Mucilaginibacter sp. Strain Metal(loid) and Antibiotic Resistance Isolated from Estuarine Soil Contaminated Mine Tailing from the Fundão Dam
Abstract
:1. Introduction
2. Materials and Methods
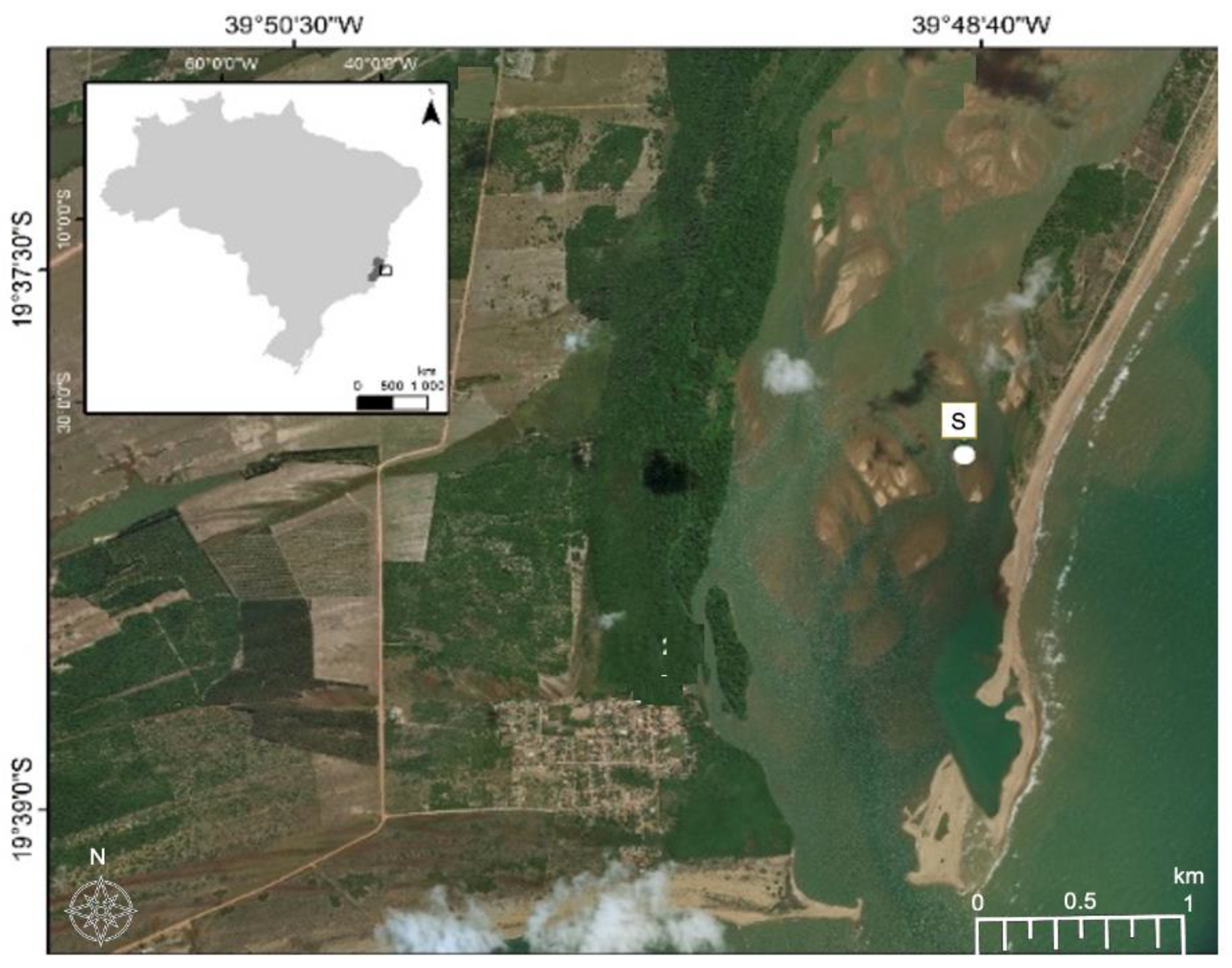
2.1. Site Description and Sampling
2.2. Isolation and Inhibitory Tests
2.3. DNA Extraction and 16S rDNA Sequencing
2.4. Bioinformatic Analysis of 16S and Phylogeny
2.5. DNA Extraction and Whole Genome Sequencing of Mucilaginibacter sp.
2.6. Whole Genome Hybrid Assembly
2.7. Taxonomic Affiliation and Phylogenetic Analysis
2.8. Annotation, Pan-Genome Analysis, and Genomic Resistance Profile
3. Results
3.1. Diversity of Culturable Resistant Bacteria from Mine Tailing Contained Soil
3.2. A potential Novel Metal(loid) Resistant Species from Mucilaginibacter Genus
3.3. Antibiotic Resistance Genetic Profile of Mucilaginibacter 21p
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Escobar, H. In Brazil, researchers struggle to fend off deepening budget cuts. Science 2017, 90, 1–2. [Google Scholar] [CrossRef]
- Segura, F.R.; Nunes, E.A.; Paniz, F.P.; Paulelli, A.C.C.; Rodrigues, G.B.; Braga, G.Ú.L.; dos Reis Pedreira Filho, W.; Barbosa, F.; Cerchiaro, G.; Silva, F.F.; et al. Potential risks of the residue from samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ. Pollut. 2016, 218, 813–825. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Nóbrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Bernardino, A.F.; Otero, X.L. The samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ. 2018, 637, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, F.; Hauser-Davis, R.A.; Soares, L.; Mazzuco, A.C.A.; Chavez Rocha, R.C.; Saint Pierre, T.D.; Saggioro, E.; Correia, F.V.; Ferreira, T.O.; Bernardino, A.F. Contamination and oxidative stress biomarkers in estuarine fish following a mine tailing disaster. PeerJ 2020, 8, e10266. [Google Scholar] [CrossRef] [PubMed]
- Carpio, I.E.M.; Franco, D.C.; Sato, M.I.Z.; Sakata, S.; Pellizari, V.H.; Ferreira Filho, S.S.; Rodrigues, D.F. Biostimulation of metal-resistant microbial consortium to remove zinc from contaminated environments. Sci. Total Environ. 2016, 550, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef]
- Fang, L.; Wei, X.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Role of extracellular polymeric substances in Cu(II) adsorption on Bacillus Subtilis and Pseudomonas Putida. Bioresour. Technol. 2011, 102, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, T.A.; Tindall, B.J.; Liesack, W.; Dedysh, S.N. Mucilaginibacter paludis gen. nov., sp. nov. and Mucilaginibacter Gracilis sp. nov., pectin-, xylan and laminarin-degrading members of the family Sphingobacteriaceae from acidic sphagnum peat bog. Int. J. Syst. Evol. Microbiol. 2007, 57, 2349–2354. [Google Scholar] [CrossRef]
- Joung, Y.; Kim, H.; Kang, H.; Lee, B.-I.; Ahn, T.S.; Joh, K. Mucilaginibacter soyangensis sp. nov., isolated from a lake. Int. J. Syst. Evol. Microbiol. 2014, 64, 413–416. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Xie, Y.R.; Chen, W.M. Mucilaginibacter Limnophilus sp. Nov., Isolated from a Lake. J. Microbiol. 2019, 57, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, S.J.; Jung, Y.T.; Oh, T.K.; Yoon, J.H. Mucilaginibacter lutimaris sp. nov., isolated from a tidal flat sediment. Int. J. Syst. Evol. Microbiol. 2012, 62, 515–519. [Google Scholar] [CrossRef]
- Aydogan, E.L.; Busse, H.J.; Moser, G.; Müller, C.; Kämpfer, P.; Glaeser, S.P. Proposal of mucilaginibacter phyllosphaerae sp. nov. isolated from the phyllosphere of galium album. Int. J. Syst. Evol. Microbiol. 2016, 66, 4138–4147. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, Y.; Xia, X.; Wu, S.; Huang, Y.; Liao, S.; Wang, G. Mucilaginibacter terrenus sp. nov., isolated from manganese mine soil. Int. J. Syst. Evol. Microbiol. 2019, 69, 3074–3079. [Google Scholar] [CrossRef]
- Khan, S.R.; Abbasi, M.K.; Hussan, A.U. Effect of induced soil compaction on changes in soil properties and wheat productivity under sandy loam and sandy clay loam soils: A greenhouse experiment. Commun. Soil Sci. Plant Anal. 2012, 43, 2550–2563. [Google Scholar] [CrossRef]
- An, D.S.; Yin, C.R.; Lee, S.T.; Cho, C.H. Mucilaginibacter daejeonensis sp. nov., isolated from dried rice straw. Int. J. Syst. Evol. Microbiol. 2009, 59, 1122–1125. [Google Scholar] [CrossRef]
- Matyar, F.; Kaya, A.; Dinçer, S. Antibacterial agents and heavy metal resistance in gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Sci. Total Environ. 2008, 407, 279–285. [Google Scholar] [CrossRef]
- Jardine, J.L.; Abia, A.L.K.; Mavumengwana, V.; Ubomba-Jaswa, E. Phylogenetic analysis and antimicrobial profiles of cultured emerging opportunistic pathogens (phyla actinobacteria and proteobacteria) identified in hot springs. Int. J. Environ. Res. Public Health 2017, 14, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 1989. [Google Scholar]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid Determination of 16S Ribosomal RNA Sequences for Phylogenetic Analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (ITOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the genome taxonomy database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef]
- Price Morgan, N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Bayliss, S.C.; Thorpe, H.A.; Coyle, N.M.; Sheppard, S.K.; Feil, E.J. PIRATE: A fast and scalable pangenomics toolbox for clustering diverged orthologues in bacteria. GigaScience 2019, 8, 10. [Google Scholar] [CrossRef]
- Wattam, A.R.; Abraham, D.; Dalay, O.; Disz, T.L.; Driscoll, T.; Gabbard, J.L.; Gillespie, J.J.; Gough, R.; Hix, D.; Kenyon, R.; et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014, 42, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Eren, A.M.; Vineis, J.H.; Morrison, H.G.; Sogin, M.L. A filtering method to generate high quality short reads using illumina paired-end technology. PLoS ONE 2013, 8, 6. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef]
- Kersters, K.; de Vos, P.; Gillis, M.; Swings, J.; Vandamme, P.; Stackebrandt, E. Introduction to the proteobacteria. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 3–37. ISBN 978-0-387-30745-9. [Google Scholar]
- Bernardino, A.F.; Pais, F.S.; Oliveira, L.S.; Gabriel, F.A.; Ferreira, T.O.; Queiroz, H.M.; Mazzuco, A.C.A. Chronic trace metals effects of mine tailings on estuarine assemblages revealed by environmental DNA. PeerJ 2019, 7, e8042. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.S.; de Souza Monteiro, A.; Júlio, A.D.L.; Queiroz, A.L.L.; dos Santos, V.L. Diversity of metal-resistant and tensoactive-producing culturable heterotrophic bacteria isolated from a copper mine in Brazilian Amazonia. Sci. Rep. 2020, 10, 6171. [Google Scholar] [CrossRef]
- Dick, G.J.; Torpey, J.W.; Beveridge, T.J.; Tebo, B.M. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine bacillus species. Appl. Environ. Microbiol. 2008, 74, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handtke, S.; Schroeter, R.; Jürgen, B.; Methling, K.; Schlüter, R.; Albrecht, D.; van Hijum, S.A.F.T.; Bongaerts, J.; Maurer, K.H.; Lalk, M.; et al. Bacillus Pumilus reveals a remarkably high resistance to hydrogen peroxide provoked oxidative stress. PLoS ONE 2014, 9, e85625. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Filippidou, S.; Junier, T.; Muñoz Rufatt, P.; Jeanneret, N.; Wunderlin, T.; Sieber, N.; Dorador, C.; Junier, P. Manganese-II oxidation and copper-II resistance in endospore forming firmicutes isolated from uncontaminated environmental sites. AIMS Environ. Sci. 2016, 3, 220–238. [Google Scholar] [CrossRef]
- Kumar, A.; Tripti; Maleva, M.; Bruno, L.B.; Rajkumar, M. Synergistic effect of ACC deaminase producing Pseudomonas sp. TR15a and siderophore producing bacillus aerophilus TR15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 2021, 276, 130038. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, C.; Bakshi, U.; Mallick, I.; Mukherji, S.; Bera, B.; Ghosh, A. Genome-guided insights into the plant growth promotion capabilities of the physiologically versatile bacillus aryabhattai strain AB211. Front. Microbiol. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Castillo, A.; Mejía-Escobedo, Y.; Rojas-Avelizapa, N. Study of bacillus megaterium potential application for high metal content residues biotreatment. Open J. Bacteriol. 2018, 2, 4–8. [Google Scholar] [CrossRef] [Green Version]
- CONAMA Resolução No 420, de 28 de Dezembro de 2009; Brazilian National Environment Council: Brasília, Brazil, 2009.
- Gomes, L.E.D.O.; Correa, L.B.; Sá, F.; Neto, R.R.; Bernardino, A.F.; Rodrigues-Neto, R.; Bernardino, A.F. The impacts of the samarco mine tailing spill on the rio doce estuary, Eastern Brazil. Mar. Pollut. Bull. 2017, 120, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.F.; de Freitas, M.B.D.; Szinwelski, N.; Vicente, N.; Medeiros, L.C.C.; Schaefer, C.E.G.R.; Dergam, J.A.; Sperber, C.F. Impacts of the samarco tailing dam collapse on metals and arsenic concentration in freshwater fish muscle from Doce river, Southeastern Brazil. Integr. Environ. Assess. Manag. 2020, 16, 622–630. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ying, S.C.; Abernathy, M.; Barcellos, D.; Gabriel, F.A.; Otero, X.L.; Nóbrega, G.N.; Bernardino, A.F.; Ferreira, T.O. Manganese: The overlooked contaminant in the world largest mine tailings dam collapse. Environ. Int. 2021, 146, 106284. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.; Tang, K.; Veeranagouda, Y.; Boente, R.; Patrick, S.; Blakely, G.; Wexler, H.M. Novel large-scale chromosomal transfer in bacteroides fragilis contributes to its pan-genome and rapid environmental adaptation. Microb. Genom. 2017, 3, e000136. [Google Scholar] [CrossRef] [Green Version]
- Banach, A.M.; Kuźniar, A.; Grządziel, J.; Wolińska, A. Azolla Filiculoides L. as a source of metal-tolerant microorganisms. PLoS ONE 2020, 15, e0232699. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Luo, L.Y.; Xie, L.L.; Jin, D.C.; Mi, B.B.; Wang, D.H.; Li, X.F.; Dai, X.Z.; Zou, X.X.; Zhang, Z.; Ma, Y.Q.; et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Appl. Soil Ecol. 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Soares, R.; Trejo, J.; Lorite, M.J.; Figueira, E.; Sanjuán, J.; Castro, I.V.E. Diversity, phylogeny and plant growth promotion traits of nodule associated bacteria isolated from Lotus Parviflorus. Microorganisms 2020, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Cameotra, S.S. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 7367–7376. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.M.; Siddiqi, M.Z.; Im, W.-T. Mucilaginibacter ginsenosidivorans sp. nov., isolated from soil of ginseng field. Curr. Microbiol. 2017, 74, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.S.; Park, S.C.; Kim, E.M.; Lim, C.H.; Seong, C.N. Mucilaginibacter rigui sp. nov., isolated from Wetland freshwater, and emended description of the genus Mucilaginibacter. Int. J. Syst. Evol. Microbiol. 2010, 60, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, M.M.; Tang, J.; Kane, M.; Emerson, D. Captured diversity in a culture collection: Case study of the geographic and habitat distributions of environmental isolates held at the american type culture collection. Appl. Environ. Microbiol. 2005, 71, 2813–2823. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.P.; Carraro, N.; Yang, N.; Liu, B.; Xia, X.; Feng, R.; Saquib, Q.; Al-Wathnani, H.A.; van der Meer, J.R.; Rensing, C. Genomic islands confer heavy metal resistance in Mucilaginibacter Kameinonensis and Mucilaginibacter Rubeus isolated from a gold/copper mine. Genes 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiat, B.; Sauviac, L.; Picheraux, C.; Rossignol, M.; Bruand, C. Sinorhizobium meliloti sigma factors RpoE1 and RpoE4 are activated in stationary phase in response to sulfite. PLoS ONE 2012, 7, e50768. [Google Scholar] [CrossRef] [Green Version]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B12: A paradigm for protein metalation. Biochim. Biophys. Acta 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Ferrer, A.; Rivera, J.; Zapata, C.; Norambuena, J.; Sandoval, Á.; Chávez, R.; Orellana, O.; Levicán, G. Cobalamin protection against oxidative stress in the acidophilic iron-oxidizing Bacterium Leptospirillum group II CF-1. Front. Microbiol. 2016, 7, 748. [Google Scholar] [CrossRef] [Green Version]
- Pieck, J.C.; Hennecke, U.; Pierik, A.J.; Friedel, M.G.; Carell, T. Characterization of a new thermophilic spore photoproduct lyase from Geobacillus Stearothermophilus (SplG) with defined lesion containing DNA substrates. J. Biol. Chem. 2006, 281, 36317–36326. [Google Scholar] [CrossRef] [Green Version]
- Perreten, V.; Schwarz, F.V.; Teuber, M.; Levy, S.B. Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus Lactis and Escherichia Coli. Antimicrob. Agents Chemother. 2001, 45, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Nishino, K.; Nikaido, E.; Yamaguchi, A. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella Enterica Serovar Typhimurium. J. Bacteriol. 2007, 189, 9066–9075. [Google Scholar] [CrossRef] [Green Version]
- Perron, K.; Caille, O.; Rossier, C.; van Delden, C.; Dumas, J.L.; Köhler, T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 8761–8768. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Núñez-Montero, K.; Quezada-Solís, D.; Khalil, Z.G.; Capon, R.J.; Andreote, F.D.; Barrientos, L. Genomic and metabolomic analysis of antarctic bacteria revealed culture and elicitation conditions for the production of antimicrobial compounds. Biomolecules 2020, 10, 673. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ. Health Perspect. 2013, 121, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Fierer, N.; Dantas, G.; Biology, E. HHS public access. Ann. Glob. Health 2014, 509, 612–616. [Google Scholar] [CrossRef]
- Graham, D.W.; Olivares-Rieumont, S.; Knapp, C.W.; Lima, L.; Werner, D.; Bowen, E. Antibiotic resistance gene abundances associated with waste discharges to the Almendares river near Havana, Cuba. Environ. Sci. Technol. 2011, 45, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Elena, S.F.; Lenski, R.E. Evolution experiments with microorganisms: The dynamics and genetic bases of adaptation. Nat. Rev. Genet. 2003, 4, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Fan, Z.; Lu, S.; Ma, Y.; Nie, X.; Tong, F.; Peng, X. Changes in rhizosphere bacterial communities during remediation of heavy metal-accumulating plants around the Xikuangshan mine in Southern China. Sci. Rep. 2019, 9, 1947. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, K.S.; Hung, C.S.; Crowley, J.R.; Stapleton, A.E.; Henderson, J.P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012, 8, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal resistance and its association with antibiotic resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, T.O.; Barcellos, D.; Nóbrega, G.N.; Antelo, J.; Otero, X.L.; Bernardino, A.F. From sinks to sources: The role of fe oxyhydroxide transformations on phosphorus dynamics in estuarine soils. J. Environ. Manag. 2021, 278, 111575. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; de Oliveira Filho, R.L.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in Northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Subramanian, S.; Smith, D.L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis Thaliana. Sci. Rep. 2020, 10, 12740. [Google Scholar] [CrossRef]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Tang, J.; Nie, L.; Huang, J.; Wang, G. High-quality-draft genome sequence of the heavy metal resistant and exopolysaccharides producing bacterium Mucilaginibacter pedocola TBZ30T 06 biological sciences 0604 genetics. Stand. Genom. Sci. 2018, 13, 34. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Qiao, Z.; Wang, R.; Wang, G. Mucilaginibacter pedocola sp. nov isolated from a heavy-metal-contaminated paddy field. Int. J. Syst. Evol. Microbiol. 2016, 66, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Poonguzhali, S.; Lee, J.S.; Senthilkumar, M.; Lee, K.C.; Sundaram, S. Mucilaginibacter gossypii sp. nov. and Mucilaginibacter gossypiicola sp. nov., plant-growth-promoting bacteria isolated from cotton rhizosphere soils. Int. J. Syst. Evol. Microbiol. 2010, 60, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, F.; Huang, Y.; Zhou, M.; Gao, J.; Yan, T.; Sheng, H.; An, L. Sphingomonas sp. cra20 increases plant growth rate and alters rhizosphere microbial community structure of arabidopsis thaliana under drought stress. Front. Microbiol. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.J.; Park, G.T.; Cha, M.S.; Heo, M.S. Solubilization of insoluble inorganic phosphates by a novel salt- and PH-tolerant Pantoea Agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef]
- Ten, L.N.; Jeon, N.Y.; Li, W.; Cho, Y.J.; Kim, M.K.; Lee, S.Y.; Rooney, A.P.; Jung, H.Y. Mucilaginibacter terrigena sp. nov. sp., a novel member of the family Sphingobacteriaceae. Curr. Microbiol. 2019, 76, 1152–1160. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, C.; Muras, A.; Romero, M.; López, M.; Tomás, M.; Otero, A. Multiple quorum quenching enzymes are active in the nosocomial pathogen acinetobacter baumannii ATCC17978. Front. Cell. Infect. Microbiol. 2018, 8, 310. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Prindle, A.; Liu, J.; Asally, M.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Ion channels enable electrical communication in bacterial communities. Nature 2015, 527, 59–63. [Google Scholar] [CrossRef] [Green Version]


| Isolate | Metal | Antimicrobial | Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance Profile (mmol/L) | Minimum Inhibitory Concentration (µg/mL) | Cell/Colony | ||||||||||
| Cd2+ | Zn2+ | Co2+ | Mn2+ | Amp | Cl | St | NA | Tet | Ka | Gram | Color | |
| 1p | 500 | 800 | 3200 | 200 | 100 | 100 | + | White | ||||
| 2p | 800 | 3200 | 100 | + | White | |||||||
| 3p | 150 | 500 | 800 | 3200 | 70 | 100 | 30 | + | White | |||
| 4p | 800 | 3200 | 100 | + | White | |||||||
| 5p | 150 | 500 | 800 | 3200 | 200 | + | White | |||||
| 6p | 800 | 3200 | 100 | + | White | |||||||
| 7p | 800 | 3200 | + | White | ||||||||
| 8p | 800 | 3200 | + | White | ||||||||
| 9p | 30 | 500 | 800 | 3200 | 10 | 50 | 100 | 30 | + | White | ||
| 10p | 800 | 3200 | 300 | 10 | + | White | ||||||
| 11p | 800 | 3200 | 100 | + | White | |||||||
| 12p | 800 | 3200 | + | White | ||||||||
| 13p | 200 | 800 | 3200 | 300 | 10 | 100 | 200 | + | White | |||
| 14p | 800 | 3200 | 100 | + | White | |||||||
| 15p | 800 | 3200 | + | White | ||||||||
| 16p | 800 | 3200 | + | White | ||||||||
| 17p | 800 | 3200 | + | White | ||||||||
| 18p | 500 | 800 | 3200 | 10 | + | White | ||||||
| 19p | 800 | 3200 | − | Pink | ||||||||
| 20p | 300 | 10 | 800 | 3200 | 300 | 10 | 50 | 50 | 10 | − | Pink | |
| 21p | 300 | 10 | 800 | 3200 | 300 | 10 | 100 | 100 | 10 | 50 | − | Pink |
| Gene * | Description | Frequency | Other Species with the Gene | GI (Loci) |
|---|---|---|---|---|
| cotSA | Spore coat protein cotAS | rare | M. ginsenosidivorans | |
| dltC | D-alanyl carrier protein | unique | ||
| endOF2 | Endo-β-N-acetylglucosaminidase F2 | rare | M. lappiensis | |
| cusC | Cation efflux system protein | rare | M. gossypiicola | |
| fabG | 3-oxoacyl-[acyl-carrier-protein] reductase | rare | M. ginsenosidivorans | |
| glmE | Glutamate mutase epsilon subunit | unique | ||
| lgrB | Linear gramicidin synthase subunit B | rare | M. rubeus | |
| lgrE | Linear gramicidin dehydrogenase | rare | ||
| menE | 2-succinylbenzoate--CoA ligase | unique | ||
| mmgB | putative 3-hydroxybutyryl-CoA dehydrogenase | unique | ||
| rcsB | Transcriptional regulatory protein | rare | M. gossypii; M. corticis | Yes (2,368,463) |
| rfbD | UDP-galactopyranose mutase | unique | ||
| rpoE_4 | ECF RNA polymerase sigma-E factor | unique | Yes (2,473,906) | |
| splG | Spore photoproduct lyase | unique | Yes (2,484,144) | |
| srfAA | Surfactin synthase subunit 1 | unique | ||
| srfAB | Surfactin synthase subunit 2 | unique | ||
| tmoS | Sensor histidine kinase | rare | M. pineti; M. polytrichastri | Yes (2,596,785) |
| yojI | ABC transporter ATP-binding/permease protein | rare | M. lappiensis; M. oryzae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, A.L.S.; Andreote, F.D.; Defalco, T.; Delbaje, E.; Barrientos, L.; Dias, A.C.F.; Gabriel, F.A.; Bernardino, A.F.; Núñez-Montero, K. Mucilaginibacter sp. Strain Metal(loid) and Antibiotic Resistance Isolated from Estuarine Soil Contaminated Mine Tailing from the Fundão Dam. Genes 2022, 13, 174. https://doi.org/10.3390/genes13020174
Vasconcelos ALS, Andreote FD, Defalco T, Delbaje E, Barrientos L, Dias ACF, Gabriel FA, Bernardino AF, Núñez-Montero K. Mucilaginibacter sp. Strain Metal(loid) and Antibiotic Resistance Isolated from Estuarine Soil Contaminated Mine Tailing from the Fundão Dam. Genes. 2022; 13(2):174. https://doi.org/10.3390/genes13020174
Chicago/Turabian StyleVasconcelos, Ana L. S., Fernando Dini Andreote, Thaiane Defalco, Endrews Delbaje, Leticia Barrientos, Armando C. F. Dias, Fabricio Angelo Gabriel, Angelo F. Bernardino, and Kattia Núñez-Montero. 2022. "Mucilaginibacter sp. Strain Metal(loid) and Antibiotic Resistance Isolated from Estuarine Soil Contaminated Mine Tailing from the Fundão Dam" Genes 13, no. 2: 174. https://doi.org/10.3390/genes13020174
APA StyleVasconcelos, A. L. S., Andreote, F. D., Defalco, T., Delbaje, E., Barrientos, L., Dias, A. C. F., Gabriel, F. A., Bernardino, A. F., & Núñez-Montero, K. (2022). Mucilaginibacter sp. Strain Metal(loid) and Antibiotic Resistance Isolated from Estuarine Soil Contaminated Mine Tailing from the Fundão Dam. Genes, 13(2), 174. https://doi.org/10.3390/genes13020174






