Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sodium Valproate Exposure
2.3. Confirmation of ASD-like Traits in VPA Rats
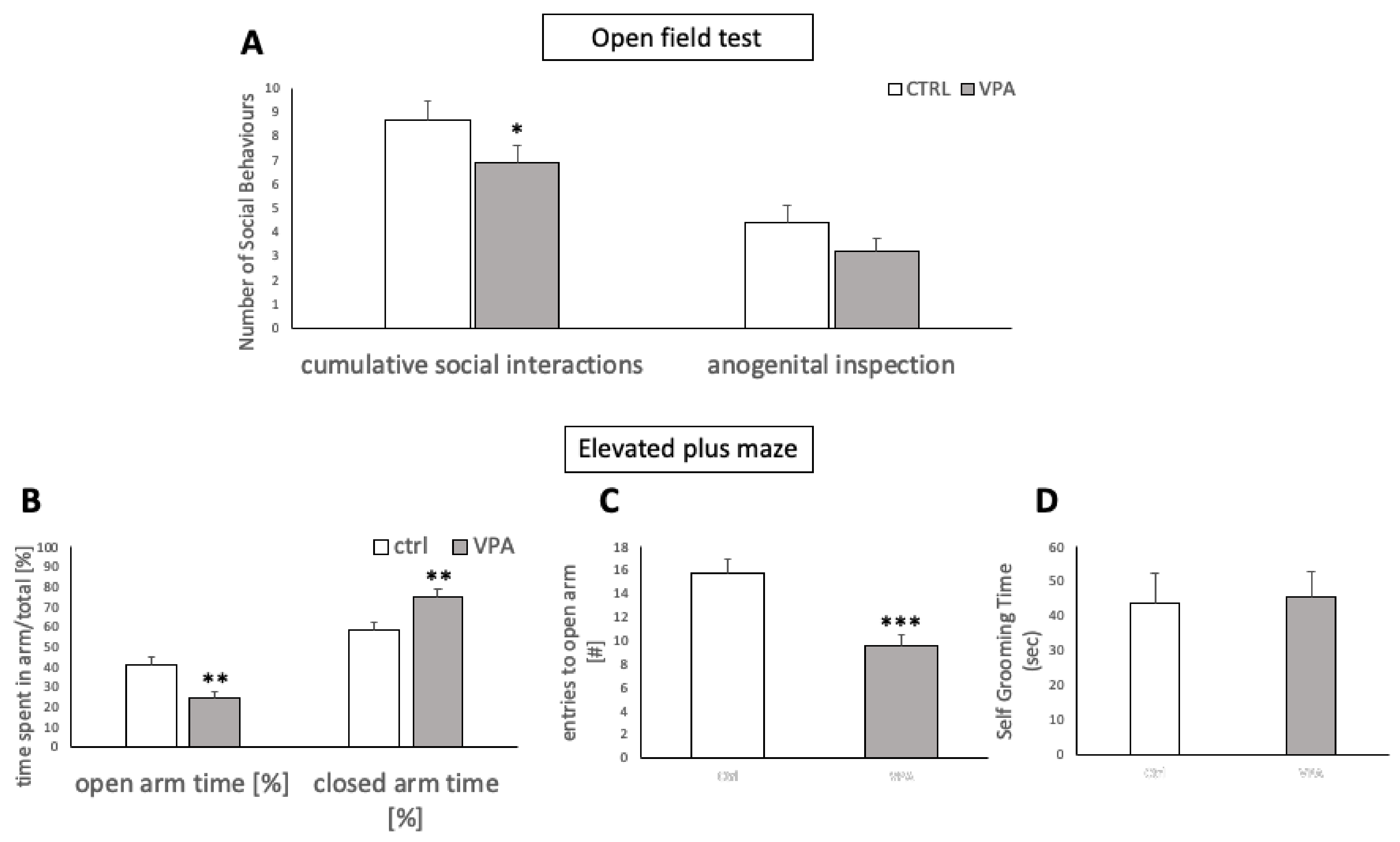
2.3.1. Open Field Test for Social Interactions
2.3.2. Elevated-Plus Maze Test
2.4. Feeding Studies
2.4.1. Ad Libitum Standard Chow and Water Intake in VPA Rats versus Healthy Controls
2.4.2. Energy Deprivation-Induced Feeding
2.4.3. Feeding after Overnight Food and Water Deprivation
2.4.4. Body Weight Trajectory
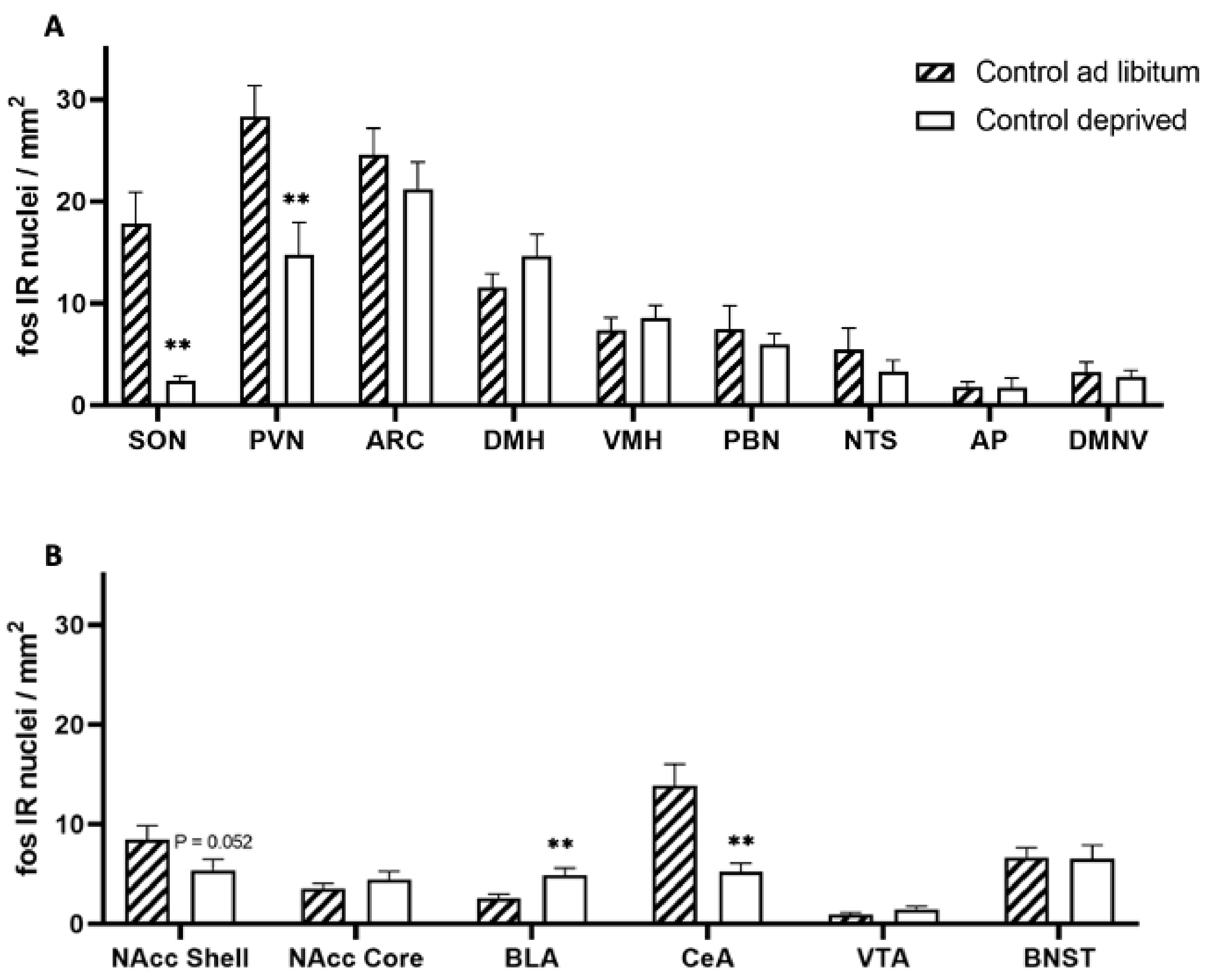
2.5. Neuronal Activation in Hungry vs. Fed Animals
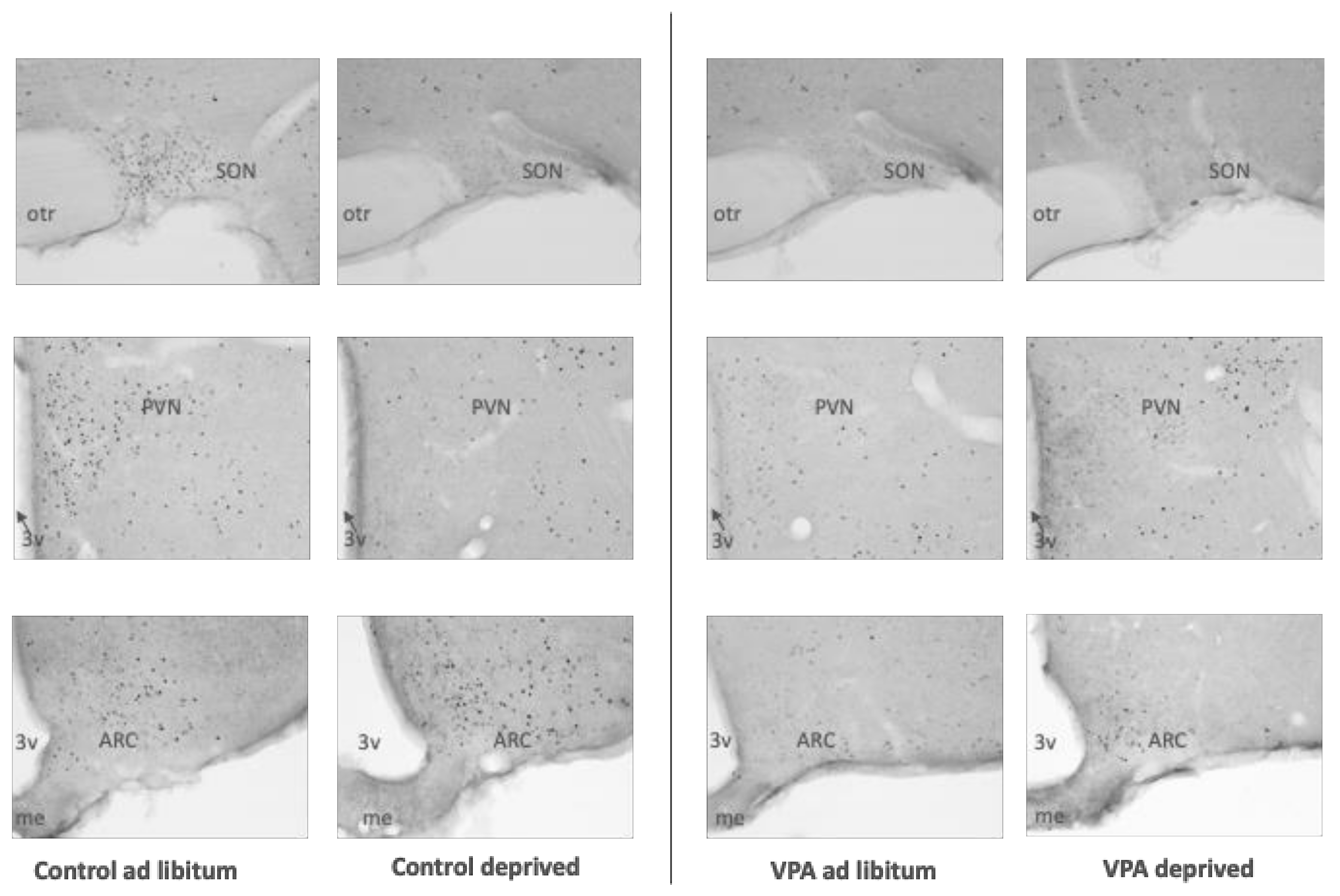
2.5.1. Deprivation and Tissue Dissection
2.5.2. Immunohistochemistry
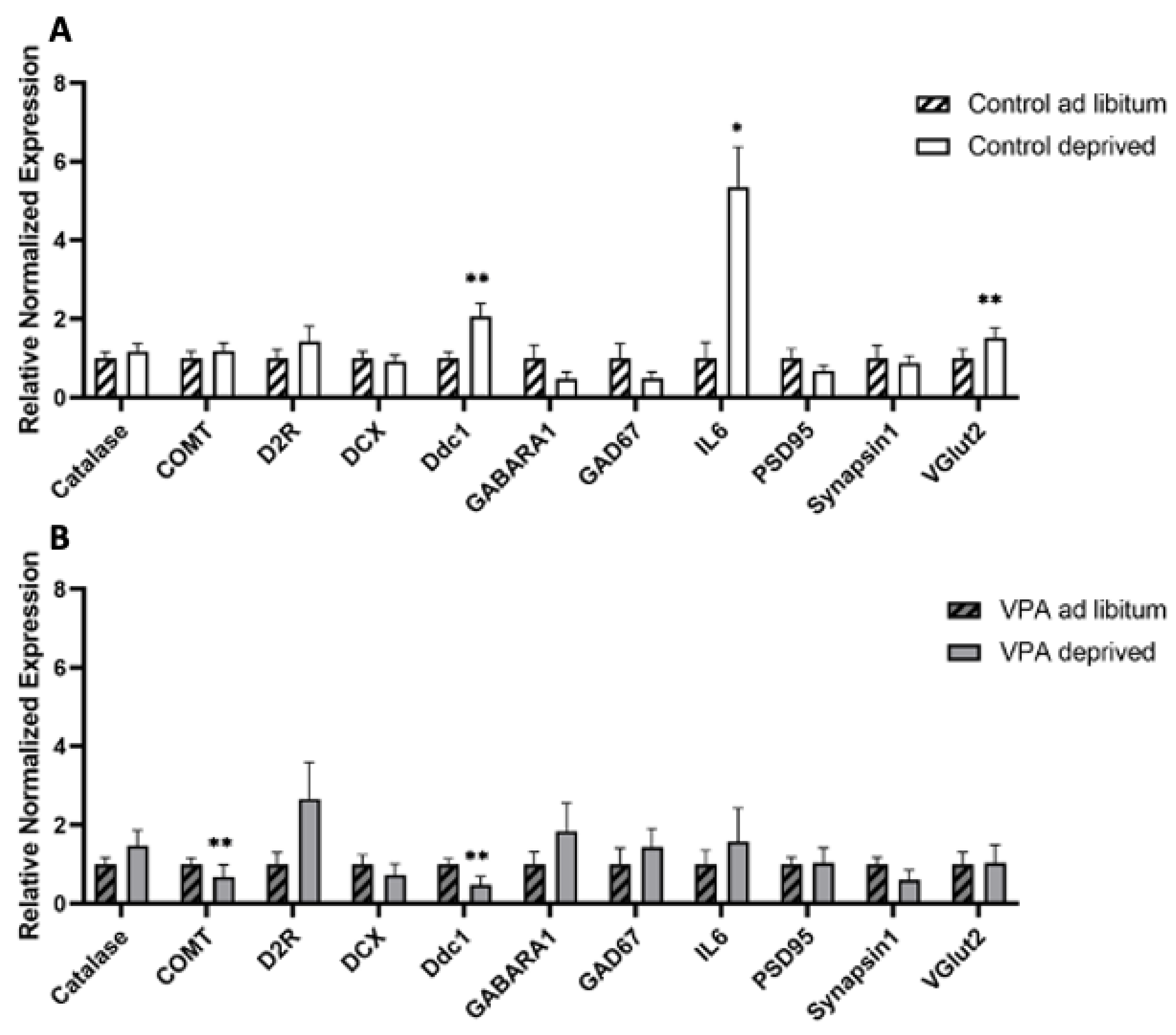
2.6. Gene Expression in Hungry vs. Fed Animals
2.6.1. Deprivation and Dissection of Hypothalamus and Brainstem
2.6.2. rtPCR Protocol and Data Analysis
2.7. Data Analysis
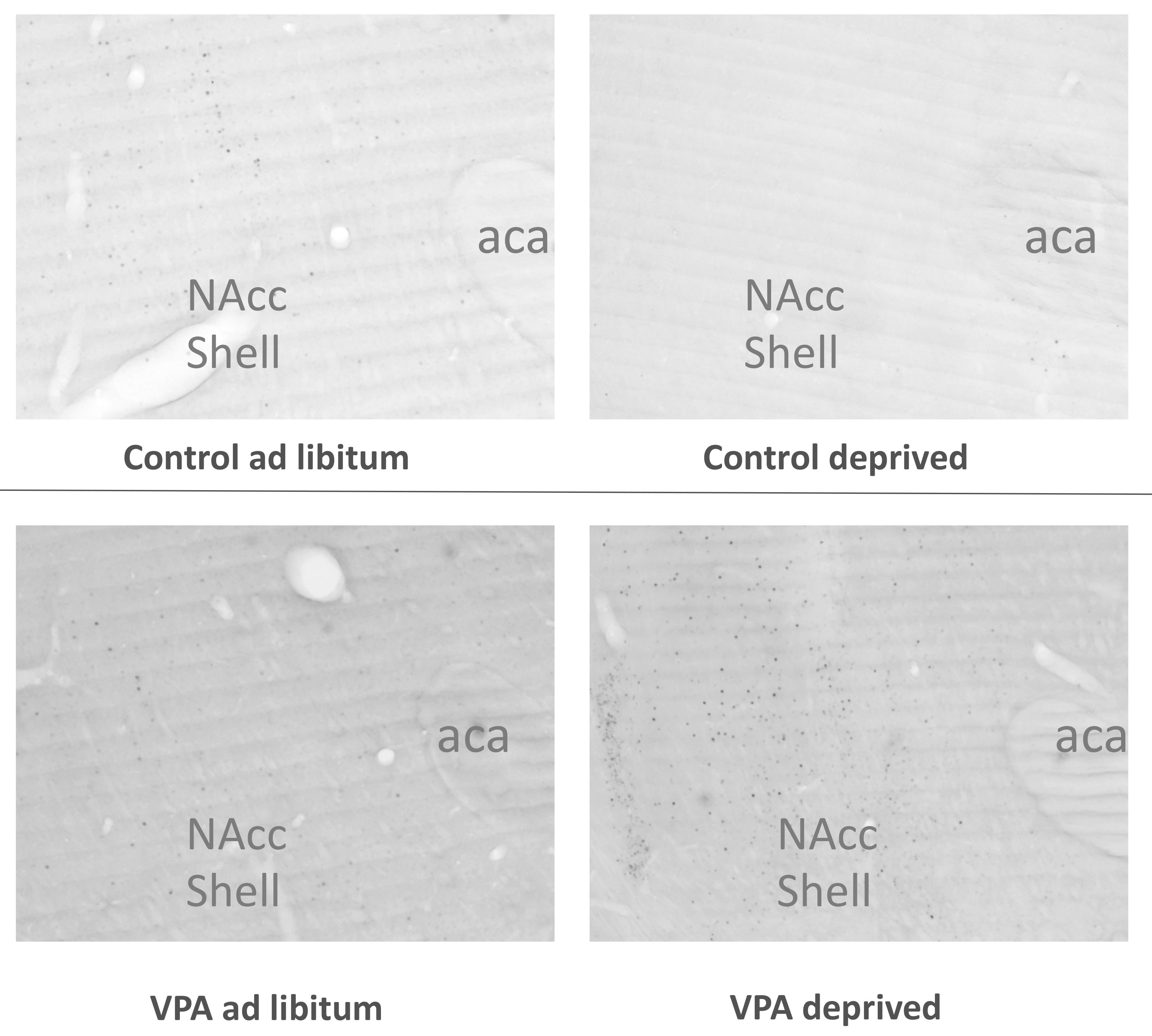
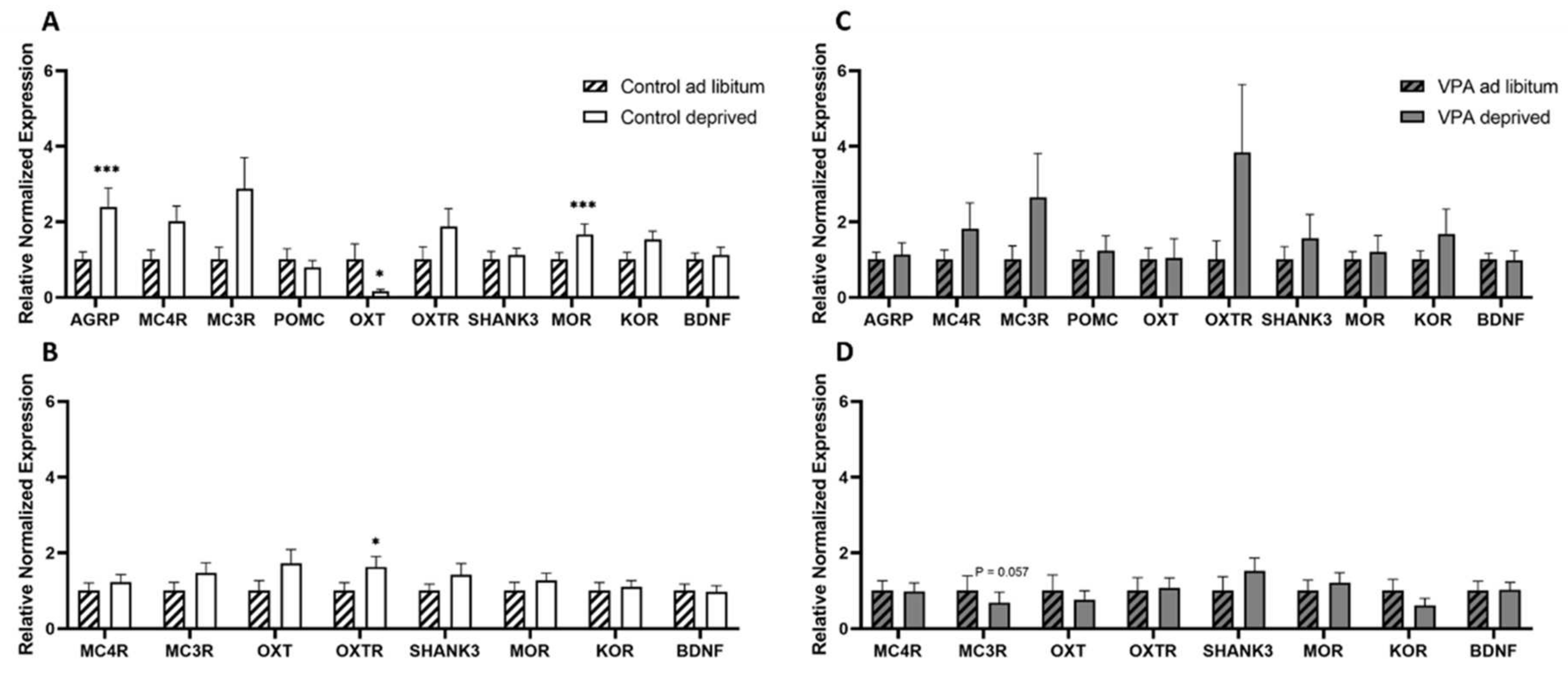
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dufour-Rainfray, D.; Vourc’h, P.; Le Guisquet, A.M.; Garreau, L.; Ternant, D.; Bodard, S.; Jaumain, E.; Gulhan, Z.; Belzung, C.; Andres, C.R.; et al. Behavior and serotonergic disorders in rats exposed prenatally to valproate: A model for autism. Neurosci. Lett. 2010, 470, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, D.; Kaur, G. Valproic acid alters GnRH-GABA interactions in cycling female rats. Cell. Mol. Neurobiol. 2007, 27, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Baf, M.H.; Subhash, M.N.; Lakshmana, K.M.; Rao, B.S. Sodium valproate induced alterations in monoamine levels in different regions of the rat brain. Neurochem. Int. 1994, 24, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zieminska, E.; Toczylowska, B.; Diamandakis, D.; Hilgier, W.; Filipkowski, R.K.; Polowy, R.; Orzel, J.; Gorka, M.; Lazarewicz, J.W. Glutamate, Glutamine and GABA Levels in Rat Brain Measured Using MRS, HPLC and NMR Methods in Study of Two Models of Autism. Front. Mol. Neurosci. 2018, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Kanner, L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968, 35, 100–136. [Google Scholar]
- Sharp, W.G.; Berry, R.C.; McCracken, C.; Nuhu, N.N.; Marvel, E.; Saulnier, C.A.; Klin, A.; Jones, W.; Jaquess, D.L. Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. J. Autism Dev. Disord. 2013, 43, 2159–2173. [Google Scholar] [CrossRef]
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food selectivity in children with autism spectrum disorders and typically developing children. J. Pediatr. 2010, 157, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Diolordi, L.; del Balzo, V.; Bernabei, P.; Vitiello, V.; Donini, L.M. Eating habits and dietary patterns in children with autism. Eat. Weight Disord. 2014, 19, 295–301. [Google Scholar] [CrossRef]
- Williams, P.G.; Dalrymple, N.; Neal, J. Eating habits of children with autism. Pediatr. Nurs. 2000, 26, 259–264. [Google Scholar]
- Gray, H.L.; Chiang, H.M. Brief Report: Mealtime Behaviors of Chinese American Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 892–897. [Google Scholar] [CrossRef]
- Zickgraf, H.F.; Richard, E.; Zucker, N.L.; Wallace, G.L. Rigidity and Sensory Sensitivity: Independent Contributions to Selective Eating in Children, Adolescents, and Young Adults. J. Clin. Child Adolesc. Psychol. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Strand, M. Eggs, sugar, grated bones: Colour-based food preferences in autism, eating disorders, and beyond. Med. Humanit. 2021, 47, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Kochalka, J.; Schaer, M.; Wakeman, H.; Qin, S.; Padmanabhan, A.; Menon, V. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain 2018, 141, 2795–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, C.J.; Foss-Feig, J.H.; Heacock, J.L.; Newsom, C.R.; Cowan, R.L.; Benningfield, M.M.; Rogers, B.P.; Cao, A. Response of neural reward regions to food cues in autism spectrum disorders. J. Neurodev. Disord. 2012, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, N.; Rubia, K.; van Amelsvoort, T.; Daly, E.; Smith, A.; Murphy, D.G. Neural correlates of reward in autism. Br. J. Psychiatry 2008, 192, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, E.G.; Crawford, M.L. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 1977, 7, 49–55. [Google Scholar] [CrossRef]
- Croonenberghs, J.; Bosmans, E.; Deboutte, D.; Kenis, G.; Maes, M. Activation of the inflammatory response system in autism. Neuropsychobiology 2002, 45, 1–6. [Google Scholar] [CrossRef]
- Brigida, A.L.; Schultz, S.; Cascone, M.; Antonucci, N.; Siniscalco, D. Endocannabinod Signal Dysregulation in Autism Spectrum Disorders: A Correlation Link between Inflammatory State and Neuro-Immune Alterations. Int. J. Mol. Sci. 2017, 18, 1425. [Google Scholar] [CrossRef] [Green Version]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Harutyunyan, H.; Harutyunyan, A. A certain role of SOD/CAT imbalance in pathogenesis of autism spectrum disorders. Free. Radic. Biol. Med. 2018, 123, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24, 193–206. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Yang, H.Y.; Ko, T.L.; Hsu, Y.F.; Sheu, J.R.; Ou, G.; Hsu, M.J. Valproic acid suppresses lipopolysaccharide-induced cyclooxygenase-2 expression via MKP-1 in murine brain microvascular endothelial cells. Biochem. Pharmacol. 2014, 88, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Rowe, M.; Ren, M.; Hong, J.S.; Chen, P.S.; Chuang, D.M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007, 321, 892–901. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Zhou, M.; Li, X.; Fang, Z.; Gao, H.; Li, Y.; Hu, W. Valproic Acid Attenuates Traumatic Brain Injury-Induced Inflammation in Vivo: Involvement of Autophagy and the Nrf2/ARE Signaling Pathway. Front. Mol. Neurosci. 2018, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Chu, L.W.; Cheng, K.I.; Hsieh, S.L.; Juan, Y.S.; Wu, B.N. Valproate reduces neuroinflammation and neuronal death in a rat chronic constriction injury model. Sci. Rep. 2018, 8, 16457. [Google Scholar] [CrossRef] [PubMed]
- Gassowska-Dobrowolska, M.; Cieslik, M.; Czapski, G.A.; Jesko, H.; Frontczak-Baniewicz, M.; Gewartowska, M.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Babiec, L.; et al. Prenatal Exposure to Valproic Acid Affects Microglia and Synaptic Ultrastructure in a Brain-Region-Specific Manner in Young-Adult Male Rats: Relevance to Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3576. [Google Scholar] [CrossRef]
- Mirza, R.; Sharma, B. Benefits of Fenofibrate in prenatal valproic acid-induced autism spectrum disorder related phenotype in rats. Brain Res. Bull. 2019, 147, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, V.C.; Engel, D.F.; Jara, C.P.; Mendes, N.F.; Haddad-Tovolli, R.; Prado, T.P.; Sidarta-Oliveira, D.; Morari, J.; Velloso, L.A.; Araujo, E.P. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J. Neuroinflamm. 2021, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ferreras, L.; Longo, F.; Richard, J.E.; Eerola, K.; Shevchouk, O.T.; Tuzinovic, M.; Skibicka, K.P. Key role for hypothalamic interleukin-6 in food-motivated behavior and body weight regulation. Psychoneuroendocrinology 2021, 131, 105284. [Google Scholar] [CrossRef]
- Buchner, D.A.; Geisinger, J.M.; Glazebrook, P.A.; Morgan, M.G.; Spiezio, S.H.; Kaiyala, K.J.; Schwartz, M.W.; Sakurai, T.; Furley, A.J.; Kunze, D.L.; et al. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mamm. Genome 2012, 23, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, S.; Nakajima, H.; Sugimoto, S.; Kodo, K.; Shigehara, K.; Morimoto, H.; Tsuma, Y.; Moroto, M.; Mori, J.; Kosaka, K.; et al. High-fat diet accelerates extreme obesity with hyperphagia in female heterozygous Mecp2-null mice. PLoS ONE 2019, 14, e0210184. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Rozman, J.; Jacobsson, J.A.; Rathkolb, B.; Stromberg, S.; Hans, W.; Klockars, A.; Alsio, J.; Riserus, U.; Becker, L.; et al. Neurobeachin, a regulator of synaptic protein targeting, is associated with body fat mass and feeding behavior in mice and body-mass index in humans. PLoS Genet. 2012, 8, e1002568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klockars, A.; Pal, T.; Levine, A.S.; Olszewski, P.K. Neural Basis of Dysregulation of Palatability-Driven Appetite in Autism. Curr. Nutr. Rep. 2021, 10, 391–398. [Google Scholar] [CrossRef]
- Schneider, T.; Przewlocki, R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Favre, M.R.; Barkat, T.R.; Lamendola, D.; Khazen, G.; Markram, H.; Markram, K. General developmental health in the VPA-rat model of autism. Front. Behav. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, T.; Silberberg, G.; Markram, H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb. Cortex 2008, 18, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Park, J.H.; Kim, H.J.; Jeon, S.J.; Dela Pena, I.C.; Han, S.H.; Cheong, J.H.; et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neurochem. 2013, 124, 832–843. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Understanding sex bias in autism spectrum disorder. Proc. Natl. Acad. Sci. USA 2013, 110, 4868–4869. [Google Scholar] [CrossRef] [Green Version]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Spadaro, P.A.; Flavell, C.R.; Widagdo, J.; Ratnu, V.S.; Troup, M.; Ragan, C.; Mattick, J.S.; Bredy, T.W. Long Noncoding RNA-Directed Epigenetic Regulation of Gene Expression Is Associated With Anxiety-like Behavior in Mice. Biol. Psychiatry 2015, 78, 848–859. [Google Scholar] [CrossRef] [Green Version]
- Head, M.A.; Jewett, D.C.; Gartner, S.N.; Klockars, A.; Levine, A.S.; Olszewski, P.K. Effect of Oxytocin on Hunger Discrimination. Front. Endocrinol. (Lausanne) 2019, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Klockars, A.; Wood, E.L.; Gartner, S.N.; McColl, L.K.; Levine, A.S.; Carpenter, E.A.; Prosser, C.G.; Olszewski, P.K. Palatability of Goat’s versus Cow’s Milk: Insights from the Analysis of Eating Behavior and Gene Expression in the Appetite-Relevant Brain Circuit in Laboratory Animal Models. Nutrients 2019, 11, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postorino, V.; Sanges, V.; Giovagnoli, G.; Fatta, L.M.; De Peppo, L.; Armando, M.; Vicari, S.; Mazzone, L. Clinical differences in children with autism spectrum disorder with and without food selectivity. Appetite 2015, 92, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory Sensitivity and Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet 2018, 118, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Figuerola, P.; Canals, J.; Fernandez-Cao, J.C.; Arija Val, V. Differences in food consumption and nutritional intake between children with autism spectrum disorders and typically developing children: A meta-analysis. Autism 2019, 23, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, E.; Norton, C.; Pimblett, C.; Stewart, C.; Tchanturia, K. Eating as an autistic adult: An exploratory qualitative study. PLoS ONE 2019, 14, e0221937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, S.; Ozkara, N.; Poustka, F. Autism spectrum disorders and low body weight: Is there really a systematic association? Int. J. Eat. Disord. 2002, 31, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Sedgewick, F.; Leppanen, J.; Tchanturia, K. Autistic adult outcomes on weight and body mass index: A large-scale online study. Eat. Weight Disord. 2020, 25, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarelli, J.; Pappas, D.; Welchons, L.; Augustyn, M. Autism Spectrum Disorder and Avoidant/Restrictive Food Intake Disorder. J. Dev. Behav. Pediatr. 2017, 38, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.M.; Brooks, L.L.; Waas, J.R.; Levine, A.S.; Olszewski, P.K. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport 2014, 25, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Wing, L. Excessive drinking of fluids in children and adults on the autism spectrum: A brief report. Adv. Autism 2015, 1, 51–60. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engstrom-Ruud, L.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Bruning, J.C. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Pazos, P.; Lima, L.; Casanueva, F.F.; Dieguez, C.; Garcia, M.C. Interleukin 6 deficiency modulates the hypothalamic expression of energy balance regulating peptides during pregnancy in mice. PLoS ONE 2013, 8, e72339. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Zhou, Z.; He, J.; Yan, C.; Ding, S. IL-6 Inhibits Starvation-induced Autophagy via the STAT3/Bcl-2 Signaling Pathway. Sci. Rep. 2015, 5, 15701. [Google Scholar] [CrossRef] [Green Version]
- Hersrud, S.L.; Stoltenberg, S.F. Epistatic interaction between COMT and DAT1 genes on eating behavior: A pilot study. Eat. Behav. 2009, 10, 131–133. [Google Scholar] [CrossRef] [Green Version]
- Angeles-Castellanos, M.; Aguilar-Roblero, R.; Escobar, C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R158–R165. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, L.E.; Fong, T.M.; Leng, G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006, 4, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Kroemer, N.B.; Veldhuizen, M.G.; Babbs, A.E.; de Araujo, I.E.; Gitelman, D.R.; Sherwin, R.S.; Sinha, R.; Small, D.M. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J. Neurosci. 2015, 35, 7964–7976. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D.A.; Neill, D.B. Functional interaction between the basolateral amygdala and the nucleus accumbens underlies incentive motivation for food reward on a fixed ratio schedule. Neuroscience 2009, 159, 1264–1273. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Cedernaes, J.; Olsson, F.; Levine, A.S.; Schioth, H.B. Analysis of the network of feeding neuroregulators using the Allen Brain Atlas. Neurosci. Biobehav. Rev. 2008, 32, 945–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Bibbo, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Daniele Fallin, M. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Borody, T.J.; Kang, D.W.; Khoruts, A.; Krajmalnik-Brown, R.; Sadowsky, M.J. Microbiota transplant therapy and autism: Lessons for the clinic. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1033–1037. [Google Scholar] [CrossRef]




| Housekeeping Genes | ||
|---|---|---|
| Gene | Forward | Reverse |
| TBP | 5′-AGAACAATCCAGATACAGCA-3′ | 5′-GGGAACTTCACATCACAGCTC-3′ |
| Tubulin β | 5′-TGCTGGCCATTCAGAGTAAGA-3′ | 5′-ACTCAGACACCAGGTCGTTCA-3′ |
| Actin b | 5’-AGTGTGACGTTGACATCCGT-3’ | 5’-TGCTAGGAGCCAGAGCAGTA-3’ |
| Genes of interest | ||
| Gene | Forward | Reverse |
| AgRP | 5′- CAGAGTTCTCAGGTCTAAGTC-3′ | 5′-TTGAAGAAGCGGCAGTAGCAC-3′ |
| BDNF | 5′-TGCAGGGGCATAGCAAAAGG-3′ | 5′-CTTATGAATCGCCAGCCAATTCTC-3′ |
| Catalase | 5′-GGCTCTCACATAGCTGCCAA-3′ | 5′-TTACTGGTGAGGCTTGTGCC-3′ |
| COMT | 5′-TGTGTGCGGAACCTAAACGA-3′ | 5′-GAAGGTCGCGTGTTCCAGTA-3′ |
| D2R | 5′-ACCTGTCCTGGTACGATGATG-3′ | 5′-GCATGGCATAGTAGTTGTAGTGG-3′ |
| DCX | 5′-TCGTAGTTTTGATGCGTTGC-3′ | 5′-GCTTTCCCCTTCTTCCAGTT-3′ |
| Ddc1 | 5′-ATGTGGCGTCATGTGTGTCT-3′ | 5′-CACGGCCACACAAAGAACAG-3′ |
| GABARA1 | 5′-GGCTTGGGAGAGCGTGTAAC-3′ | 5′-CGTGACCCATCTTCTGCCAC-3′ |
| GAD67 | 5′-GTGGCGTAGCCCATGGATG-3′ | 5′-ACTGGTGTGGGTGGTGGAAG-3′ |
| IL6 | 5′- CTGCAAGAGACTTCCATCCAG-3′ | 5′- AGTGGTATAGACAGGTCTGTTGG-3′ |
| KOR | 5′-AGACCGCAACAACATCTACAT-3′ | 5′-GCACAGAACATCTCCAAAAGG-3′ |
| MC3R | 5’-TCCGATGCTGCCTAACCTCT-3’ | 5’-GGATGTTTTCCATCAGACTGACG-3’ |
| MC4R | 5’-CTTATGATGATCCCAACCCG-3’ | 5’-GTAGCTCCTTGCTTGCATCC-3’ |
| MOR | 5′-CGGACTCGGTAGGCTGTAAC-3′ | 5′-CCTGCCGCTCTTCTCTGG-3′ |
| OXT | 5′-GACGGTGGATCTCGGACTGAA-3′ | 5′-CGCCCCTAAAGGTATCATCACAAA-3′ |
| OXTR | 5′-GATCACGCTCGCCGTCTA-3′ | 5′-CCGTCTTGAGTCGCAGATTC-3′ |
| POMC | 5′-CAGGACCTCACCACGGAAAG-3′ | 5′-GTTCATCTCCGTTGCCTGGA-3′ |
| PSD95 | 5′-CTTCTCAGCCATCGTAGAGG-3′ | 5′-GAGAGGTCTTCAATGACACG-3′ |
| Shank3 | 5′-TACAGCACTTGGAGCACCTG-3′ | 5′-GTAATTGCGGACGTCCTTGT-3′ |
| Synapsin1 | 5′-CACCAGGATGAAGACAAGCA-3′ | 5′-GTCGTTGTTGAGCAGGAGGT-3′ |
| VGlut2 | 5′-CGTGAAGAATGGCAGTATGTCTTC-3′ | 5′-TGAGGCAAATAGTGCATAAAATATGACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, T.; Laloli, K.J.; Moscrip, C.A.; Olszewski, P.K.; Klockars, A. Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism. Genes 2022, 13, 259. https://doi.org/10.3390/genes13020259
Pal T, Laloli KJ, Moscrip CA, Olszewski PK, Klockars A. Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism. Genes. 2022; 13(2):259. https://doi.org/10.3390/genes13020259
Chicago/Turabian StylePal, Tapasya, Kathryn J. Laloli, Cushla A. Moscrip, Pawel K. Olszewski, and Anica Klockars. 2022. "Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism" Genes 13, no. 2: 259. https://doi.org/10.3390/genes13020259
APA StylePal, T., Laloli, K. J., Moscrip, C. A., Olszewski, P. K., & Klockars, A. (2022). Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism. Genes, 13(2), 259. https://doi.org/10.3390/genes13020259







