TWIST1 Plays Role in Expression of Stemness State Markers in ESCC
Abstract
:1. Introduction
2. Materials and methods
2.1. In Silico Sequence Analysis
2.2. Cell Lines and Culture Condition
2.3. Retroviral Production and Enforced TWIST1 Overexpression
2.4. RNA Extraction, cDNA Synthesis, Comparative Real-Time PCR, and Statistical Analysis
3. Results
3.1. Sequence Analysis of Cancer Stem Cell Self-Renewal Genes Promoter
3.2. Upregulation of TWIST1 in ESCC Cell Line KYSE-30
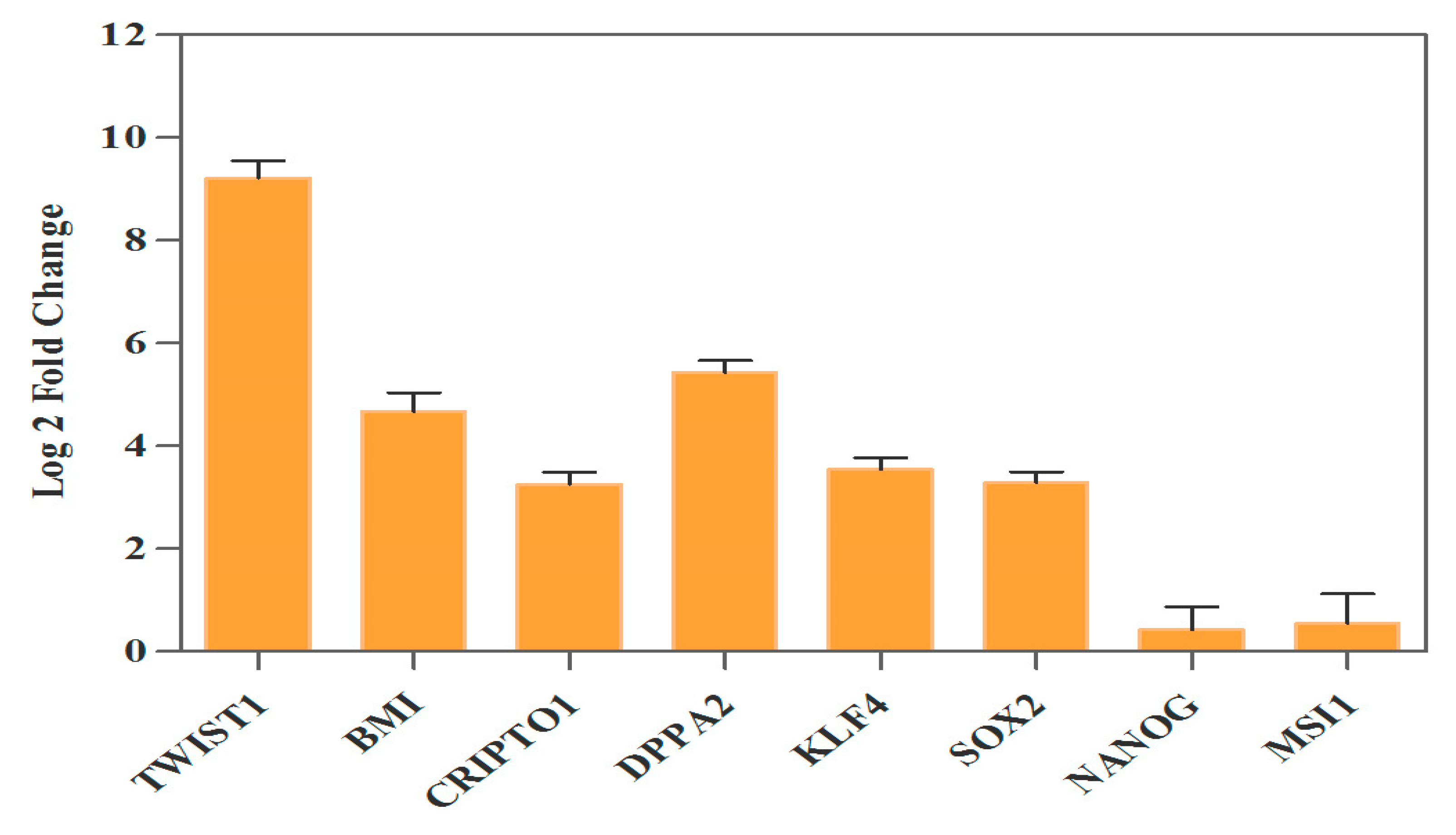
3.3. Ectopic Expression of TWIST1 Increased Expression of Cancer Stem Cell Self-Renewal Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.-L.; Wang, S.-S.; Jiang, J.; Liang, X.-H. Links between cancer stem cells and epithelial– mesenchymal transition. OncoTargets Ther. 2015, 8, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savagner, P. The epithelial–mesenchymal transition (EMT) phenomenon. Ann. Oncol. 2010, 21, vii89–vii92. [Google Scholar] [CrossRef]
- Martín, A.; Cano, A. Tumorigenesis: Twist1 links EMT to self-renewal. Nat. Cell Biol. 2010, 12, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-M.; Hu, S.-Q.; Jiang, H.; Chen, Y.-L.; Feng, J.-H.; Chen, Z.-Q.; Wen, K.-M. OCT4B1 Promoted EMT and Regulated the Self-Renewal of CSCs in CRC: Effects Associated with the Balance of miR-8064/PLK1. Mol. Ther.-Oncolytics 2019, 15, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Weidenfeld, K.; Barkan, D. EMT and Stemness in Tumor Dormancy and Outgrowth: Are They Intertwined Processes? Front. Oncol. 2018, 8, 381. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Li, B.; Liu, F.; Zhang, M.; Wang, Q.; Liu, Y.; Yao, Y.; Li, D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer 2017, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Abell, A.N.; Johnson, G.L. Implications of Mesenchymal Cells in Cancer Stem Cell Populations: Relevance to EMT. Curr. Pathobiol. Rep. 2014, 2, 21–26. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khales, S.A.; Abbaszadegan, M.R.; Majd, A.; Forghanifard, M.M. Linkage between EMT and stemness state through molecular association between TWIST1 and NY-ESO1 in esophageal squamous cell carcinoma. Biochimie 2019, 163, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Forghanifard, M.M.; Rad, A.; Farshchian, M.; Khaleghizadeh, M.; Gholamin, M.; Moghbeli, M.; Abbaszadegan, M.R. TWIST1 upregulates the MAGEA4 oncogene. Mol. Carcinog. 2016, 56, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Forghanifard, M.M.; Khales, S.A.; Farshchian, M.; Rad, A.; Homayouni-Tabrizi, M.; Abbaszadegan, M.R. Negative Regulatory Role of TWIST1 on SNAIL Gene Expression. Pathol. Oncol. Res. 2016, 23, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, R.A.; Bahadori, B.; Rad, A.; Abbaszadegan, M.R.; Forghanifard, M.M. MEIS1 knockdown may promote differentiation of esophageal squamous carcinoma cell line KYSE-30. Mol. Genet. Genom. Med. 2019, 7, e00746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadpanah, M.H.; Abbaszadegan, M.R.; Fahim, Y.; Forghanifard, M.M. Ectopic expression of TWIST1 upregulates the stemness marker OCT4 in the esophageal squamous cell carcinoma cell line KYSE30. Cell. Mol. Biol. Lett. 2017, 22, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayachandran, A.; Dhungel, B.; Steel, J.C. Epithelial-to-mesenchymal plasticity of cancer stem cells: Therapeutic targets in hepatocellular carcinoma. J. Hematol. Oncol. 2016, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Rahman, M.A.; Chen, Z.G.; Shin, D.M. Multiple biological functions of Twist1 in various cancers. Oncotarget 2017, 8, 20380–20393. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Du, P.; Ge, Z.; Jin, Y.; Ding, D.; Liu, X.; Zou, Q. TWIST1 and BMI1 in Cancer Metastasis and Chemoresistance. J. Cancer 2016, 7, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-Y.; Liu, X.-Y.; Wang, N.; Wang, L.-N.; Yang, B.-X.; Ren, Q.; Liang, H.-Y.; Ma, X.-T. Twist-1, A Novel Regulator of Hematopoietic Stem Cell Self-Renewal and Myeloid Lineage Development. Stem Cells 2014, 32, 3173–3182. [Google Scholar] [CrossRef]
- Mahmoudian, R.A.; Abbaszadegan, M.R.; Forghanifard, M.M.; Moghbeli, M.; Moghbeli, F.; Chamani, J.; Gholamin, M. Biological and Clinicopathological Significance of Cripto-1 Expression in the Progression of Human ESCC. Rep. Biochem. Mol. Biol. 2017, 5, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Moghbeli, M.; Forghanifard, M.M.; Aarabi, A.; Mansourian, A.; Abbaszadegan, M.R. Clinicopathological Sex- Related Relevance of Musashi1 mRNA Expression in Esophageal Squamous Cell Carcinoma Patients. Pathol. Oncol. Res. 2013, 20, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Forghanifard, M.M.; Khales, S.A.; Javdani-Mallak, A.; Rad, A.; Farshchian, M.; Abbaszadegan, M.R. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med. Oncol. 2014, 31, 922. [Google Scholar] [CrossRef] [PubMed]
- Forghanifard, M.M.; Moaven, O.; Farshchian, M.; Montazer, M.; Raeisossadati, R.; Abdollahi, A.; Moghbeli, M.; Nejadsattari, T.; Parivar, K.; Abbaszadegan, M.R. Expression Analysis Elucidates the Roles of MAML1 and Twist1 in Esophageal Squamous Cell Carcinoma Aggressiveness and Metastasis. Ann. Surg. Oncol. 2011, 19, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Noh, K.H.; Lee, Y.-H.; Song, K.-H.; Oh, S.J.; Kim, S.Y.; Kim, T.W. NANOG signaling promotes metastatic capability of immunoedited tumor cells. Clin. Exp. Metastasis 2015, 32, 429–439. [Google Scholar] [CrossRef]
- Paranjape, A.N.; Balaji, S.A.; Mandal, T.; Krushik, E.V.; Nagaraj, P.; Mukherjee, G.; Rangarajan, A. Bmi1 regulates self-renewal and epithelial to mesenchymal transition in breast cancer cells through Nanog. BMC Cancer 2014, 14, 785. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Rzemieniewska, N.; Bednarek, I.A. The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biol. Ther. 2015, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.-H.; Hsu, D.S.-S.; Wang, H.-W.; Wang, H.-J.; Lan, H.-Y.; Yang, W.-H.; Huang, C.-H.; Kao, S.-Y.; Tzeng, C.-H.; Tai, S.-K.; et al. Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nature 2010, 12, 982–992. [Google Scholar] [CrossRef]
- Cho, J.-H.; Dimri, M.; Dimri, G.P. A Positive Feedback Loop Regulates the Expression of Polycomb Group Protein BMI1 via WNT Signaling Pathway. J. Biol. Chem. 2013, 288, 3406–3418. [Google Scholar] [CrossRef] [Green Version]
- Kamijo, T. Role of stemness-related molecules in neuroblastoma. Pediatr. Res. 2012, 71, 511–515. [Google Scholar] [CrossRef]
- Hadjimichael, C.; Chanoumidou, K.; Papadopoulou, N.; Arampatzi, P.; Papamatheakis, J.; Kretsovali, A. Common stemness regulators of embryonic and cancer stem cells. World J. Stem Cells 2015, 7, 1150–1184. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.Y.; Wong, E.S.Y.; Kong, D.S.H.; Chan, H.Y.; Jiang, L.; Wong, O.G.W.; Lam, E.W.-F.; Chan, K.K.L.; Ngan, H.Y.S.; Le, X.-F.; et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene 2012, 32, 3500–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-F.; Wu, K.-J. Endothelial Transdifferentiation of Tumor Cells Triggered by the Twist1-Jagged1-KLF4 Axis: Relationship between Cancer Stemness and Angiogenesis. Stem Cells Int. 2015, 2016, 6439864. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Katz, J.P. KLF4 is downregulated but not mutated during human esophageal squamous cell carcinogenesis and has tumor stage-specific functions. Cancer Biol. Ther. 2016, 17, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-F.; Huang, C.-H.; Liu, C.-J.; Hung, J.-J.; Hsu, C.-C.; Teng, S.-C.; Wu, K.-J. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat. Commun. 2014, 5, 4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ye, X.; Wang, P.; Jung, K.; Wu, C.; Douglas, D.; Kneteman, N.; Bigras, G.; Ma, Y.; Lai, R. Sox2 suppresses the invasiveness of breast cancer cells via a mechanism that is dependent on Twist1 and the status of Sox2 transcription activity. BMC Cancer 2013, 13, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velpula, K.K.; Dasari, V.R.; Tsung, A.J.; Dinh, D.H.; Rao, J.S. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget 2011, 2, 1028–1042. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts. Cell Stem Cell 2010, 7, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Rangel, M.C.; Karasawa, H.; Castro, N.P.; Nagaoka, T.; Salomon, D.S.; Bianco, C. Role of Cripto-1 during Epithelial-to-Mesenchymal Transition in Development and Cancer. Am. J. Pathol. 2012, 180, 2188–2200. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, X.; Yu, X.; Bian, B.-S.; Qian, F.; Hu, X.-G.; Ji, C.-D.; Yang, L.; Ren, Y.; Cui, W.; et al. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol. Cancer 2017, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Guo, Y.-Z.; Yue, X.; Zhang, G.-P.; Zhang, Y.; Kuang, M.; Peng, B.-G.; Li, S.-Q. Cripto-1 promotes tumor invasion and predicts poor outcomes in hepatocellular carcinoma. Carcinogenesis 2019, 41, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-J.; Chen, S.-N.; Chen, W.-G.; Wu, G.-Q.; Liao, Y.-F.; Xu, J.-B.; Tang, H.; Yang, S.-H.; He, S.-Y.; Luo, Y.-F.; et al. Cripto-1 expression in patients with clear cell renal cell carcinoma is associated with poor disease outcome. J. Exp. Clin. Cancer Res. 2019, 38, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.S.; Salem, A.M.; Ahmed, R.Z.; Amer, S.A.; Abozaid, M.M. Prognostic and predictive values of Twist-1 and Cripto-1 expressions in non-small cell carcinoma of lung: An immunohistochemical study. Egypt. J. Pathol. 2018, 38, 190–198. [Google Scholar]
- Khaleghizadeh, M.; Forghanifard, M.M.; Rad, A.; Farshchian, M.; Hejazi, Z.; Gholamin, M.; Memar, B.; Abbaszadegan, M.R. Ectopic Expression of Human DPPA2 Gene in ESCC Cell Line Using Retroviral System. Avicenna J. Med. Biotechnol. 2018, 10, 75–82. [Google Scholar]
- Shabestarian, H.; Ghodsi, M.; Mallak, A.J.; Jafarian, A.H.; Montazer, M.; Forghanifard, M.M. DPPA2 Protein Expression is Associated with Gastric Cancer Metastasis. Asian Pac. J. Cancer Prev. 2016, 16, 8461–8465. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, C.; Wang, Z.; Ramazanov, B.; Tang, Y.; Mehta, S.; Dambrot, C.; Lee, Y.-W.; Tessema, K.; Kumar, I.; Astudillo, M. Dppa2/4 facilitate epigenetic remodeling during reprogramming to pluripotency. Cell Stem Cell 2018, 23, 396–411.e8. [Google Scholar] [CrossRef] [Green Version]
- John, T.; Caballero, O.L.; Svobodová, S.J.; Kong, A.; Chua, R.; Browning, J.; Fortunato, S.; Deb, S.; Hsu, M.; Gedye, C.A.; et al. ECSA/DPPA2 is an Embryo-Cancer Antigen that Is Coexpressed with Cancer-Testis Antigens in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2008, 14, 3291–3298. [Google Scholar] [CrossRef] [Green Version]
- Tchabo, N.E.; Mhawech-Fauceglia, P.; Caballero, O.L.; Villella, J.; Beck, A.F.; Miliotto, A.J.; Liao, J.; Andrews, C.; Lele, S.; Old, L.J.; et al. Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun. 2009, 9, 6. [Google Scholar]
- Luo, W.; Li, S.; Peng, B.; Ye, Y.; Deng, X.; Yao, K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e56324. [Google Scholar]
- Moghbeli, M.; Sadrizadeh, A.; Forghanifard, M.M.; Mozaffari, H.M.; Golmakani, E.; Abbaszadegan, M.R. Role of Msi1 and PYGO2 in esophageal squamous cell carcinoma depth of invasion. J. Cell Commun. Signal. 2015, 10, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghbeli, M.; Forghanifard, M.M.; Sadrizadeh, A.; Mozaffari, H.M.; Golmakani, E.; Abbaszadegan, M.R. Role of Msi1 and MAML1 in Regulation of Notch Signaling Pathway in Patients with Esophageal Squamous Cell Carcinoma. J. Gastrointest. Cancer 2015, 46, 365–369. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence | Annealing T, °C | Amplicon Size (bp) |
|---|---|---|---|
| BMI1 | F: CGTGTATTGTTCGTTACCTGGAGAC R: CATTGGCAGCATCAGCAGAAGG | 63 | 204 |
| CRIPTO1 | F: GGGATACAGCACAGTAAGGAG R: ACGGTGGTAGTTGTCGAGTC | 61 | 295 |
| DPPA2 | F: AGAAATACAATCCAGGTCATCTACTTC R: GCATATCTTGCCGTTGTTCAGG | 62 | 237 |
| KLF4 | F: TCTTCTCTTCGTTGACTTTG R: GCCAGCGGTTATTCGG | 55 | 210 |
| NANOG | F: GGCAATGGTGTGACGCAGAAGGC R:GCTCCAGGTTGAATTGTTCCAGGTC | 65 | 137 |
| MSI1 | F: TGAGCAGTTTGGGAAGGTG R: TCACACACTTTCTCCACGATG | 62 | 117 |
| SOX2 | F: AACAGCCCGGACCGCGTCAA R: TCGCAGCCGCTTAGCCTCGT | 62 | 189 |
| TWIST1 | F: GGAGTCCGCAGTCTTACGAG R: TCTGGAGGACCTGGTAGAGG | 58 | 201 |
| GAPDH | F: GGAAGGTGAAGGTCGGAGTCA R: GTCATTGATGGCAACAATATCCACT | 60 | 101 |
| Sequence | Number | Positions |
| CACTTG | 4 | 1294-99, 1731-36, 1825-30, 4914-19 |
| CAGGTG | ||
| CAAGTG | 6 | 1125-30, 1942-47, 6084-89, 8852-57 |
| CATCTG | 2 | 6458-63, 6811-16 |
| CAGCTG | 1 | 5220-25 |
| CACCTG | 1 | 5394-99 * |
| CATTTG | 8 | 1356-61, 1668-73, 2111-16, 2495-2500, 3853-58, 7739-44, 7809-14, 10114-19 |
| CATATG | 1 | 1715-20 |
| CAGATG | 3 | 6641-46, 6888-93 *, 9107-12 |
| CAGTTG | 5 | 1334-39, 2046-51, 3434-39-4183-88, 4620-25 |
| CAAATG | 5 | 1936-41, 5040-45, 6279-75, 6614-19, 8194-99 |
| CACATG | 3 | 998-03-3923-28, 5774-79 |
| CAACTG | 3 | 2476-81-2611-16, 9243-48, |
| CATGTG | 4 | 2971-76, 7219-24, 8530-35, 8597-8602 |
| CAATTG | 1 | 9351-56 |
| Sequence | Number | Positions |
|---|---|---|
| CAGGTG | 2 | 4940-45, 6478-83 |
| CAAGTG | 2 | 6447-52, 7838-43 * |
| CATCTG | 4 | 2312-17, 5186-91, 6687-92 *, 7493-98 * |
| CAGCTG | 1 | 2565-70 |
| CACCTG | 11 | 636-41, 1493-98, 2009-14, 2604-09, 3144-49, 3542-47, 3961-66, 4048-53, 4920-25, 5442-47 *, 6103-08 |
| CATTTG | 6 | 2143-48, 3549-54, 4760-65 *, 6312-17, 6531-36, 6815-20 * |
| CATATG | ||
| CAGATG | 2 | 2062-2067, 6596-6601 |
| CAAATG | 2 | 324-329, 1339-1344 |
| CACATG | 2 | 2246-51, 3626-31 |
| CAACTG | 730-735, 1546-1551, 7263-7268 * | |
| CATGTG | 5 | 1646-1651, 1977-1982, 4274-4279, 4874-4879, 7121-71268 * |
| CACGTG | 1 | 7255-60 * |
| Sequence | Number | Positions |
|---|---|---|
| CACTTG | 8 | 1070-75, 1454-59, 4400-05, 5478-83, 5613-18, 10561-66, 16659-64, 20675-80 |
| CAGGTG | 20 | 4994-99, 6239-44, 6505-10, 8682-87, 9174-79-9768-73, 15373-78, 10959-64, 11852-57 *, 12504-09, 15912-17, 16955-60, 17376-81, 17665-70, 18194-99, 18530-35, 19210-15, 19551-56, 20059-64, 21256-61 |
| CAAGTG | 7 | 705-10, 1385-90, 7490-95, 15082-87, 19075-80, 19646-51, 22042-47 |
| CATCTG | 11 | 1855-60, 4637-42, 10316-21, 12695-700, 13351-56, 19349-54, 20068-73, 20688-93, 20992-97, 22177-82, 22367-72 |
| CAGCTG | 4 | 7701-06, 12359-64, 19869-74, 19954-59 |
| CACCTG | 14 | 980-85, 6429-34, 9608-13, 9777-82, 11186-91, 12911-16, 13324-29, 13786-91, 15389-94, 16617-22, 16704-09, 17385-90, 21127-32, 21272-77 |
| CATTTG | 10 | 1883-85, 2193-98, 2914-19, 7956-61, 8216-21, 8575-80, 12762-67, 20541-46, 21034-39, 21448-53 |
| CATATG | 1 | 6471-76 |
| CAGATG | 3 | 1980-85 *, 1440-45, 15296-301 |
| CAGTTG | 6 | 5328-33, 7712-17, 8349-54 *, 13242-47, 13293-98, 22140-45 |
| CAAATG | 6 | 2501-06, 3792-97, 3907-12 *, 7203-08 *, 15645-50, 16308-13, 18294-99 |
| CACATG | 5 | 4534-39, 8006-11, 8611-16, 12999-13004, 22124-29 |
| CAACTG | 1 | 15259-64 |
| CATGTG | 10 | 2171-76, 3457-62, 6417-22, 11499-504, 11708-13, 13915-20, 14636-41, 17135-40, 18799-804, 19970-74 |
| CAATTG | 6 | 2446-51, 10305-10, 11738-43, 16200-05, 16583-88, 22717-22 * |
| CACGTG | 1 | 9599-604 |
| Sequence | Number | Positions |
|---|---|---|
| CACTTG | 2 | 2948-53 *, 3379-84 * |
| CAGGTG | 4 | 1259-64 *, 1537-42 *, 2751-56 *, 4554-59 |
| CAGCTG | 3 | 489-94, 1268-73 *, 1715-20 * |
| CACCTG | 5 | 950-55 *, 1438-43 *, 2527-32 *, 3437-42 *, 4146-51 |
| CAGATG | 4 | 3152-57 *, 4611-16 *, 4923-28 *, 5401-06 * |
| CAAATG | 3 | 1988-93, 4255-60, 5455-60 * |
| CAACTG | 1 | 4856-61 * |
| CACGTG | 2 | 557-62, 2905-10 * |
| Sequence | Number | Positions |
|---|---|---|
| CAGCTG | 965-70 *, 1548-53 *, 1972-77 * | |
| CAGATG | 1013-18 * | |
| CAGTTG | 1988-93 * | |
| CAAATG | 1541-46 * | |
| CACATG | 737-42 *, 920-25 *, 1313-18 *, 1382-87 * | |
| CATGTG | 1384-89 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izadpanah, M.H.; Forghanifard, M.M. TWIST1 Plays Role in Expression of Stemness State Markers in ESCC. Genes 2022, 13, 2369. https://doi.org/10.3390/genes13122369
Izadpanah MH, Forghanifard MM. TWIST1 Plays Role in Expression of Stemness State Markers in ESCC. Genes. 2022; 13(12):2369. https://doi.org/10.3390/genes13122369
Chicago/Turabian StyleIzadpanah, Mohammad Hossein, and Mohammad Mahdi Forghanifard. 2022. "TWIST1 Plays Role in Expression of Stemness State Markers in ESCC" Genes 13, no. 12: 2369. https://doi.org/10.3390/genes13122369





