The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder
Abstract
:1. Introduction
2. miR-708 Overview
3. The Villain: MiR-708 Encouraging Tumor Growth and Progression
4. MiR-708 as a Hero: Its Role as Tumor Suppressor
5. In Silico hsa-miR-708-5p Expression Analysis in Human Cancers
miR-708 mRNAs Targets in Human Cancers
6. Experimental Evidence for mir-708 Intervention
7. Final Considerations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA Expression Profiles Classify Human Cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Jorge, A.L.; Pereira, E.R.; de Oliveira, C.S.; Ferreira, E.D.S.; Menon, E.T.N.; Diniz, S.N.; Pezuk, J.A. MicroRNAs: Understanding Their Role in Gene Expression and Cancer. Einstein 2021, 19, eRB5996. [Google Scholar] [CrossRef]
- Pezuk, J.A.; Salomão, K.B.; Baroni, M.; Pereira, C.A.; Geron, L.; Brassesco, M.S. Aberrantly Expressed MicroRNAs and Their Implications in Childhood Central Nervous System Tumors. Cancer Metastasis Rev. 2019, 38, 813–828. [Google Scholar] [CrossRef]
- Delsin, L.E.A.; Salomao, K.B.; Pezuk, J.A.; Brassesco, M.S. Expression Profiles and Prognostic Value of MiRNAs in Retinoblastoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1–10. [Google Scholar] [CrossRef]
- Salomão, K.B.; Pezuk, J.A.; de Souza, G.R.; Chagas, P.; Pereira, T.C.; Valera, E.T.; Brassesco, M.S. MicroRNA Dysregulation Interplay with Childhood Abdominal Tumors. Cancer Metastasis Rev. 2019, 38, 783–811. [Google Scholar] [CrossRef]
- Viera, G.M.; Salomao, K.B.; de Sousa, G.R.; Baroni, M.; Delsin, L.E.A.; Pezuk, J.A.; Brassesco, M.S. MiRNA Signatures in Childhood Sarcomas and Their Clinical Implications; Springer: Berlin/Heidelberg, Germany, 2019; Volume 21, ISBN 0123456789. [Google Scholar]
- Ghanbarian, H.; Yıldız, M.T.; Tutar, Y. MicroRNA Targeting. Methods Mol. Biol. 2022, 2257, 105–130. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Scrideli, C.A.; Brassesco, M.S.; Morales, A.G.; Pezuk, J.A.; de Queiroz, R.P.; Yunes, J.A.; Brandalise, S.R.; Tone, L.G. Differential MiRNA Expression in Childhood Acute Lymphoblastic Leukemia and Association with Clinical and Biological Features. Leuk. Res. 2012, 36, 293–298. [Google Scholar] [CrossRef]
- van Roosbroeck, K.; Calin, G.A. Cancer Hallmarks and MicroRNAs: The Therapeutic Connection. Adv. Cancer Res. 2017, 135, 119–149. [Google Scholar] [CrossRef]
- Pezuk, J.A.; Brassesco, M.S.; de Oliveira, R.S.; Machado, H.R.; Neder, L.; Scrideli, C.A.; Tone, L.G. PLK1-Associated MicroRNAs Are Correlated with Pediatric Medulloblastoma Prognosis. Childs Nerv. Syst. 2017, 33, 609–615. [Google Scholar] [CrossRef]
- Wang, S.; Olson, E.N. AngiomiRs—Key Regulators of Angiogenesis. Curr. Opin. Genet. Dev. 2009, 19, 205–211. [Google Scholar] [CrossRef]
- Hurst, D.R.; Edmonds, M.D.; Welch, D.R. Metastamir: The Field of Metastasis-Regulatory MicroRNA Is Spreading. Cancer Res. 2009, 69, 7495–7498. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, N.J.; Lutz, C.S.; Monteleone, N.J.; Lutz, C.S. MiR-708-5p: A MicroRNA with Emerging Roles in Cancer. Oncotarget 2017, 8, 71292–71316. [Google Scholar] [CrossRef] [Green Version]
- Lui, W.O.; Pourmand, N.; Patterson, B.K.; Fire, A. Patterns of Known and Novel Small RNAs in Human Cervical Cancer. Cancer Res. 2007, 67, 6031–6043. [Google Scholar] [CrossRef] [Green Version]
- Peppino, G.; Riccardo, F.; Arigoni, M.; Bolli, E.; Barutello, G.; Cavallo, F.; Quaglino, E. Role and Involvement of TENM4 and MiR-708 in Breast Cancer Development and Therapy. Cells 2022, 11, 172. [Google Scholar] [CrossRef]
- Quaglino, E.; Conti, L.; Cavallo, F. Breast Cancer Stem Cell Antigens as Targets for Immunotherapy. Semin. Immunol. 2020, 47, 101386. [Google Scholar] [CrossRef]
- Graumann, R.; Capua, G.A.D.; Oyarzún, J.E.; Vásquez, M.A.; Liao, C.; Brañes, J.A.; Roa, I.; Casanello, P.; Corvalán, A.H.; Owen, G.I.; et al. Expression of Teneurins Is Associated with Tumor Differentiation and Patient Survival in Ovarian Cancer. PLoS ONE 2017, 12, e0177244. [Google Scholar] [CrossRef] [Green Version]
- Ngollo, M.; Lebert, A.; Daures, M.; Judes, G.; Rifai, K.; Dubois, L.; Kemeny, J.L.; Penault-Llorca, F.; Bignon, Y.J.; Guy, L.; et al. Global Analysis of H3K27me3 as an Epigenetic Marker in Prostate Cancer Progression. BMC Cancer 2017, 17, 261. [Google Scholar] [CrossRef]
- Peppino, G.; Ruiu, R.; Arigoni, M.; Riccardo, F.; Iacoviello, A.; Barutello, G.; Quaglino, E. Teneurins: Role in Cancer and Potential Role as Diagnostic Biomarkers and Targets for Therapy. Int. J. Mol. Sci. 2021, 22, 2321. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian MicroRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Guan, W.; Han, S.; Hong, D.K.; Kim, L.S.; Kim, H. MicroRNA-708-3p Mediates Metastasis and Chemoresistance through Inhibition of Epithelial-to-Mesenchymal Transition in Breast Cancer. Cancer Sci. 2018, 109, 1404–1413. [Google Scholar] [CrossRef]
- Cao, T.; Lu, Y.; Wang, Q.; Qin, H.; Li, H.; Guo, H.; Ge, M.; Glass, S.E.; Singh, B.; Zhang, W.; et al. A CGA/EGFR/GATA2 Positive Feedback Circuit Confers Chemoresistance in Gastric Cancer. J. Clin. Investig. 2022, 132, 1–17. [Google Scholar] [CrossRef]
- Leidinger, P.; Hart, M.; Backes, C.; Rheinheimer, S.; Keck, B.; Wullich, B.; Keller, A.; Meese, E. Differential Blood-Based Diagnosis between Benign Prostatic Hyperplasia and Prostate Cancer: MiRNA as Source for Biomarkers Independent of PSA Level, Gleason Score, or TNM Status. Tumour. Biol. 2016, 37, 10177–10185. [Google Scholar] [CrossRef]
- Behrman, S.; Acosta-Alvear, D.; Walter, P. A CHOP-Regulated MicroRNA Controls Rhodopsin Expression. J. Cell Biol. 2011, 192, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, M.L.; Shaban, M.S.; Albert, B.V.; Gökçen, A.; Kracht, M. The Crosstalk of Endoplasmic Reticulum (ER) Stress Pathways with NF-ΚB: Complex Mechanisms Relevant for Cancer, Inflammation and Infection. Biomedicines 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, K.J.; Gokila Vani, M.; Hsieh, H.W.; Lin, C.C.; Liao, J.W.; Chueh, P.J.; Wang, S.Y. MicroRNA-708 Activation by Glucocorticoid Receptor Agonists Regulate Breast Cancer Tumorigenesis and Metastasis via Downregulation of NF-ΚB Signaling. Carcinogenesis 2019, 40, 335–348. [Google Scholar] [CrossRef]
- Ryu, S.; McDonnell, K.; Choi, H.; Gao, D.; Hahn, M.; Joshi, N.; Park, S.M.; Catena, R.; Do, Y.; Brazin, J.; et al. Suppression of MiRNA-708 by Polycomb Group Promotes Metastases by Calcium-Induced Cell Migration. Cancer Cell 2013, 23, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Shan, J.; Al-Muftah, M.A.; Al-Kowari, M.K.; Abuaqel, S.W.J.; Al-Rumaihi, K.; Al-Bozom, I.; Li, P.; Chouchane, L. Targeting Wnt/EZH2/MicroRNA-708 Signaling Pathway Inhibits Neuroendocrine Differentiation in Prostate Cancer. Cell Death Discov. 2019, 5, 139. [Google Scholar] [CrossRef] [Green Version]
- Hanaki, S.; Shimada, M. Targeting EZH2 as Cancer Therapy. J. Biochem. 2021, 170, 1–4. [Google Scholar] [CrossRef]
- Baer, C.; Claus, R.; Frenzel, L.P.; Zucknick, M.; Park, Y.J.; Gu, L.; Weichenhan, D.; Fischer, M.; Pallasch, C.P.; Herpel, E.; et al. Extensive Promoter DNA Hypermethylation and Hypomethylation Is Associated with Aberrant MicroRNA Expression in Chronic Lymphocytic Leukemia. Cancer Res. 2012, 72, 3775–3785. [Google Scholar] [CrossRef] [Green Version]
- Baer, C.; Oakes, C.C.; Ruppert, A.S.; Claus, R.; Kim-Wanner, S.Z.; Mertens, D.; Zenz, T.; Stilgenbauer, S.; Byrd, J.C.; Plass, C. Epigenetic Silencing of MiR-708 Enhances NF-ΚB Signaling in Chronic Lymphocytic Leukemia. Int. J. Cancer 2015, 137, 1352–1361. [Google Scholar] [CrossRef]
- Chen, S.; Dai, M. Lipopolysaccharide-Induced LncRNA TMC3-AS1 Is Highly Expressed in Osteoporosis and Promotes Osteoblast Apoptosis by Suppressing the Formation of Mature MiR-708. Int. J. Gen. Med. 2022, 15, 3345–3352. [Google Scholar] [CrossRef]
- Murray, M.J.; Bailey, S.; Raby, K.L.; Saini, H.K.; de Kock, L.; Burke, G.A.A.; Foulkes, W.D.; Enright, A.J.; Coleman, N.; Tischkowitz, M. Serum Levels of Mature MicroRNAs in DICER1-Mutated Pleuropulmonary Blastoma. Oncogenesis 2014, 3, e87. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, S.; Wu, Y.; Gao, F. MiRNA-708 Functions as a Tumour Suppressor in Hepatocellular Carcinoma by Targeting SMAD3. Oncol. Lett. 2017, 14, 2552–2558. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhou, H.; Ren, X.; Teng, J. Inhibition of JAK1 by MicroRNA-708 Promotes SH-SY5Y Neuronal Cell Survival after Oxygen and Glucose Deprivation and Reoxygenation. Neurosci. Lett. 2018, 664, 43–50. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, L.; Sun, L.; Fan, S.; Li, H.; Zhao, Y.; Guo, Z.; Lin, L.; Cui, L.; Wang, K.; et al. MYCN Is a Novel Oncogenic Target in Adult B-ALL That Activates the Wnt/β-Catenin Pathway by Suppressing DKK3. J. Cell Mol. Med. 2018, 22, 3627–3637. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Xiong, L.; Yu, L.; Li, Z.; Guo, Q.; Li, Z.; Li, B.; Lin, N. Comprehensive Analysis of MicroRNA-Regulated Protein Interaction Network Reveals the Tumor Suppressive Role of MicroRNA-149 in Human Hepatocellular Carcinoma via Targeting AKT-MTOR Pathway. Mol. Cancer 2014, 13, 253. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Zhang, X.; Zhang, L.; Dong, J.; Cai, W.; Gao, J.; Hong, B. MiR-708 Promotes the Development of Bladder Carcinoma via Direct Repression of Caspase-2. J. Cancer Res. Clin. Oncol. 2013, 139, 1189–1198. [Google Scholar] [CrossRef]
- Dileepan, M.; Jude, J.A.; Rao, S.P.; Walseth, T.F.; Panettieri, R.A.; Subramanian, S.; Kannan, M.S. MicroRNA-708 Regulates CD38 Expression through Signaling Pathways JNK MAP Kinase and PTEN/AKT in Human Airway Smooth Muscle Cells. Respir. Res. 2014, 15, 107. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Fan, W.; Ma, L.; Geng, X. MiR-708-5p Promotes Fibroblast–like Synoviocytes’ Cell Apoptosis and Ameliorates Rheumatoid Arthritis by Inhibition of Wnt3a/β-Catenin Pathway. Drug. Des. Devel. Ther. 2018, 12, 3439–3447. [Google Scholar] [CrossRef] [Green Version]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Mäkelä, R.; Krohn, M.; Aure, M.R.; Nunes-Xavier, C.E.; Perälä, M.; Tramm, T.; Alsner, J.; et al. Identifying MicroRNAs Regulating B7-H3 in Breast Cancer: The Clinical Impact of MicroRNA-29c. Br. J. Cancer 2014, 110, 2072–2080. [Google Scholar] [CrossRef]
- Yang, J.; Lu, C.; Wei, J.; Guo, Y.; Liu, W.; Luo, L.; Fisch, G.; Li, X. Inhibition of KPNA4 Attenuates Prostate Cancer Metastasis. Oncogene 2017, 36, 2868–2878. [Google Scholar] [CrossRef] [Green Version]
- Guedes, A.G.P.; Deshpande, D.A.; Dileepan, M.; Walseth, T.F.; Panettieri, R.A.; Subramanian, S.; Kannan, M.S. CD38 and Airway Hyper-Responsiveness: Studies on Human Airway Smooth Muscle Cells and Mouse Models. Can. J. Physiol. Pharmacol. 2015, 93, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Baghdadi, M.B.; Firmino, J.; Soni, K.; Evano, B.; di Girolamo, D.; Mourikis, P.; Castel, D.; Tajbakhsh, S. Notch-Induced MiR-708 Antagonizes Satellite Cell Migration and Maintains Quiescence. Cell Stem. Cell 2018, 23, 859–868.e5. [Google Scholar] [CrossRef]
- Sun, S.N.; Hu, S.; Shang, Y.P.; Li, L.Y.; Zhou, H.; Chen, J.S.; Yang, J.F.; Li, J.; Huang, Q.; Shen, C.P.; et al. Relevance Function of MicroRNA-708 in the Pathogenesis of Cancer. Cell Signal 2019, 63, 109390. [Google Scholar] [CrossRef]
- Monteleone, N.J.; Lutz, C.S. MiR-708-5p Enhances Erlotinib/Paclitaxel Efficacy and Overcomes Chemoresistance in Lung Cancer Cells. Oncotarget 2020, 11, 4699–4721. [Google Scholar] [CrossRef]
- Bagheri, F.; Tanha, H.M.; Naeini, M.M.; Ghaedi, K.; Azadeh, M. Tumor-Promoting Function of Single Nucleotide Polymorphism Rs1836724 (C3388T) Alters Multiple Potential Legitimate MicroRNA Binding Sites at the 3′-Untranslated Region of ErbB4 in Breast Cancer. Mol. Med. Rep. 2016, 13, 4494–4498. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, R.; Zhang, J.; Meng, C.; Zhang, J.; Song, X.; Lv, C. MicroRNA-708-3p as a Potential Therapeutic Target via the ADAM17-GATA/STAT3 Axis in Idiopathic Pulmonary Fibrosis. Exp. Mol. Med. 2018, 50, e465. [Google Scholar] [CrossRef]
- Guo, L.; Gao, S.; Sun, W.; Wang, Y.; Zhao, J. Elevated LINC01232 Is Associated with Poor Prognosis and HBV Infection in Hepatocellular Carcinoma Patients and Contributes to Tumor Progression in Vitro. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101813. [Google Scholar] [CrossRef]
- Cheng, D.L.; Xiang, Y.Y.; Ji, L.J.; Lu, X.J. Competing Endogenous RNA Interplay in Cancer: Mechanism, Methodology, and Perspectives. Tumor Biol. 2015, 36, 479–488. [Google Scholar] [CrossRef]
- Chen, J.; Lu, C.; Wang, X.; Wang, L.; Chen, J.; Ji, F. LncRNA NONRATT009773.2 Promotes Bone Cancer Pain Progression through the MiR-708-5p/CXCL13 Axis. Eur. J. Neurosci. 2022, 55, 661–674. [Google Scholar] [CrossRef]
- Liu, X.; Shen, X.; Zhang, J. Long Non-Coding RNA LINC00514 Promotes the Proliferation and Invasion through the MiR-708-5p/HOXB3 Axis in Cervical Squamous Cell Carcinoma. Environ. Toxicol. 2022, 37, 161–170. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Yu, L.; Liu, B.; Ma, J.; Yang, W. LINC00665 Facilitates the Malignant Processes of Osteosarcoma by Increasing the RAP1B Expression via Sponging MiR-708 and MiR-142-5p. Anal. Cell Pathol. 2021, 2021, 5525711. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, W.W.; Wei, W.; Zhan, L.X.; Huang, X. Long Noncoding RNA Meg3 Sponges MiR-708 to Inhibit Intestinal Tumorigenesis via SOCS3-Repressed Cancer Stem Cells Growth. Cell Death Dis. 2021, 13, 25. [Google Scholar] [CrossRef]
- Dong, H.T.; Liu, Q.; Zhao, T.; Yao, F.; Xu, Y.; Chen, B.; Wu, Y.; Zheng, X.; Jin, F.; Li, J.; et al. Long Non-Coding RNA LOXL1-AS1 Drives Breast Cancer Invasion and Metastasis by Antagonizing MiR-708-5p Expression and Activity. Mol. Ther. Nucleic Acids 2020, 19, 696–705. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Li, F.; Li, H.; Bei, S.; Zhang, X.; Feng, L. LncRNA LOXL1-AS1 Facilitates the Tumorigenesis and Stemness of Gastric Carcinoma via Regulation of MiR-708-5p/USF1 Pathway. Cell Prolif. 2019, 52, e12687. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chang, Z.; Han, C.; Zhuang, L.; Zhou, C.; Qi, X.; Peng, Z. Long Non-Coding RNA MINCR Aggravates Colon Cancer via Regulating MiR-708-5p-Mediated Wnt/β-Catenin Pathway. Biomed. Pharmacother. 2020, 129, 110292. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Tong, X.; Zhang, Y. Schisandrin B Inhibits Cell Viability and Migration, and Induces Cell Apoptosis by Circ_0009112/MiR-708-5p Axis Through PI3K/AKT Pathway in Osteosarcoma. Front. Genet. 2020, 11, 588670. [Google Scholar] [CrossRef]
- Lei, S.L.; Zhao, H.; Yao, H.L.; Chen, Y.; Lei, Z.D.; Liu, K.J.; Yang, Q. Regulatory Roles of MicroRNA-708 and MicroRNA-31 in Proliferation, Apoptosis and Invasion of Colorectal Cancer Cells. Oncol. Lett. 2014, 8, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Necela, B.M.; Carr, J.M.; Asmann, Y.W.; Thompson, E.A. Differential Expression of MicroRNAs in Tumors from Chronically Inflamed or Genetic (APCMin/+) Models of Colon Cancer. PLoS ONE 2011, 6, e18501. [Google Scholar] [CrossRef] [Green Version]
- Pizzini, S.; Bisognin, A.; Mandruzzato, S.; Biasiolo, M.; Facciolli, A.; Perilli, L.; Rossi, E.; Esposito, G.; Rugge, M.; Pilati, P.; et al. Impact of MicroRNAs on Regulatory Networks and Pathways in Human Colorectal Carcinogenesis and Development of Metastasis. BMC Genom. 2013, 14, 589. [Google Scholar] [CrossRef]
- Fedatto, P.F.; de Carvalho, T.I.; de Oliveira, J.C.; Antônio, D.S.M.; Pezuk, J.A.; da Cunha Tirapelli, D.P.; Féres, O.; da Rocha, J.J.R.; Scrideli, C.A.; Tone, L.G.; et al. MiR-708-5p as a Predictive Marker of Colorectal Cancer Prognosis. J. Anal. Oncol. 2016, 5, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Wang, S.; Sun, Z.; Lin, Y.; Sun, S.; Chen, J.; Chen, W. Identification of MicroRNAs as Potential Biomarker for Gastric Cancer by System Biological Analysis. BioMed Res. Int. 2014, 2014, 901428. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Guo, H.; Cao, Y.; Xiong, J. MiR-708-5p Inhibits the Progression of Pancreatic Ductal Adenocarcinoma by Targeting Sirt3. Pathol. Res. Pract. 2019, 215, 794–800. [Google Scholar] [CrossRef]
- Frampton, A.E.; Gall, T.M.; Giovannetti, E.; Stebbing, J.; Castellano, L.; Jiao, L.R.; Krell, J. Distinct MiRNA Profiles Are Associated with Malignant Transformation of Pancreatic Cystic Tumors Revealing Potential Biomarkers for Clinical Use. Expert Rev. Mol. Diagn. 2014, 13, 325–329. [Google Scholar] [CrossRef]
- Yu, J.; Li, A.; Hong, S.M.; Hruban, R.H.; Goggins, M. MicroRNA Alterations of Pancreatic Intraepithelial Neoplasias. Clin. Cancer Res. 2012, 18, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Zhou, L.; Zhang, J.; Zheng, F.; Wang, H.; Ma, D.; Tian, J. Comprehensive Expression Profiling of MicroRNAs in Laryngeal Squamous Cell Carcinoma. Head Neck 2013, 35, 720–728. [Google Scholar] [CrossRef]
- Jang, J.S.; Jeon, H.S.; Sun, Z.; Aubry, M.C.; Tang, H.; Park, C.H.; Rakhshan, F.; Schultz, D.A.; Kolbert, C.P.; Lupu, R.; et al. Increased MiR-708 Expression in NSCLC and Its Association with Poor Survival in Lung Adenocarcinoma from Never Smokers. Clin. Cancer Res. 2012, 18, 3658–3667. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Xuan, Z.; Yang, X.; Ye, X.; Pan, Z.; Fang, Q. Identification of Key MicroRNAs and Hub Genes in Non-Small-Cell Lung Cancer Using Integrative Bioinformatics and Functional Analyses. J. Cell Biochem. 2020, 121, 2690–2703. [Google Scholar] [CrossRef]
- Molina-Pinelo, S.; Gutiérrez, G.; Pastor, M.D.; Hergueta, M.; Moreno-Bueno, G.; García-Carbonero, R.; Nogal, A.; Suárez, R.; Salinas, A.; Pozo-Rodríguez, F.; et al. MicroRNA-Dependent Regulation of Transcription in Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e90524. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Todd, N.W.; Yu, L.; Fang, H.; Jiang, F. Early Detection of Squamous Cell Lung Cancer in Sputum by a Panel of MicroRNA Markers. Mod. Pathol. 2010, 23, 1157–1164. [Google Scholar] [CrossRef]
- Song, T.; Xia, W.; Shao, N.; Zhang, X.; Wang, C.; Wu, Y.; Dong, J.; Cai, W.; Li, H. Differential MiRNA Expression Profiles in Bladder Urothelial Carcinomas. Asian Pac. J. Cancer Prev. 2010, 11, 905–911. [Google Scholar] [PubMed]
- Blick, C.; Ramachandran, A.; Mccormick, R.; Wigfield, S.; Cranston, D.; Catto, J.; Harris, A.L. Identification of a Hypoxia-Regulated MiRNA Signature in Bladder Cancer and a Role for MiR-145 in Hypoxia-Dependent Apoptosis. Br. J. Cancer 2015, 113, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.C.; Brassesco, M.S.; Morales, A.G.; Pezuk, J.A.; Fedatto, P.F.; da Silva, G.N.; Scrideli, C.A.; Tone, L.G. MicroRNA-100 Acts as a Tumor Suppressor in Human Bladder Carcinoma 5637 Cells. Asian Pac. J. Cancer Prev. 2011, 12, 3001–3004. [Google Scholar] [PubMed]
- Schotte, D.; Chau, J.C.K.; Sylvester, G.; Liu, G.; Chen, C.; van der Velden, V.H.J.; Broekhuis, M.J.C.; Peters, T.C.J.M.; Pieters, R.; den Boer, M.L. Identification of New MicroRNA Genes and Aberrant MicroRNA Profiles in Childhood Acute Lymphoblastic Leukemia. Leukemia 2008, 23, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, J.C.; Scrideli, C.A.; Brassesco, M.S.; Yunes, J.A.; Brandalise, S.R.; Tone, L.G. MiR-708-5p Is Differentially Expressed in Childhood Acute Lymphoblastic Leukemia but Not Strongly Associated to Clinical Features. Pediatr. Blood Cancer 2015, 62, 177–178. [Google Scholar] [CrossRef]
- Han, B.W.; Feng, D.D.; Li, Z.G.; Luo, X.Q.; Zhang, H.; Li, X.J.; Zhang, X.J.; Zheng, L.L.; Zeng, C.W.; Lin, K.Y.; et al. A Set of MiRNAs That Involve in the Pathways of Drug Resistance and Leukemic Stem-Cell Differentiation Is Associated with the Risk of Relapse and Glucocorticoid Response in Childhood ALL. Hum. Mol. Genet. 2011, 20, 4903–4915. [Google Scholar] [CrossRef]
- The Expression and Regulatory Mechanism of MicroRNA-708 in Pediatric Common B-Cell Acute Lymphoblastic Leukemia—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23611221/ (accessed on 19 November 2022).
- Liu, C.; Iqbal, J.; Teruya-Feldstein, J.; Shen, Y.; Dabrowska, M.J.; Dybkaer, K.; Lim, M.S.; Piva, R.; Barreca, A.; Pellegrino, E.; et al. MicroRNA Expression Profiling Identifies Molecular Signatures Associated with Anaplastic Large Cell Lymphoma. Blood 2013, 122, 2083–2092. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Yamamura, S.; Majid, S.; Shahryari, V.; Hirata, H.; Tanaka, Y.; Dahiya, R. MicroRNA-708 Induces Apoptosis and Suppresses Tumorigenicity in Renal Cancer Cells. Cancer Res. 2011, 71, 6208–6219. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Lan, J.; Ge, J.; Nie, Q.; Mao, Q.; Qiu, Y. MiR-708 Acts as a Tumor Suppressor in Human Glioblastoma Cells. Oncol. Rep. 2013, 30, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yang, F.; Xu, H.; Yue, Z.; Fang, X.; Liu, J. MicroRNA-708 Is Downregulated in Hepatocellular Carcinoma and Suppresses Tumor Invasion and Migration. Biomed. Pharmacother. 2015, 73, 154–159. [Google Scholar] [CrossRef]
- Mroweh, M.; Roth, G.; Decaens, T.; Marche, P.N.; Lerat, H.; Jílková, Z.M. Targeting Akt in Hepatocellular Carcinoma and Its Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 1794. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, S.; Zhao, G.; Yang, L.; Zhang, P.; Yi, Q.; Cheng, S. MiR-708/LSD1 Axis Regulates the Proliferation and Invasion of Breast Cancer Cells. Cancer Med. 2016, 5, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, S.; Majid, S.; Shahryari, V.; Arora, S.; Yamamura, S.; Chang, I.; Zaman, M.S.; Deng, G.; Tanaka, Y.; Dahiya, R. MiRNA-708 Control of CD44+ Prostate Cancer-Initiating Cells. Cancer Res. 2012, 72, 3618–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldrup, C.; Kosaka, N.; Ochiya, T.; Borre, M.; Høyer, S.; Orntoft, T.F.; Sorensen, K.D. Profiling of Circulating MicroRNAs for Prostate Cancer Biomarker Discovery. Drug Deliv. Transl. Res. 2014, 4, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Delsin, L.E.A.; Roberto, G.M.; Fedatto, P.F.; Engel, E.E.; Scrideli, C.A.; Tone, L.G.; Brassesco, M.S. Downregulated Adhesion-Associated MicroRNAs as Prognostic Predictors in Childhood Osteosarcoma. Pathol. Oncol. Res. 2017, 25, 11–20. [Google Scholar] [CrossRef]
- Lin, K.T.; Yeh, Y.M.; Chuang, C.M.; Yang, S.Y.; Chang, J.W.; Sun, S.P.; Wang, Y.S.; Chao, K.C.; Wang, L.H. Glucocorticoids Mediate Induction of MicroRNA-708 to Suppress Ovarian Cancer Metastasis through Targeting Rap1B. Nat. Commun. 2015, 6, 5917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Xiang, D.; Lin, Y. MicroRNA-708 Inhibits the Proliferation and Invasion of Osteosarcoma Cells by Directly Targeting ZEB1. Mol. Med. Rep. 2019, 49, 3948–3954. [Google Scholar] [CrossRef]
- Feng, T.; Zhu, Z.; Jin, Y.; Wang, H.; Mao, X.; Liu, D.; Li, Y.; Lu, L.; Zuo, G. The MicroRNA-708-5p/ZEB1/EMT Axis Mediates the Metastatic Potential of Osteosarcoma. Oncol. Rep. 2020, 43, 491–502. [Google Scholar] [CrossRef]
- Sui, C.; Liu, D.; Hu, Y.; Zhang, L. MicroRNA-708-5p Affects Proliferation and Invasion of Osteosarcoma Cells by Targeting URGCP. Exp. Ther. Med. 2019, 17, 2235–2241. [Google Scholar] [CrossRef]
- Roberto, G.M.; Vieira, G.M.; Delsin, L.E.A.; de Silva, M.O.; Hakime, R.G.; Engel, E.E.; Scrideli, C.A.; Tone, L.G.; Brassesco, M.S. MiR-708-5p Is Inversely Associated with EWS/FLI1 Ewing Sarcoma but Does Not Represent a Prognostic Predictor. Cancer Genet. 2019, 230, 21–27. [Google Scholar] [CrossRef]
- Robin, T.P.; Smith, A.; McKinsey, E.; Reaves, L.; Jedlicka, P.; Ford, H.L. EWS/FLI1 Regulates EYA3 in Ewing Sarcoma via Modulation of MiRNA-708, Resulting in Increased Cell Survival and Chemoresistance. Mol. Cancer Res. 2012, 10, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Liu, H. Both a Hypoxia-Inducible EYA3 and a Histone Acetyltransferase P300 Function as Coactivators of SIX5 to Mediate Tumorigenesis and Cancer Progression. Ann. Transl. Med. 2022, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kim, S.-W.; Nam, J.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y.; Kwon, T.K.; Lee, T.-J.; Kim, E.-A.; Kim, S.-W.; et al. Inhibition of C-FLIPL Expression by MiRNA-708 Increases the Sensitivity of Renal Cancer Cells to Anti-Cancer Drugs. Oncotarget 2016, 7, 31832–31846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, R.S.; Costa e Silva, M.; Coutinho, L.L.; Garcia Gomes, R.; Pedrosa, F.; Massaro, J.D.; Donadi, E.A.; Lucena-Silva, N. MicroRNA Expression Profiles Discriminate Childhood T- from B-Acute Lymphoblastic Leukemia. Hematol. Oncol. 2019, 37, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yang, G.; Feng, M.; Zheng, S.; Cao, Z.; Qiu, J.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; et al. NF-ΚB in Pancreatic Cancer: Its Key Role in Chemoresistance. Cancer Lett. 2018, 421, 127–134. [Google Scholar] [CrossRef]
- Zheng, H.-C. The Molecular Mechanisms of Chemoresistance in Cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.; Saghari, S.; Bassiri, F.; Raesi, R.; Zarrabi, A.; Hushmandi, K.; Sethi, G.; Tergaonkar, V. NF-ΚB as a Regulator of Cancer Metastasis and Therapy Response: A Focus on Epithelial–Mesenchymal Transition. J. Cell Physiol. 2022, 237, 2770–2795. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The MultiMiR R Package and Database: Integration of MicroRNA–Target Interactions along with Their Disease and Drug Associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in Body Fluids—The Mix of Hormones and Biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ye, T.; Liu, H.; Lv, P.; Duan, C.; Wu, X.; Jiang, K.; Lu, H.; Xia, D.; Peng, E.; et al. Expression Profiles, Biological Functions and Clinical Significance of CircRNAs in Bladder Cancer. Mol. Cancer 2021, 20, 4. [Google Scholar] [CrossRef]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating MiRNA Panels for Specific and Early Detection in Bladder Cancer. Cancer Sci. 2019, 110, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Pezuk, J.A.; Miller, T.L.A.; Bevilacqua, J.L.B.; de Barros, A.C.S.D.; de Andrade, F.E.M.; e Macedo, L.F.D.A.; Aguilar, V.; Claro, A.N.M.; Camargo, A.A.; Galante, P.A.F.; et al. Measuring Plasma Levels of Three MicroRNAs Can Improve the Accuracy for Identification of Malignant Breast Lesions in Women with BI-RADS 4 Mammography. Oncotarget 2017, 8, 83940–83948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backes, C.; Meese, E.; Keller, A. Specific MiRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Q.; Zhang, M.; Su, W.; Wang, Z.; Li, Y.; Zhang, J.; Beer, D.G.; Yang, S.; Chen, G. Serum MicroRNA Signature Is Capable of Early Diagnosis for Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2019, 15, 1712–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezuk, J.A. The Importance of Circulating MiRNAs and Its Limitation on the Clinic. Hum. J. 2017, 8, 278–283. [Google Scholar]
- Yen, R.; Grasedieck, S.; Wu, A.; Lin, H.; Su, J.; Rothe, K.; Nakamoto, H.; Forrest, D.L.; Eaves, C.J.; Jiang, X. Identification of Key MicroRNAs as Predictive Biomarkers of Nilotinib Response in Chronic Myeloid Leukemia: A Sub-Analysis of the ENESTxtnd Clinical Trial. Leukemia 2022, 36, 2443–2452. [Google Scholar] [CrossRef]
- Qin, X.; Sun, L.; Wang, J. Restoration of MicroRNA-708 Sensitizes Ovarian Cancer Cells to Cisplatin via IGF2BP1/Akt Pathway. Cell Biol. Int. 2017, 41, 1110–1118. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Barkat, M.A.; Harshita; Ansari, M.A.; Javed, M.N.; Sajid Jamal, Q.M.; Kamal, M.A. Nanotechnological Based MiRNA Intervention in the Therapeutic Management of Neuroblastoma. Semin. Cancer Biol. 2021, 69, 100–108. [Google Scholar] [CrossRef]
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. MiRNA Nanotherapeutics for Cancer. Drug. Discov. Today 2017, 22, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Zhao, Q.; Zhang, C.; Zhen, L.; Liu, C.; Wang, G.; Zhang, L.; Bao, L.; Lu, Y.; Meng, L.; et al. Neonatal Heart-Enriched MiR-708 Promotes Proliferation and Stress Resistance of Cardiomyocytes in Rodents. Theranostics 2017, 7, 1953–1965. [Google Scholar] [CrossRef]
- Ramchandani, D.; Lee, S.K.; Yomtoubian, S.; Han, M.S.; Tung, C.H.; Mittal, V. Nanoparticle Delivery of MiR-708 Mimetic Impairs Breast Cancer Metastasis. Mol. Cancer Ther. 2019, 18, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.P.; Rajapakshe, K.; Hartig, S.M.; Reva, B.; McLellan, M.D.; Kandoth, C.; Ding, L.; Zack, T.I.; Gunaratne, P.H.; Wheeler, D.A.; et al. Identification of a Pan-Cancer Oncogenic MicroRNA Superfamily Anchored by a Central Core Seed Motif. Nat. Commun. 2013, 4, 3730. [Google Scholar] [CrossRef] [PubMed]






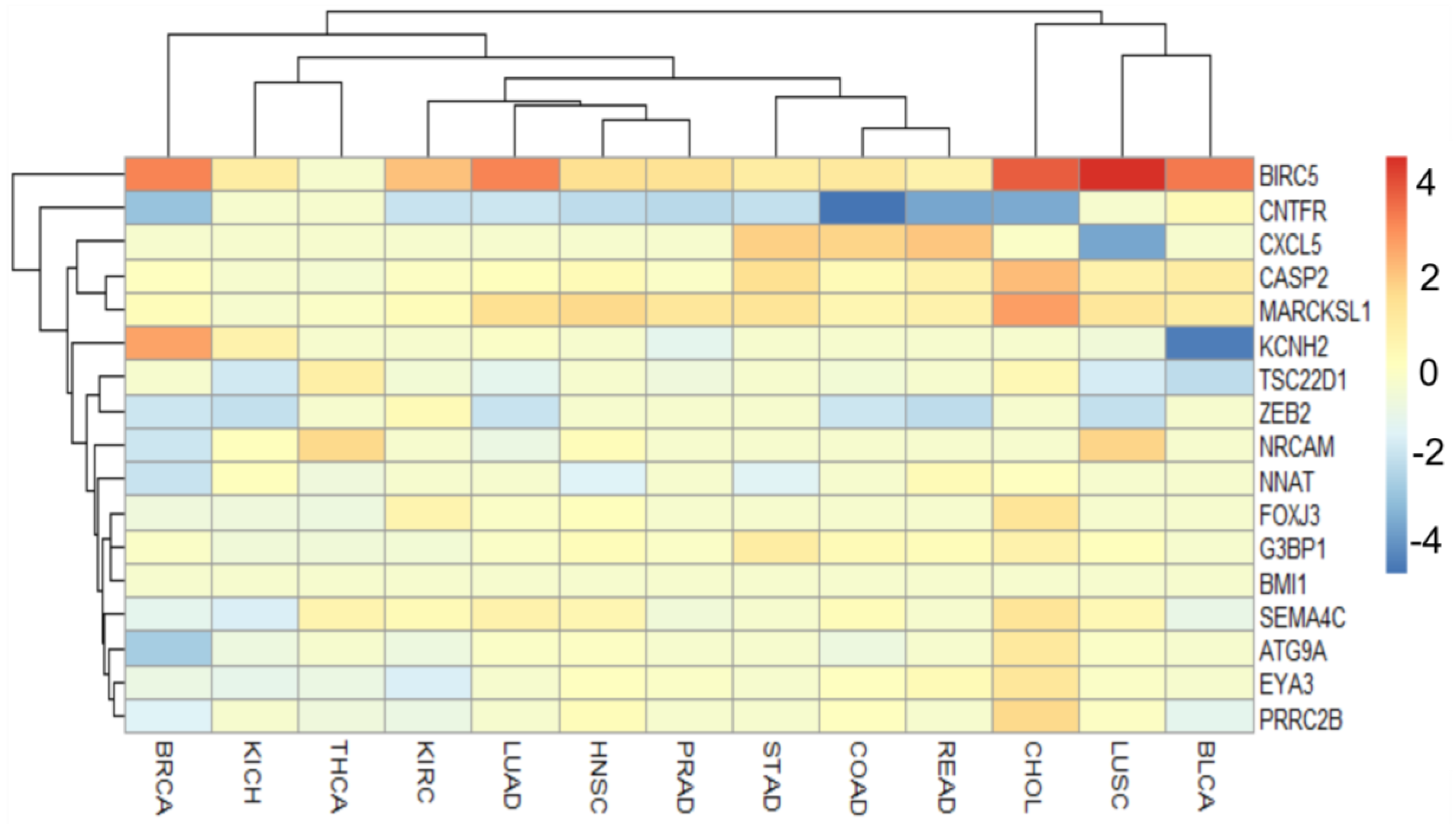

| Target | Role in Cancer | Mechanism |
|---|---|---|
| BIRC5 | oncogene | apoptosis |
| ATG9A | ? oncogene | autophagy |
| CASP2 | tumor suppressor | apoptosis |
| CNTFR | tumor suppressor | immune response |
| CXL5 | oncogene | immune response |
| EYA3 | oncogene | transcriptional activator |
| FOXJ3 | ? oncogene | transcriptional regulator |
| G3BP1 | oncogene | element of Ras signal |
| KCNH2 | oncogene | component of potassium channel |
| MARCKSL | oncogene | cell motility |
| NNAT | ? tumor suppressor | component of ion channel |
| NRCAM | oncogene | EMT |
| PRRC2B | ? oncogene | cell differentiation |
| SEMA4C | oncogene | element of MAPK cascate |
| TSC22D1 | tumor suppressor | transcript factor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho de Oliveira, J.; Mathias, C.; Oliveira, V.C.; Pezuk, J.A.; Brassesco, M.S. The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder. Genes 2022, 13, 2375. https://doi.org/10.3390/genes13122375
Carvalho de Oliveira J, Mathias C, Oliveira VC, Pezuk JA, Brassesco MS. The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder. Genes. 2022; 13(12):2375. https://doi.org/10.3390/genes13122375
Chicago/Turabian StyleCarvalho de Oliveira, Jaqueline, Carolina Mathias, Verônica Cristina Oliveira, Julia Alejandra Pezuk, and María Sol Brassesco. 2022. "The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder" Genes 13, no. 12: 2375. https://doi.org/10.3390/genes13122375






