Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Population and Design
2.2. Phenotypes and Sample Collection
2.3. RNA Isolation
2.4. RNA-Seq Library Preparation and Analysis
2.5. Differential Expression Genes Analysis
2.6. Gene Ontology (G.O.) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.7. Weighted Gene Co-Expression Network Analysis
2.8. Statistical Analysis
3. Results
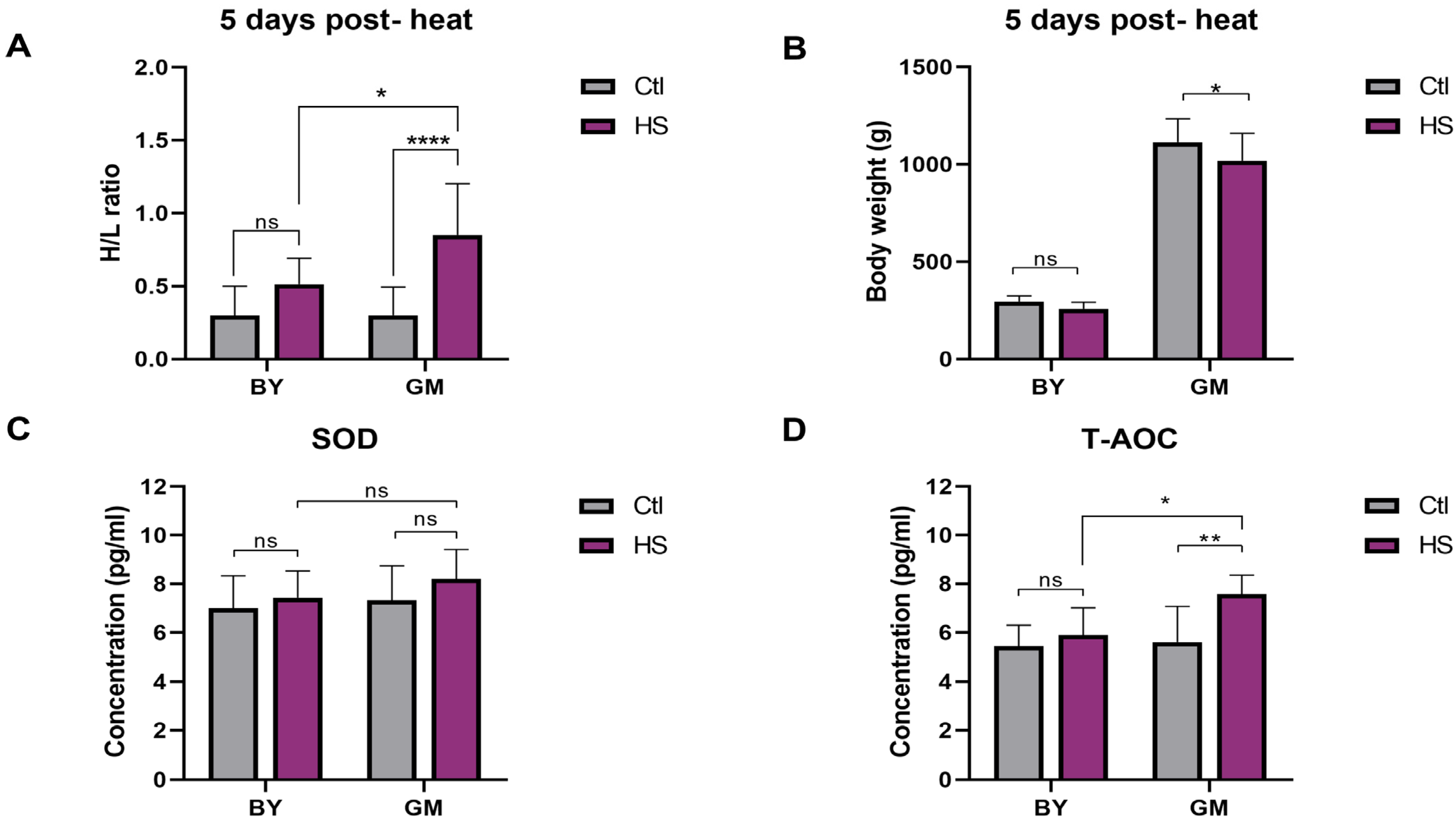
3.1. Effects of Heat Stress on the Phenotypical and Physiological Parameters between Beijing You and Guang Ming Chickens
3.2. Liver Transcriptome Sequencing Profiling
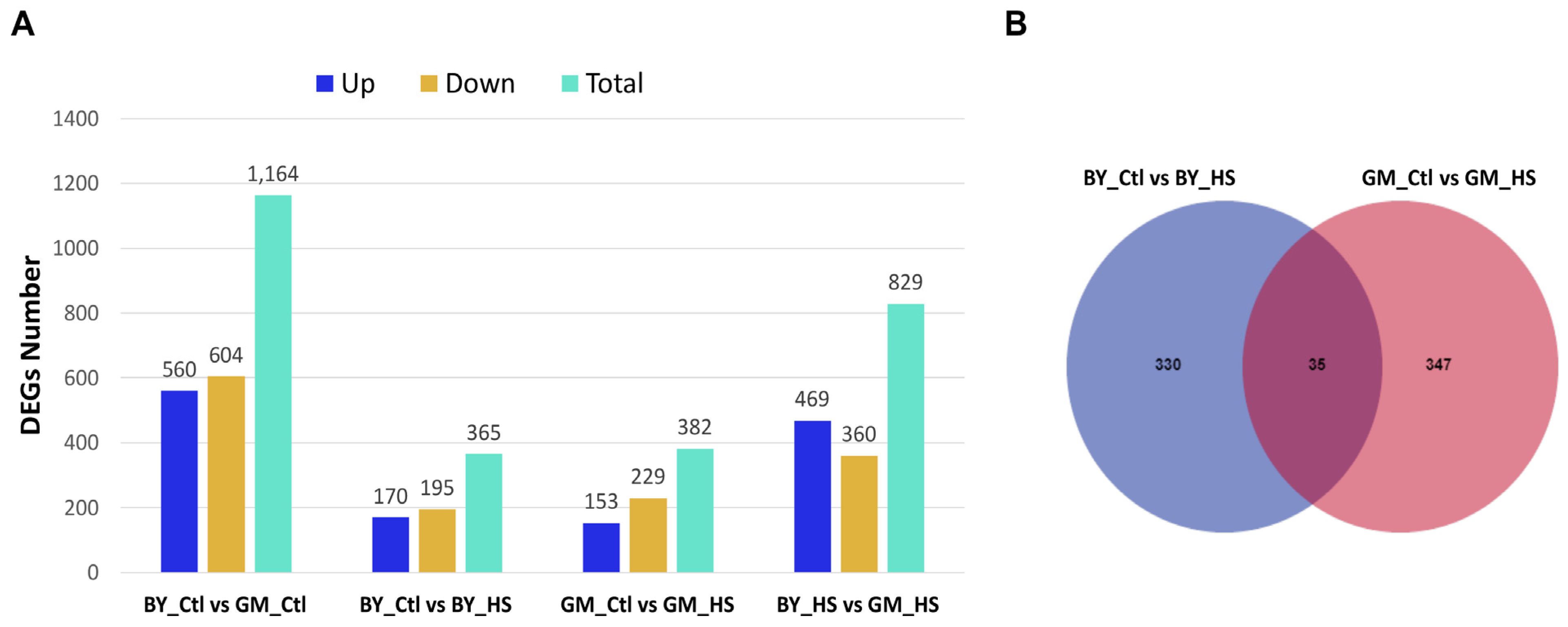
3.3. Identification of Differential Expressed Genes (DEGs) from Beijing You and Guang Ming Chickens between Control and Heat Stress Groups
3.4. Gene Ontology (GO) Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways Analysis of Beijing You and Guang Ming Chickens
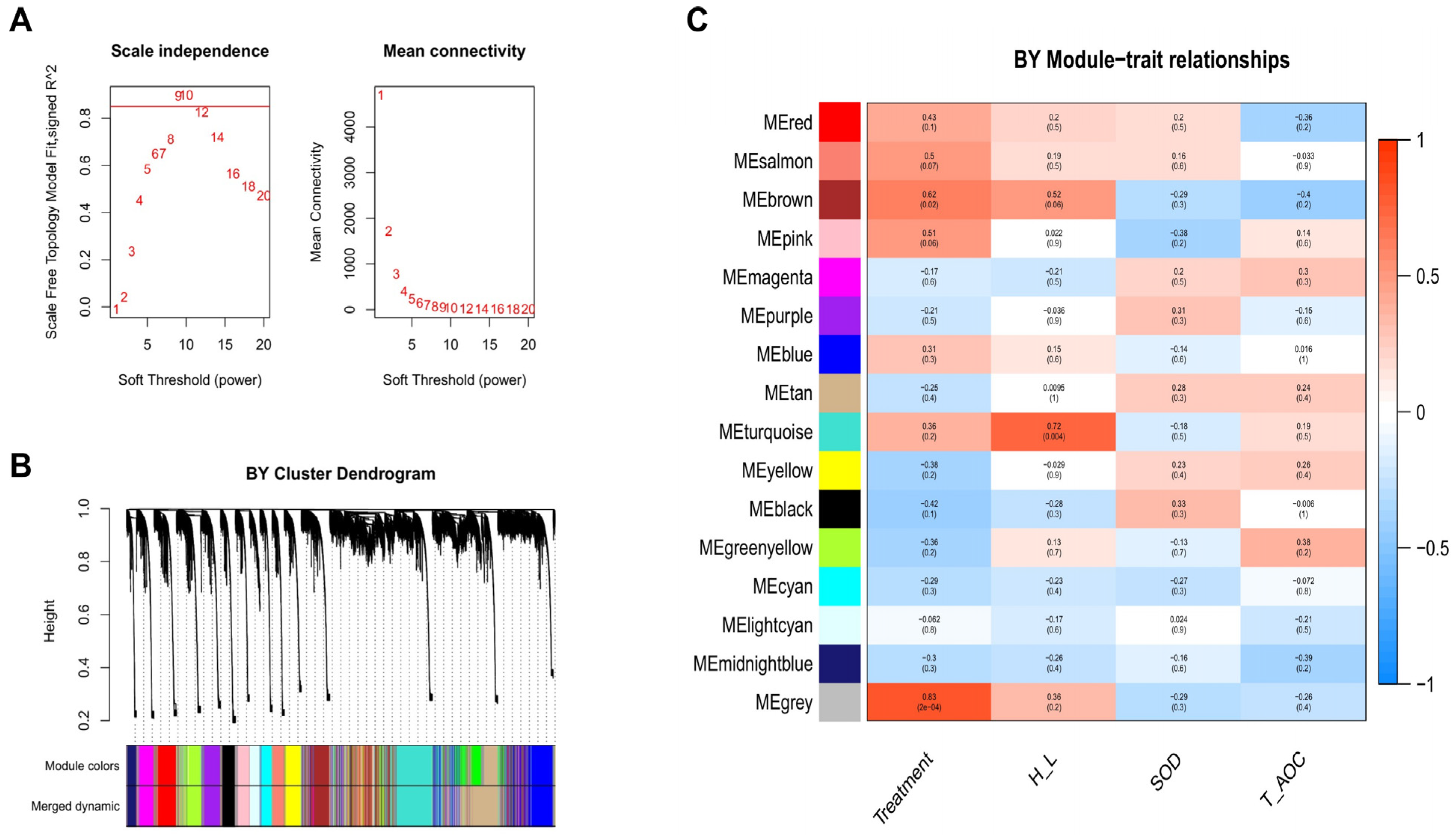
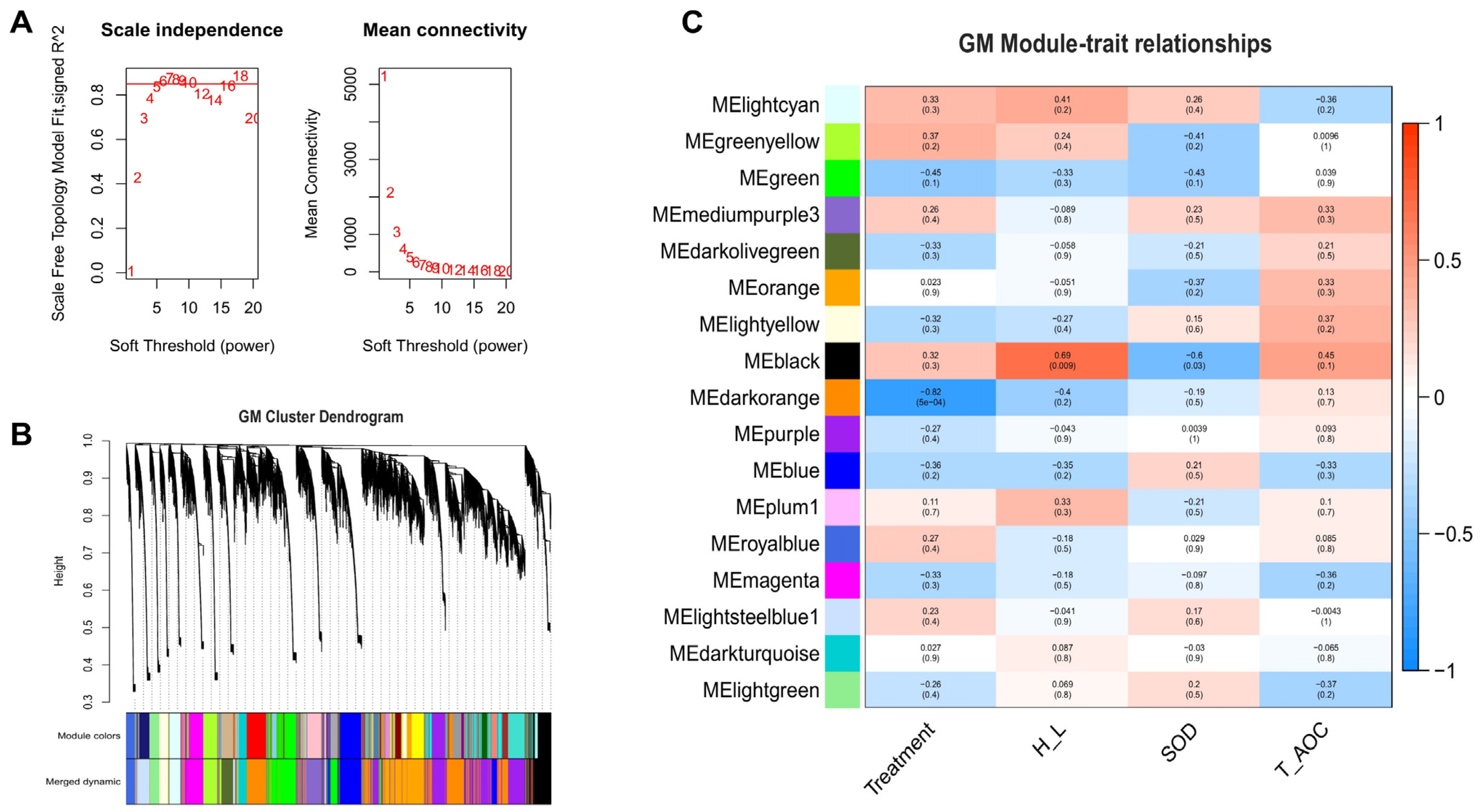
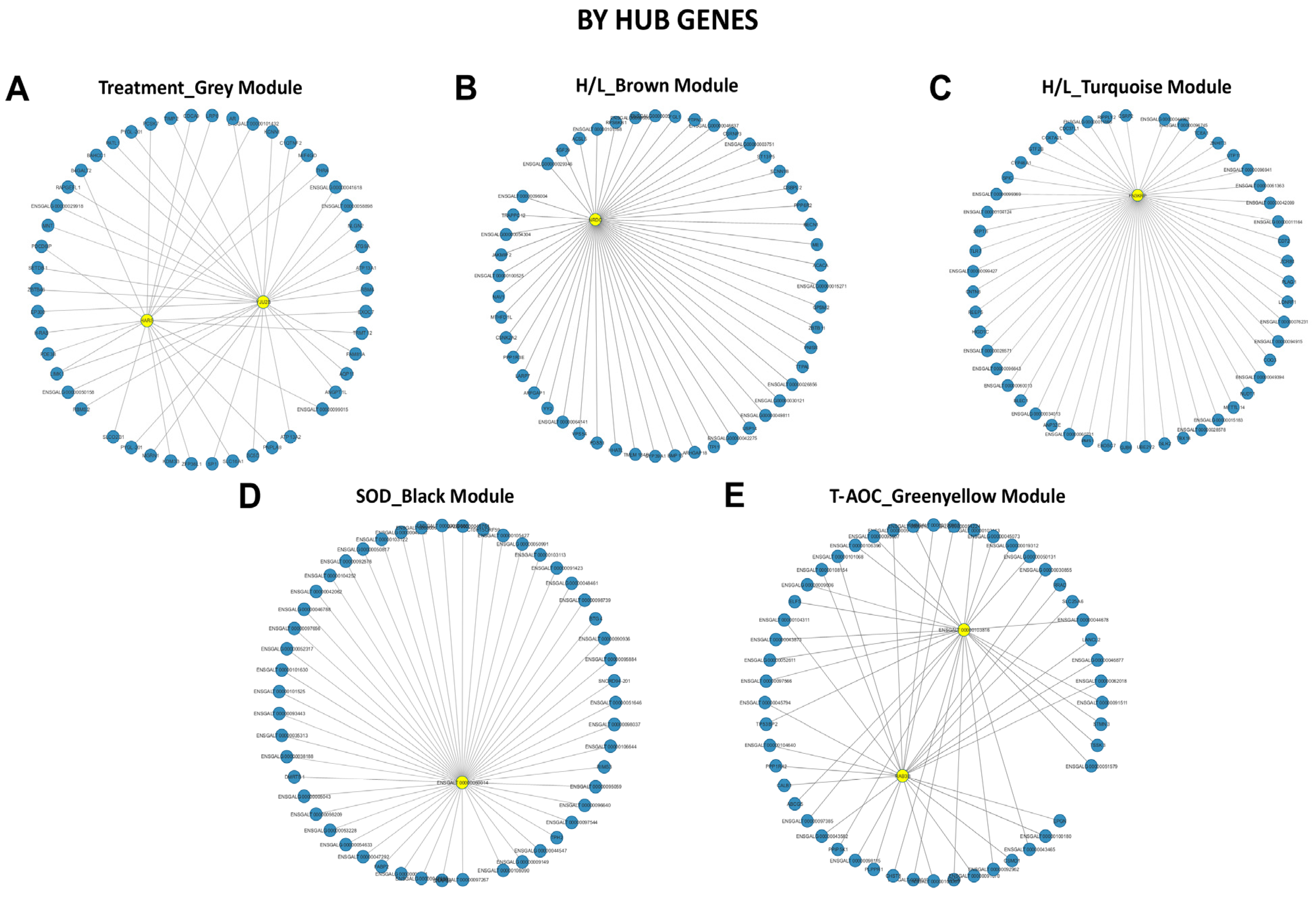
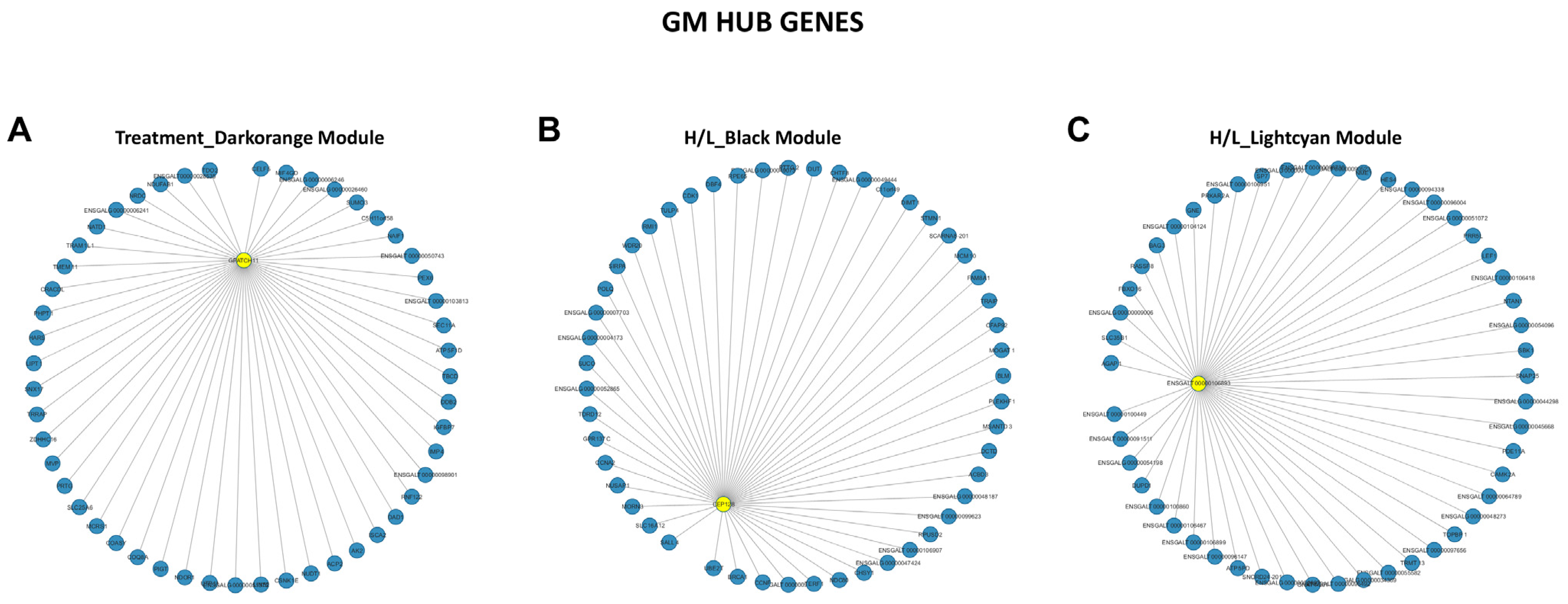
3.5. Weighted Gene Co-Expression Network Analysis (WGCNA) of Beijing You and Guang Ming Chickens
3.6. Screening of Hub Genes Related to Heat Stress in Beijing You and Guang Ming Chickens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abd El-Hack, M.E.; Patra, A. Heat stress: Effects on productive and reproductive performance of quail. World’s Poult. Sci. J. 2017, 73, 747–756. [Google Scholar] [CrossRef]
- Yahav, S.; Goldfeld, S.; Plavnik, I.; Hurwitz, S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995, 20, 245–253. [Google Scholar] [CrossRef]
- Settar, P.; Yalçin, S.; Türkmut, L.; Ozkan, S.; Cahanar, A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999, 78, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animal 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, M.S.; El-Hindawy, M.M.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S.A.A. Use of acetylsalicylic acid as an allostatic modulator in the diets of growing Japanese quails exposed to heat stress. J. Therm. Biol. 2018, 74, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Saelao, P.; Kern, C.; Jin, S.; Gallardo, R.A.; Kelly, T.; Dekkers, J.M.; Lamont, S.J.; Zhou, H. Liver Transcriptome Responses to Heat Stress and Newcastle Disease Virus Infection in Genetically Distinct Chicken Inbred Lines. Genes 2020, 11, 1067. [Google Scholar] [CrossRef]
- Cangar, O.; Aerts, J.M.; Buyse, J.; Berckmans, D. Quantification of the spatial distribution of surface temperatures of broilers. Poult. Sci. 2008, 87, 2493–2499. [Google Scholar] [CrossRef]
- Yahav, S.; Straschnow, A.; Luger, D.; Shinder, D.; Tanny, J.; Cohen, S. Ventilation, sensible heat loss, broiler energy, and water balance under harsh environmental conditions. Poult. Sci. 2004, 83, 253–258. [Google Scholar] [CrossRef]
- Wang, S.; Edens, F.W. Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poult. Sci. 1998, 77, 1636–1645. [Google Scholar] [CrossRef]
- Soleimani Farjam, A.; Idrus, Z.; Hair-Bejo, M.; Omar, A.; Raha, A. The role of heat shock protein 70 in resistance to Salmonella enteritidis in broiler chickens subjected to neonatal feed restriction and thermal stress. Poult. Sci. 2012, 91, 340–345. [Google Scholar] [CrossRef]
- McDaniel, C.D.; Bramwell, R.K.; Wilson, J.L.; Howarth, B., Jr. Fertility of male and female broiler breeders following exposure to elevated ambient temperatures. Poult. Sci. 1995, 74, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, M.M.; Hendricks, G.L., 3rd; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Star, L.; Kemp, B.; van den Anker, I.; Parmentier, H.K. Effect of single or combined climatic and hygienic stress in four layer lines: 1. Performance. Poult. Sci. 2008, 87, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.H.; Gill, V.M.; Rice-Evans, C. Oxidative stress and heat shock protein induction in human cells. Free Radic. Res. Commun. 1987, 3, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.W.; Moseley, P.L.; Buettner, G.R. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998, 431, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, A.; Yoshiki, Y.; Akiba, Y.; Toyomizu, M. Superoxide Radical Production in Chicken Skeletal Muscle Induced by Acute Heat Stress. Poult. Sci. 2005, 84, 307–314. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Xi, L.; Liu, H.C.; Odle, J.; Luo, X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS ONE 2014, 9, e102204. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; De Vos, D.; Decuypere, E.; Buyse, J. Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallus gallus domesticus). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2008, 147, 30–35. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Tedeschi, J.N.; Kennington, W.J.; Berry, O.; Whiting, S.; Meekan, M.; Mitchell, N.J. Increased expression of Hsp70 and Hsp90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Caretta Caretta). J. Therm. Biol. 2015, 47, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Pappolla, M. Oxygen free radicals as inducers of heat shock protein synthesis in cultured human neuroblastoma cells: Relevance to neurodegenerative disease. Eur. Arch. Psychiatry Clin. Neurosci. 1993, 242, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Felver-Gant, J.N.; Mack, L.A.; Dennis, R.L.; Eicher, S.D.; Cheng, H.W. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult. Sci. 2012, 91, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Hao, Y.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012, 91, 790–799. [Google Scholar] [CrossRef]
- Tan, G.Y.; Yang, L.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010, 89, 115–122. [Google Scholar] [CrossRef]
- Kumar, B.; Manuja, A.; Aich, P. Stress and its impact on farm animals. Front. Biosci. 2012, 4, 1759–1767. [Google Scholar] [CrossRef]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002, 81, 293–301. [Google Scholar] [CrossRef]
- Altan, O.; Pabuçcuoğlu, A.; Altan, A.; Konyalioğlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef]
- McFarlane, J.M.; Curtis, S.E. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and the heterophil:lymphocyte ratio. Poult. Sci. 1989, 68, 522–527. [Google Scholar] [CrossRef]
- Honda, B.T.; Calefi, A.S.; Costola-de-Souza, C.; Quinteiro-Filho, W.M.; da Silva Fonseca, J.G.; de Paula, V.F.; Palermo-Neto, J. Effects of heat stress on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poult. Sci. 2015, 94, 2375–2381. [Google Scholar] [CrossRef]
- Post, J.; Rebel, J.M.; ter Huurne, A.A. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003, 82, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.T.; Campo, J.L. Effect of heat and several additives related to stress levels on fluctuating asymmetry, heterophil:lymphocyte ratio, and tonic immobility duration in White Leghorn chicks. Poult. Sci. 2010, 89, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef]
- Shini, S.; Huff, G.R.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 89, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, R.; Xu, S.; Zhang, Z.; Xu, G.; Zheng, J.; Qu, L. Transcriptome responses to heat stress in hypothalamus of a meat-type chicken. J. Anim. Sci. Biotechnol. 2015, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Salah, A.S.; Nassan, M.A.; El-Tarabany, M.S. Effects of Chronic Thermal Stress on Performance, Energy Metabolism, Antioxidant Activity, Brain Serotonin, and Blood Biochemical Indices of Broiler Chickens. Animal 2021, 11, 2554. [Google Scholar] [CrossRef]
- Shen, P.F.; Patterson, L.T.J.P.S. A Simplified Wrigh” s Stain Technique for Routine Avian Blood Smear Staining. Poult. Sci. 1983, 62, 923–924. [Google Scholar] [CrossRef]
- Fidan, E.; Nazlıgül, A.; Kenan, M.; Ünübol Aypak, S.; Sevil-Kilimci, F.; Karaarslan, S.; Kaya, M. Effect of photoperiod length and light intensity on some welfare criteria, carcass, and meat quality characteristics in broilers. Rev. Bras. De Zootec. 2017, 46, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Zheng, M.; Wen, J.; Zhao, G.; Wang, Q. Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animal 2021, 11, 3498. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. In 2010; Babraham Bioinformatics; Babraham Institute: Cambridge, UK, 2017. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.; Dong, J. Geometric Interpretation of Gene Coexpression Network Analysis. PLoS Comput. Biol. 2008, 4, e1000117. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Xing, S.; Liu, R.; Zhao, G.; Groenen, M.A.M.; Madsen, O.; Liu, L.; Zheng, M.; Wang, Q.; Wu, Z.; Crooijmans, R.P.M.A.; et al. Time Course Transcriptomic Study Reveals the Gene Regulation During Liver Development and the Correlation With Abdominal Fat Weight in Chicken. Front. Genet. 2021, 12, 723519. [Google Scholar] [CrossRef]
- Xing, S.; Liu, R.; Zhao, G.; Liu, L.; Groenen, M.A.M.; Madsen, O.; Zheng, M.; Yang, X.; Crooijmans, R.P.M.A.; Wen, J. RNA-Seq Analysis Reveals Hub Genes Involved in Chicken Intramuscular Fat and Abdominal Fat Deposition During Development. Front. Genet. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [Green Version]
- Cianconi, P.; Betrò, S.; Janiri, L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front. Psychiatry 2020, 11, 74. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Wong, M. Global climate change and mental health. Curr. Opin. Psychol. 2020, 32, 12–16. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, L.; Wang, Y.; Wang, G.; Zhu, Y.; Cao, R.; Qian, G.; Xie, C.; Liu, X.; Xiao, Y.; et al. Co-expression network analysis identified FCER1G in association with progression and prognosis in human clear cell renal cell carcinoma. Int. J. Biol. Sci. 2017, 13, 1361–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, A.; Upadhyay, R.C. Heat Stress and Animal Productivity. Anim. Front. 2013, 53–77. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2018, 9, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bianca, W. Rectal temperature and respiratory rate as indicators of heat tolerance in cattle. J. Agric. Sci. 1963, 60, 113–120. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. J. WVPA 2012, 41, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animal 2020, 10, 1266. [Google Scholar] [CrossRef]
- Redmond, S.B.; Chuammitri, P.; Andreasen, C.B.; Palić, D.; Lamont, S.J. Proportion of circulating chicken heterophils and CXCLi2 expression in response to Salmonella enteritidis are affected by genetic line and immune modulating diet. Vet. Immunol. Immunopathol. 2011, 140, 323–328. [Google Scholar] [CrossRef]
- al-Murrani, W.K.; Kassab, A.; al-Sam, H.Z.; al-Athari, A.M. Heterophil/lymphocyte ratio as a selection criterion for heat resistance in domestic fowls. Br. Poult. Sci. 1997, 38, 159–163. [Google Scholar] [CrossRef]
- Campo, J.L.; Davila, S.G. Estimation of heritability for heterophil:lymphocyte ratio in chickens by restricted maximum likelihood. Effects of age, sex, and crossing. Poult. Sci. 2002, 81, 1448–1453. [Google Scholar] [CrossRef]
- Gross, W.B.; Siegel, H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983, 27, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqil, A.; Zulkifli, I.; Hair Bejo, M.; Sazili, A.Q.; Rajion, M.A.; Somchit, M.N. Changes in heat shock protein 70, blood parameters, and fear-related behavior in broiler chickens as affected by pleasant and unpleasant human contact. Poult. Sci. 2013, 92, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bedanova, I.; Voslarova, E.; Chloupek, P.; Pistekova, V.; Suchy, P.; Blahova, J.; Dobsikova, R.; Vecerek, V. Stress in broilers resulting from shackling. Poult. Sci. 2007, 86, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Huth, J.C.; Archer, G.S. Comparison of Two LED Light Bulbs to a Dimmable CFL and their Effects on Broiler Chicken Growth, Stress, and Fear. Poult. Sci. 2015, 94, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Zulkifli, I.; Soleimani, A.F.; Kashiani, P. The effect of different degrees of feed restriction on heat shock protein 70, acute phase proteins, and other blood parameters in female broiler breeders. Poult. Sci. 2015, 94, 2322–2329. [Google Scholar] [CrossRef]
- Gross, W.B. Factors affecting chicken thrombocyte morphology and the relationship with heterophil:lymphocyte ratios. Br. Poult. Sci. 1989, 30, 919–925. [Google Scholar] [CrossRef]
- Stevenson, J.R.; Taylor, R. Effects of glucocorticoid and antiglucocorticoid hormones on leukocyte numbers and function. Int. J. Immunopharmacol. 1988, 10, 1–6. [Google Scholar] [CrossRef]
- Attia, Y.A.; Hassan, R.A.; Tag El-Din, A.E.; Abou-Shehema, B.M. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J. Anim. Physiol. Anim. Nutr. 2011, 95, 744–755. [Google Scholar] [CrossRef]
- Ghazi, S.; Habibian, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress. Biol. Trace Elem. Res. 2012, 146, 309–317. [Google Scholar] [CrossRef]
- Imik, H.; Ozlu, H.; Gumus, R.; Atasever, M.A.; Urcar, S.; Atasever, M. Effects of ascorbic acid and α-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br. Poult. Sci. 2012, 53, 800–808. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009, 88, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wen, J.; Zhang, H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, H.; Swennen, Q.; Everaert, N.; Geraert, P.A.; Mercier, Y.; Stinckens, A.; Decuypere, E.; Buyse, J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011, 90, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Coble, D.J.; Fleming, D.; Persia, M.E.; Ashwell, C.M.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. RNA-seq analysis of broiler liver transcriptome reveals novel responses to high ambient temperature. BMC Genom. 2014, 15, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, K.; Lee, E.; Kwan, A.; Lim, Y.; Lee, J.; Jang, G.; Chung, H. Transcriptome analysis and identification of significantly differentially expressed genes in Holstein calves subjected to severe thermal stress. Int. J. Biometeorol. 2017, 61, 1993–2008. [Google Scholar] [CrossRef]
- Yu, H.; Thun, R.; Chandrasekharappa, S.; Trent, J.M.; Zhang, J.; Meisler, M.H. Human PCK1 encoding phosphoenolpyruvate carboxykinase is located on chromosome 20q13.2. Genomics 1993, 15, 219–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, K.J.; Shelly, L.L.; Pan, C.J.; Sidbury, J.B.; Chou, J.Y. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science 1993, 262, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Skiba-Cassy, S.; Collin, A.; Chartrin, P.; Médale, F.; Simon, J.; Duclos, M.J.; Tesseraud, S. Chicken liver and muscle carnitine palmitoyltransferase 1: Nutritional regulation of messengers. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Murthy, M.S.; Pande, S.V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc. Natl. Acad. Sci. USA 1987, 84, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.L.; Neufer, P.D. Exercise attenuates the fasting-induced transcriptional activation of metabolic genes in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1078–E1086. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle. Diabetes 2003, 52, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wu, P.H.; Tarr, P.T.; Lindenberg, K.S.; St-Pierre, J.; Zhang, C.Y.; Mootha, V.K.; Jäger, S.; Vianna, C.R.; Reznick, R.M.; et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 2004, 119, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Goh, Y.Y.; Chin, H.F.; Kersten, S.; Tan, N.S. Angiopoietin-like 4: A decade of research. Biosci. Rep. 2012, 32, 211–219. [Google Scholar] [CrossRef]
- Lan, X.; Hsieh, J.C.F.; Schmidt, C.J.; Zhu, Q.; Lamont, S.J. Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genom. 2016, 17, 955. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, K.; Yoshimoto, T.; Torigoe, K.; Kurimoto, M.; Matsui, K.; Hada, T.; Okamura, H.; Nakanishi, K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int. Immunol. 2000, 12, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Piel, W.H.; Gui, L.; Bruford, E.; Monteiro, A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics 2005, 86, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tian, W.; Jiang, K.; Wang, Z.; Wang, D.; Li, Z.; Yan, F.; Wang, Y.; Tian, Y.; Ou, K.; et al. Global investigation of estrogen-responsive genes regulating lipid metabolism in the liver of laying hens. BMC Genom. 2021, 22, 428. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. RBE 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, M.K.; Koiri, R.K.; Srivastava, R. Expression of estrogen receptor alpha in response to stress and estrogen antagonist tamoxifen in the shell gland of Gallus gallus domesticus: Involvement of anti-oxidant system and estrogen. Stress 2021, 24, 261–272. [Google Scholar] [CrossRef]
- Tanabe, Y.; Nakamura, T.; Tanase, H.; Doi, O. Comparisons of plasma LH, progesterone, testosterone and estradiol concentrations in male and female chickens (Gallus domesticus) from 28 to 1141 days of age. Endocrinol. Jpn. 1981, 28, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flouriot, G.; Pakdel, F.; Valotaire, Y. Transcriptional and post-transcriptional regulation of rainbow trout estrogen receptor and vitellogenin gene expression. Mol. Cell. Endocrinol. 1996, 124, 173–183. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Handa, R.J.; Mani, S.K.; Uht, R.M. Estrogen Receptors and the Regulation of Neural Stress Responses. Neuroendocrinology 2012, 96, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M.G.; Seuwen, K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J. Recept. Signal Transduct. Res. 2002, 22, 79–110. [Google Scholar] [CrossRef]
- Schaub, M.C.; Hefti, M.A.; Zaugg, M. Integration of calcium with the signaling network in cardiac myocytes. J. Mol. Cell. Cardiol. 2006, 41, 183–214. [Google Scholar] [CrossRef]
- Renaud, S.J.; Kubota, K.; Rumi, M.A.K.; Soares, M.J. The FOS transcription factor family differentially controls trophoblast migration and invasion. J. Biol. Chem. 2014, 289, 5025–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The Bone Morphogenetic Protein System In Mammalian Reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yao, Z.; Lee, T.; Yamamoto, S.; Erickson, G.F.; Shimasaki, S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem. 2000, 275, 39523–39528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, S.; Tsai, M.Y.; Xu, Q.; Mu, X.M.; Lardy, H.; Huang, K.E.; Lin, H.; Yeh, S.D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, J.; Donnelly, R.J.; Kotenko, S.; Mariano, T.M.; Cook, J.R.; Wang, N.; Emanuel, S.; Schwartz, B.; Miki, T.; Pestka, S. Identification and sequence of an accessory factor required for activation of the human interferon gamma receptor. Cell 1994, 76, 793–802. [Google Scholar] [CrossRef]
- Talbot, S.; Tötemeyer, S.; Yamamoto, M.; Akira, S.; Hughes, K.; Gray, D.; Barr, T.; Mastroeni, P.; Maskell, D.J.; Bryant, C.E. Toll-like receptor 4 signalling through MyD88 is essential to control Salmonella enterica serovar typhimurium infection, but not for the initiation of bacterial clearance. Immunology 2009, 128, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Torres, A.; Vallance, B.A.; Bergman, M.A.; Finlay, B.B.; Cookson, B.T.; Jones-Carson, J.; Fang, F.C. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: Importance of the Kupffer cell network. J. Immunol. 2004, 172, 6202–6208. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef]
- Murphy, M.; Stinnakre, M.G.; Senamaud-Beaufort, C.; Winston, N.J.; Sweeney, C.; Kubelka, M.; Carrington, M.; Bréchot, C.; Sobczak-Thépot, J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat. Genet. 1997, 15, 83–86. [Google Scholar] [CrossRef]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef]
- Ueki, T.; Park, J.H.; Nishidate, T.; Kijima, K.; Hirata, K.; Nakamura, Y.; Katagiri, T. Ubiquitination and downregulation of BRCA1 by ubiquitin-conjugating enzyme E2T overexpression in human breast cancer cells. Cancer Res. 2009, 69, 8752–8760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, S.; Xie, Z.; Reed, J.C. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J. Biol. Chem. 1999, 274, 781–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, J.N.; Zuiderweg, E.R.; Gestwicki, J.E. Non-canonical Interactions between Heat Shock Cognate Protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) Co-Chaperones Are Important for Client Release. J. Biol. Chem. 2016, 291, 19848–19857. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Beijing You | Guang Ming Broiler | ||||||
|---|---|---|---|---|---|---|---|
| Ensembl Gene ID | Gene Name | Regulated | p-Values * | Ensembl Gene ID | Gene Name | Regulated | p-Values * |
| ENSGALG00000000619 | ANGPTL4 | up | 0.047959 | ENSGALG00000000112 | PLP1 | down | 0.013577 |
| ENSGALG00000000949 | HBEGF | down | 1.43 × 10−8 | ENSGALG00000000158 | MHCDMA | down | 0.000247 |
| ENSGALG00000001094 | ADGRD2 | up | 0.02279 | ENSGALG00000000314 | NEFL | down | 0.040557 |
| ENSGALG00000001252 | CREB3L3 | down | 9.88 × 10−10 | ENSGALG00000000318 | CSRP1 | down | 0.006843 |
| ENSGALG00000001347 | LHX6 | up | 0.040949 | ENSGALG00000000556 | UTS2 | up | 0.006796 |
| ENSGALG00000001662 | ATP10B | down | 0.030923 | ENSGALG00000000667 | EDN2 | down | 0.04102 |
| ENSGALG00000001723 | - | up | 0.040431 | ENSGALG00000000901 | - | up | 0.000927 |
| ENSGALG00000001749 | ACSBG2 | up | 0.000153 | ENSGALG00000001000 | HSPA5 | up | 2.31 × 10−5 |
| ENSGALG00000001963 | - | down | 0.019399 | ENSGALG00000001115 | MMEL1 | up | 0.008385 |
| ENSGALG00000002028 | GPRC5B | down | 4.84 × 10−6 | ENSGALG00000001136 | - | up | 0.048206 |
| Trait | Breed | Correlation | Module Color | Gene Names (GS, MM) |
|---|---|---|---|---|
| Treatment | Beijing You | Positive | Grey | HARS (0.89, 0.77), TRMT12 (0.86, 0.69), ENSGALG00000004144 (0.85, 0.78), B4GALT4 (0.83, 0.80), MSI1 (0.82, 0.88) |
| Guang Ming | Negative | Darkorange | POLR2I (−0.95, 0.93), C1orf232 (−0.94, 0.81), GGACT (−0.93, 0.88), MIF4GD (−0.92, 0.73), ECD (−0.92, 0.81) | |
| H/L | Beijing You | Positive | Turquoise | OMG (0.88, 0.80), ENSGALG00000030007 (0.88, 0.70), LONRF1 (0.88, 0.69), BRI3BP (0.87, 0.83), MMP13 (0.87, 0.72) |
| Brown | ENSGALT00000045744 (0.91, 0.64), SEC61A2 (0.83, 0.75), UGT1A1 (0.83, 0.82), RD3L (0.82, 0.76), ENSGALT00000101291 (0.82, 0.76) | |||
| Guang Ming | Positive | Black | ENSGALG00000053515 (0.91, 0.79), CILP (0.87, 0.61), ENSGALT00000106076 (0.87, 0.79), ENSGALT00000028967 (0.86, 0.83), ENSGALG00000048900 (0.86, 0.82) | |
| SOD | Beijing You | Positive | Black | ENSGALG00000031869 (0.76, 0.50), CNIH3 (0.74, 0.58), ENSGALT00000053113 (0.67, 0.66), RTCA (−0.75, −0.49) |
| Guang Ming | Negative | Black | NCAPG2 (−0.88, 0.76), ENSGALT00000098242 (−0.87, 0.77), UBE2T (−0.87, 0.57), ESCO2 (−0.85, 0.62), OVALX (−0.85, 0.62) | |
| T-AOC | Beijing You | Positive | Greenyellow | ENSGALG00000046741 (0.77, 0.62), DPH2 (0.76, 0.59), MEF2A (0.74, 0.44), ENSGALG00000047728 (0.73, 0.40), ENSGALG00000019352 (0.72, 0.71) |
| Guang Ming | Positive | Black | RUFY3 (0.83, 0.62), ENSGALG00000051746 (0.81, 0.59), ENSGALG00000037214 (0.80, 0.43), GALNT11 (0.79, 0.061), CEP112 (0.77, 0.53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto Sánchez, A.L.; Wang, Q.; Thiam, M.; Wang, Z.; Zhang, J.; Zhang, Q.; Zhang, N.; Li, Q.; Wen, J.; Zhao, G. Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers. Genes 2022, 13, 416. https://doi.org/10.3390/genes13030416
Barreto Sánchez AL, Wang Q, Thiam M, Wang Z, Zhang J, Zhang Q, Zhang N, Li Q, Wen J, Zhao G. Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers. Genes. 2022; 13(3):416. https://doi.org/10.3390/genes13030416
Chicago/Turabian StyleBarreto Sánchez, Astrid Lissette, Qiao Wang, Mamadou Thiam, Zixuan Wang, Jin Zhang, Qi Zhang, Na Zhang, Qinghe Li, Jie Wen, and Guiping Zhao. 2022. "Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers" Genes 13, no. 3: 416. https://doi.org/10.3390/genes13030416
APA StyleBarreto Sánchez, A. L., Wang, Q., Thiam, M., Wang, Z., Zhang, J., Zhang, Q., Zhang, N., Li, Q., Wen, J., & Zhao, G. (2022). Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers. Genes, 13(3), 416. https://doi.org/10.3390/genes13030416






