selvaggio, G.; Antunes, F.; Coelho, P.M.B.M.; Salvador, A. Hydrogen Peroxide Metabolism and Sensing in Human Erythrocytes: A Validated Kinetic Model and Reappraisal of the Role of Peroxiredoxin II. Free Radic. Biol. Med. 2014, 74, 35–49. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Lim, J.B.; Langford, T.F.; Huang, B.K.; Deen, W.M.; Sikes, H.D. A Reaction-Diffusion Model of Cytosolic Hydrogen Peroxide. Free Radic. Biol. Med. 2016, 90, 85–90. [Google Scholar] [CrossRef]
- Travasso, R.D.M.; Sampaio dos Aidos, F.; Bayani, A.; Abranches, P.; Salvador, A. Localized Redox Relays as a Privileged Mode of Cytoplasmic Hydrogen Peroxide Signaling. Redox Biol. 2017, 12, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Chénais, B.; Andriollo, M.; Guiraud, P.; Belhoussine, R.; Jeannesson, P. Oxidative Stress Involvement in Chemically Induced Differentiation of K562 Cells. Free Radic. Biol. Med. 2000, 28, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hietakangas, V.; Poukkula, M.; Heiskanen, K.M.; Karvinen, J.T.; Sistonen, L.; Eriksson, J.E. Erythroid Differentiation Sensitizes K562 Leukemia Cells to TRAIL-Induced Apoptosis by Downregulation of c-FLIP. Mol. Cell. Biol. 2003, 23, 1278–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isoda, H.; Motojima, H.; Onaga, S.; Samet, I.; Villareal, M.O.; Han, J. Analysis of the Erythroid Differentiation Effect of Flavonoid Apigenin on K562 Human Chronic Leukemia Cells. Chem. Biol. Interact. 2014, 220, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Malik, Z.; Chitayat, S.D.; Langzam, Y. Hemin Dependent Morphological Maturation and Endogenous Porphyrin Synthesis by K562 Leukemic Cells. Cancer Lett. 1988, 41, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, L.Y.; Mu, D.; Gong, J.H.; Chen, J. Induction of Apoptosis and Erythroid Differentiation of Human Chronic Myelogenous Leukemia K562 Cells by Low Concentrations of Lidamycin. Oncol. Rep. 2019, 41, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Menon, V.; Ghaffari, S. Following Transcriptome to Uncover FOXO Biological Functions. Methods Mol. Biol. 2019, 1890, 219–227. [Google Scholar] [CrossRef]
- Menon, V.; Ghaffari, S. Transcription Factors FOXO in the Regulation of Homeostatic Hematopoiesis. Curr. Opin. Hematol. 2018, 25, 290. [Google Scholar] [CrossRef]
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F.; Takubo, K.; Yamazaki, S.; Matsuoka, S.; Miyamoto, T.; Ito, K.; Ohmura, M.; et al. Foxo3a Is Essential for Maintenance of the Hematopoietic Stem Cell Pool. Cell Stem Cell 2007, 1, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Thanuthanakhun, N.; Nuntakarn, L.; Sampattavanich, S.; Anurathapan, U.; Phuphanitcharoenkun, S.; Pornpaiboonstid, S.; Borwornpinyo, S.; Hongeng, S. Investigation of FoxO3 Dynamics during Erythroblast Development in β-Thalassemia Major. PLoS ONE 2017, 12, e0187610. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.; Wang, S.; Zhang, Q.; Zheng, J.; Yang, Y.; Qi, H.; Qu, H.; Zhang, Z.; Liu, F.; et al. Knockdown of Transcription Factor Forkhead Box O3 (FOXO3) Suppresses Erythroid Differentiation in Human Cells and Zebrafish. Biochem. Biophys. Res. Commun. 2015, 460, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Paikari, A.; Sumazin, P.; Ginter Summarell, C.C.; Crosby, J.R.; Boerwinkle, E.; Weiss, M.J.; Sheehan, V.A. Metformin Induces FOXO3-Dependent Fetal Hemoglobin Production in Human Primary Erythroid Cells. Blood 2018, 132, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, B.; Pace, B.S. NRF2 Mediates γ-Globin Gene Regulation and Fetal Hemoglobin Induction in Human Erythroid Progenitors. Haematologica 2017, 102, e285–e288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.; Conran, N. Emerging Pharmacotherapeutic Approaches for the Management of Sickle Cell Disease. Expert Opin. Pharmacother. 2018, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugiura, S.; Kobayashi, D.; Yoshida, K.; Yabuuchi, H.; Aizawa, S.; Maeda, T.; Tamai, I. Decreased Proliferation and Erythroid Differentiation of K562 Cells by SiRNA-Induced Depression of OCTN1 (SLC22A4) Transporter Gene. Pharm. Res. 2007, 24, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ma, W.; Liu, P.; Zhou, F. Overview of Thioredoxin System and Targeted Therapies for Acute Leukemia. Mitochondrion 2019, 47, 38–46. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 Regulation of Antioxidant Genes Is Dependent on the Formation of a FoxO3a/PGC-1α Complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid. Redox Signal. 2018, 29, 541–551. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Redox Biology: Signaling via a Peroxiredoxin Sensor. Nat. Chem. Biol. 2015, 11, 5–6. [Google Scholar] [CrossRef]
- Czech, M.; Lawrence, J.; Lynn, W. Hexose Transport in Isolated Brown Fat Cells. A Model System for Investigating Insulin Action on Membrane Transport. J. Biol. Chem. 1974, 249, 5421–5427. [Google Scholar] [CrossRef]
- Mahadev, K.; Wu, X.; Zilbering, A.; Zhu, L.; Lawrence, J.T.R.; Goldstein, B.J. Hydrogen Peroxide Generated during Cellular Insulin Stimulation Is Integral to Activation of the Distal Insulin Signaling Cascade in 3T3-L1 Adipocytes. J. Biol. Chem. 2001, 276, 48662–48669. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H. Interference of Selenium and Selenoproteins with the Insulin-Regulated Carbohydrate and Lipid Metabolism. Free Radic. Biol. Med. 2013, 65, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Szypowska, A.A.; Burgering, B.M.T. The Peroxide Dilemma: Opposing and Mediating Insulin Action. Antioxid. Redox Signal. 2011, 15, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Zhang, L.; Gong, Z.; Hou, X.; Xu, Y.; Feng, X.; Wang, H.; You, H. FoxO3 Inactivation Promotes Human Cholangiocarcinoma Tumorigenesis and Chemoresistance through Keap1-Nrf2 Signaling. Hepatology 2016, 63, 1914–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dare, A.; Channa, M.L.; Nadar, A. L-Ergothioneine and Its Combination with Metformin Attenuates Renal Dysfunction in Type-2 Diabetic Rat Model by Activating Nrf2 Antioxidant Pathway. Biomed. Pharmacother. 2021, 141, 111921. [Google Scholar] [CrossRef]
- Servillo, L.; Castaldo, D.; Casale, R.; D’Onofrio, N.; Giovane, A.; Cautela, D.; Balestrieri, M.L. An Uncommon Redox Behavior Sheds Light on the Cellular Antioxidant Properties of Ergothioneine. Free Radic. Biol. Med. 2015, 79, 228–236. [Google Scholar] [CrossRef]
- Hopkins, B.L.; Nadler, M.; Skoko, J.J.; Bertomeu, T.; Pelosi, A.; Shafaei, P.M.; Levine, K.; Schempf, A.; Pennarun, B.; Yang, B.; et al. A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 MicroRNA Target Let-7. Antioxid. Redox Signal. 2018, 28, 62–77. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Kang, D.; Yu, D.Y.; Rhee, S.G. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H2O2 Accumulation for Cell Signaling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin Evolution and the Regulation of Hydrogen Peroxide Signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H2O2, and Protein Chaperones. Antioxid. Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, T.; Huang, P. Post-Translational Modifications of FOXO Family Proteins (Review). Mol. Med. Rep. 2016, 14, 4931–4941. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Reinheckel, T.; Davies, K.J.A. Degradation of Oxidized Proteins in K562 Human Hematopoietic Cells by Proteasome. J. Biol. Chem. 1996, 271, 15504–15509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Yoshida, T.; Kikuchi, S.; Nagao, K.; Kokubu, A.; Pluskal, T.; Villar-Briones, A.; Nakamura, T.; Yanagida, M. Synergistic Roles of the Proteasome and Autophagy for Mitochondrial Maintenance and Chronological Lifespan in Fission Yeast. Proc. Natl. Acad. Sci. USA 2010, 107, 3540–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozov, A.V.; Karpov, V.L. Biological Consequences of Structural and Functional Proteasome Diversity. Heliyon 2018, 4, e00894. [Google Scholar] [CrossRef]







| Dependent Variable | Independent Variable |
|---|---|
| Members of FOXO3 and Nrf2 pathways | |
| PRDX1 | R = 0.81; p < 0.00 |
| PRDX2 | R = 0.77; p < 0.00 |
| PRDX6 | R = 0.35; p = 0.24 |
| SOD1 | R = 0.46; p = 0.03 |
| CAT | R = 0.66; p < 0.00 |
| GPX1 | R = 0.45; p = 0.04 |
| TRX | R = 0.75; p < 0.00 |
| PSMB5 | R = 0.50; p = 0.01 |
| PSMB6 | R = 0.38; p = 0.15 |
| γ-globin | R = 0.29; p = 0.47 |
| Dependent Variable | Independent Variable | ||||
|---|---|---|---|---|---|
| Nrf2 | Keap1 | FOXO3 | MST1 | 14-3-3 | |
| PRDX1 | r = 0.72; p < 0.00 | r = 0.49; p < 0.00 | r = 0.23; p = 0.10 | r = −0.25; p = 0.06 | r = 0.18; p = 0.03 |
| PRDX2 | r = 0.28; p = 0.04 | r = 0.69; p < 0.00 | r = 0.29; p = 0.03 | r = 0.07; p = 0.58 | r = −0.25; p < 0.00 |
| SOD1 | r = 0.31; p = 0.02 | r = 0.15; p = 0.27 | r = 0.11; p = 0.41 | r = −0.32; p = 0.01 | r = 0.08; p = 0.49 |
| CAT | r = 0.63; p < 0.00 | r = 0.10; p = 0.45 | r = 0.08; p = 0.55 | r = −0.19; p = 0.18 | r = −0.07; p = 0.46 |
| GPX1 | r = −0.24; p = 0.08 | r = 0.33; p = 0.01 | r = 0.09; p = 0.49 | r = −0.20; p = 0.15 | r = −0.10; p = 0.41 |
| TRX | r = 0.04; p = 0.74 | r = 0.26; p = 0.06 | r = 0.45; p < 0.00 | r = −0.04; p = 0.76 | r = 0.49; p < 0.00 |
| PSMB5 | r = −0.10; p = 0.44 | r = 0.50; p < 0.00 | r = −0.00; p = 0.95 | r = −0.13; p = 0.29 | r = 0.07; p = 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, V.S.; Torres, F.F.; de Paula, C.P.; da Silva, J.P.M.d.O.; de Almeida, E.A.; da Cunha, A.F.; da Silva, D.G.H. Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells. Genes 2022, 13, 2368. https://doi.org/10.3390/genes13122368
Bernardo VS, Torres FF, de Paula CP, da Silva JPMdO, de Almeida EA, da Cunha AF, da Silva DGH. Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells. Genes. 2022; 13(12):2368. https://doi.org/10.3390/genes13122368
Chicago/Turabian StyleBernardo, Victoria Simões, Flaviene Felix Torres, Carla Peres de Paula, João Pedro Maia de Oliveira da Silva, Eduardo Alves de Almeida, Anderson Ferreira da Cunha, and Danilo Grünig Humberto da Silva. 2022. "Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells" Genes 13, no. 12: 2368. https://doi.org/10.3390/genes13122368







