Abstract
A comprehensive summary of recent knowledge in syndactyly (SD) is important for understanding the genetic etiology of SD and disease management. Thus, this review article provides background information on SD, as well as insights into phenotypic and genetic heterogeneity, newly identified gene mutations in various SD types, the role of HOXD13 in limb deformities, and recently introduced modern surgical techniques for SD. This article also proposes a procedure for genetic analysis to obtain a clearer genotype–phenotype correlation for SD in the future. We briefly describe the classification of non-syndromic SD based on variable phenotypes to explain different phenotypic features and mutations in the various genes responsible for the pathogenesis of different types of SD. We describe how different types of mutation in HOXD13 cause various types of SD, and how a mutation in HOXD13 could affect its interaction with other genes, which may be one of the reasons behind the differential phenotypes and incomplete penetrance. Furthermore, we also discuss some recently introduced modern surgical techniques, such as free skin grafting, improved flap techniques, and dermal fat grafting in combination with the Z-method incision, which have been successfully practiced clinically with no post-operative complications.
1. Background
Syndactyly (SD) is a congenital digital malformation characterized by webbing of the fingers and toes. Syndactyly is derived from the Greek word “syn”, meaning together, and “dactylos”, meaning digits. It is one of the most common hereditary limb disorders, with a prevalence of 3–10 in every 10,000 births, although higher estimates in the range of 10–40/10,000 have been reported [1,2,3,4]. Its occurrence in males is twice that in females, and mothers aged 40 years or older are more likely to produce children with inborn limb deformities compared to mothers who are 30 years of age or younger [5]. SD is genetic in origin; clinically, it is an extremely heterogeneous developmental deformity [6]. It may be symmetrical or asymmetrical and unilateral or bilateral. Moreover, inter- or intra-familial phenotypic variability is relatively common. The extent of variability of the disorder can even be observed in the same individual as he/she may have asymmetrical phenotypic features in the hands and feet, as well as between the right hand and left hand. SD can be completely or partially identified as bony or cutaneous, involving the phalanges, and may extend to the carpal and tarsal bones, even to the metacarpal and metatarsal levels of the limbs, and occasionally adjacent to the distal end of the forearm and foreleg.
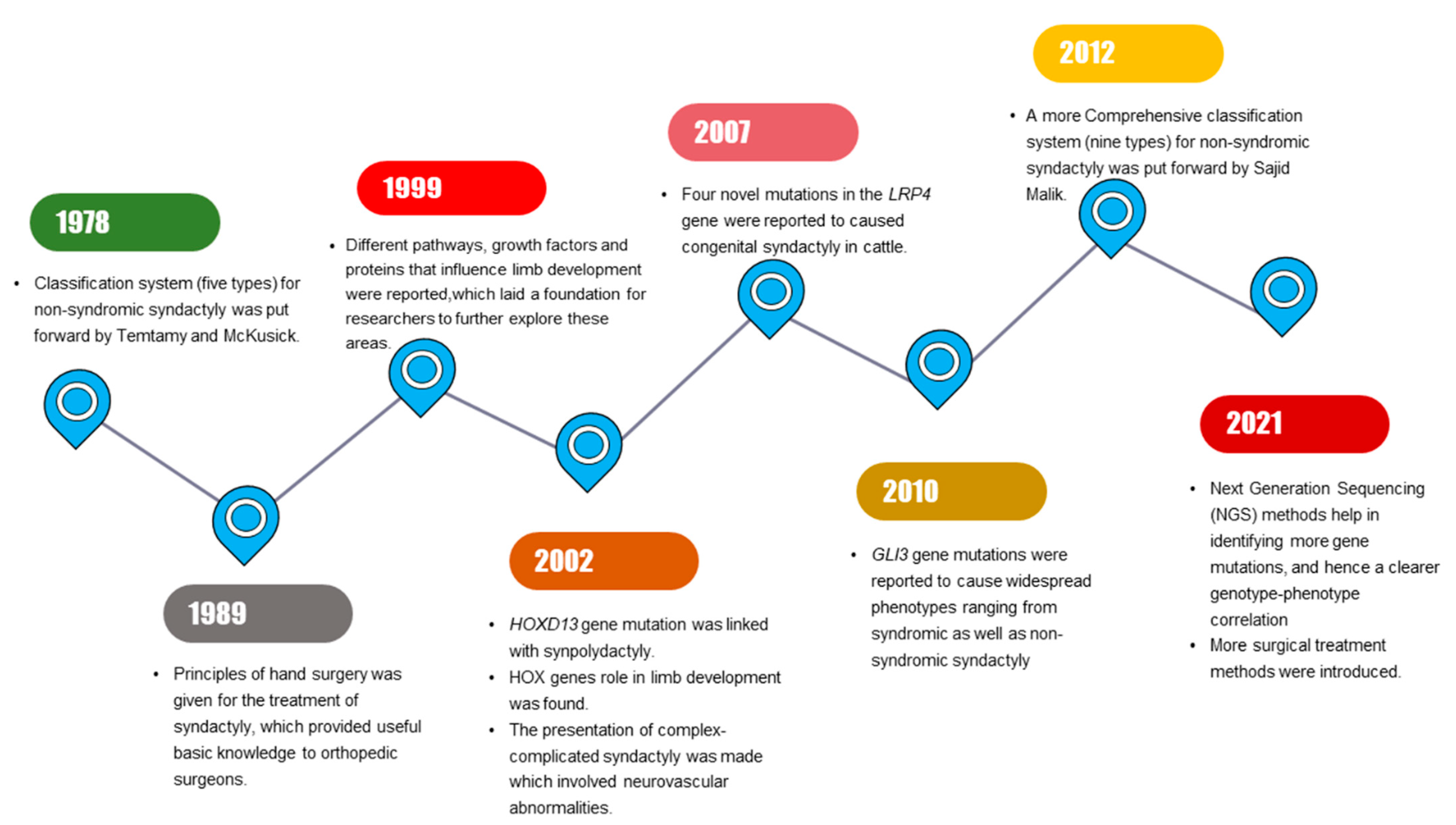
Milder phenotypic features in limbs that are related to SD might be identified by interphalangeal fold differences [7]. SD mostly segregates as an isolated (non-syndromic) limb disorder but may occur in combination with other disorders (synostosis, acro-syndactyly, cleft hand, clinodactyly, polydactyly) or syndromes (Apert syndrome, Poland’s syndrome, Pfeiffer syndrome) [3]. Significant progress has been made in SD research, with multiple milestones being achieved during the past few years (Figure 1).
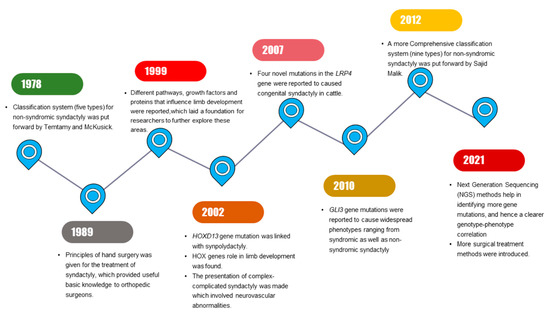
Figure 1.
Schematic diagram illustrating the series of milestones achieved in past years in syndactyly research.
2. Classification of Syndactyly
The classification of non-syndromic SD can be performed in different ways, based on the various phenotypes. It can be simple or complex, complete or incomplete, and osseous (bone-involved) or cutaneous (only skin-involved). The classification system of Temtamy and McKusick for non-syndromic syndactyly is largely based on the phenotypic appearance (nature or site of affected limbs), along with segregation of the disorder in affected families [7]. The classification system of Temtamy and McKusick provided the basis for the latest modern classification system, which additionally considers advancements made clinically, as well as in basic molecular studies. In 2012, a nine-type classification system was put forward by Malik et al. that was mainly an extended version of the Temtamy and McKusick classification system [8]. The autosomal dominant mode of inheritance is evident in most of the types [9]. We summarize the classification of non-syndromic syndactyly in Table 1.

Table 1.
List of genes and loci responsible for different types of non-syndromic syndactyly.
3. Variable Phenotypic Features of Non-Syndromic Syndactyly Types
The most-reported phenotypic features of SD are webbing of the 3rd and 4th fingers, while webbing of the 1st and 2nd digits rarely occurs because, during normal development, the thumb (1st digit) is not closely attached to the remaining fingers of the hand. There are two types of webbing. In the first type, only the skin is involved; this is referred to as simple syndactyly and can be sub-categorized into complete and partial SD; however, in the second type, the bones are also fused underneath the skin and this is called complex syndactyly [34].
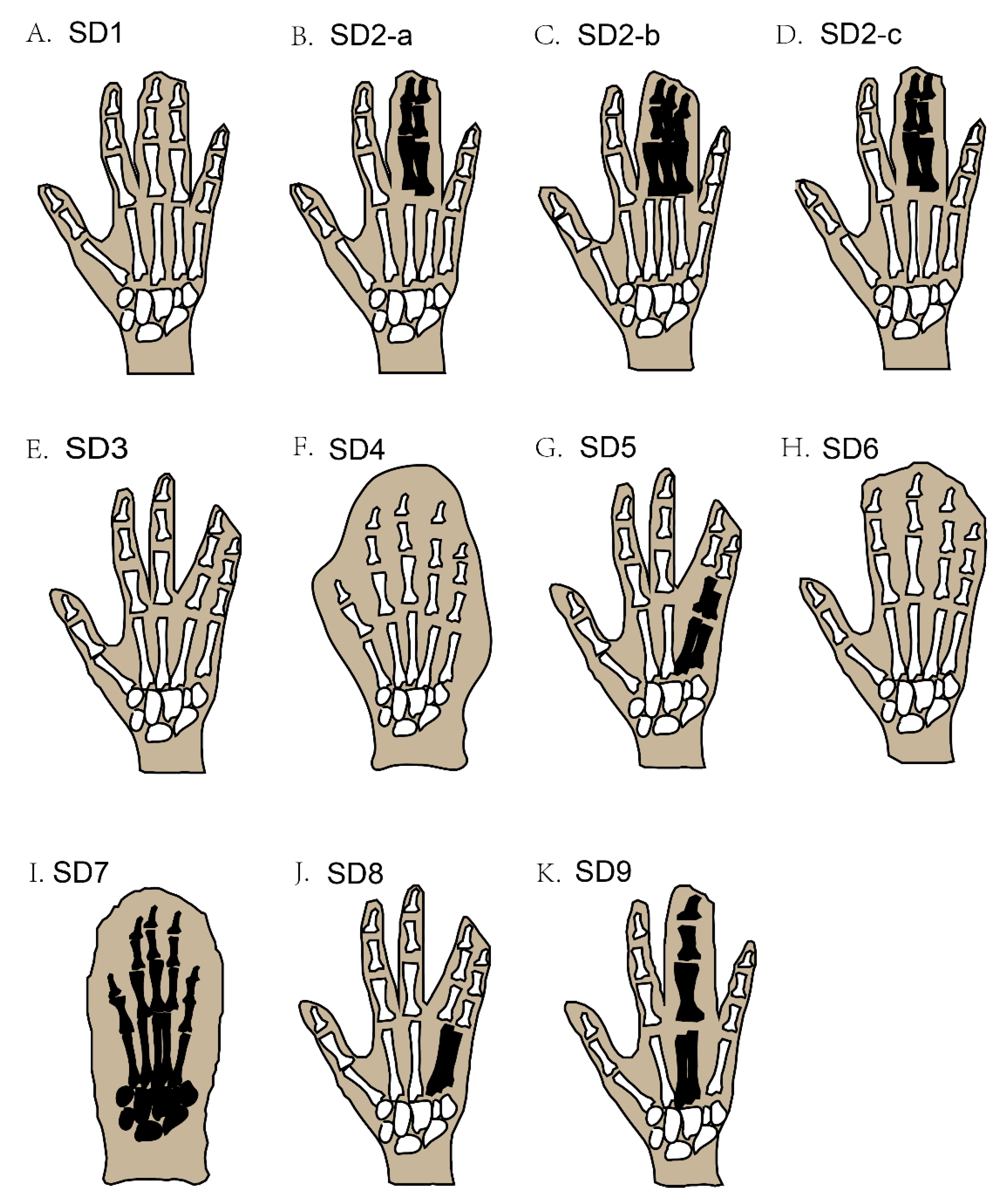
In syndactyly type I (SD1), the clinical records show a large variation in patient phenotypes; this involves mesoaxial webbing, i.e., either the complete or partial blending of either the 3rd and 4th fingers, the 2nd and 3rd toe, or both in the same individual [4,10,11] (Figure 2A). A family with almost two dozen affected members showed deformities of digits that were characteristic of SD type 1c, with webbed fingers of both hands along with normal feet, but only one member of the family showed fused toes (3rd to 5th) [8,35].
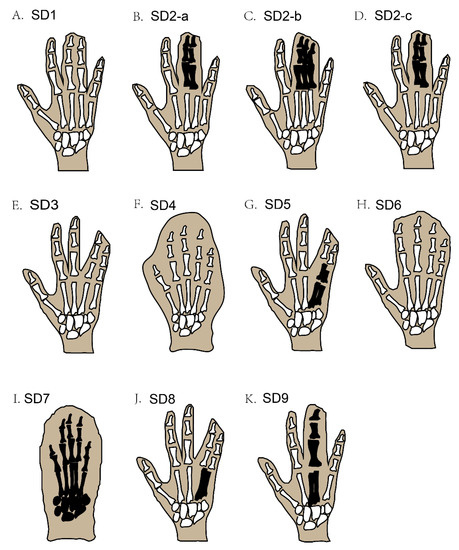
Figure 2.
Schematic diagram of different types of non-syndromic syndactyly (types 1–9). Black-coloured areas represent the blending of the bones under webbed skin, while white-coloured areas under webbed skin represent unfused bones.
Syndactyly and polydactyly (the addition of an extra digit in the limbs) may be found together in some cases. For example, syndactyly type II (SD2), also termed synpolydactyly (SPD), typically involves both webbing of the fingers and toe duplication, or an extra toe added in the feet [7], and usually involves webbing of the 3rd and 4th digits in the hands and the 4th and 5th toes, with an extra toe added [19]. The recognized phenotypic features of this type of SPD are webbing of the fingers (3rd and 4th) and toes (4th and 5th), which may be unilateral or bilateral and, rarely, evince duplicated toes and fingers [36] (Figure 2B–D). SPD is a very heterogeneous deformity among all SD types, in terms of phenotype as well as genotype [37]. The more complex type of SPD was first evident in a Belgium family with three affected family members who had irregular metacarpal as well as metatarsal synostoses [18].
The phenotypic features of syndactyly type III (SD3) are webbing of the 4th plus 5th fingers of both hands at the same time, but in some cases, the 3rd finger of each hand is also involved, accompanied by camptodactyly (Figure 2E) [21]. Similarly, syndactyly type IV (SD4) involves the webbing of the skin of all five digits of the hand, without the involvement of the bone; in most cases, polydactyly is also seen in the affected hands (Figure 2F). This type of SD is further classified into two categories, based on feet involvement along with affected hands. In the first category, no feet are involved, and in the second category, the fusion of the toes of one or both feet are involved [8,11]. Syndactyly type V (SD5) can be recognized by a bony combination of the 4th and 5th metacarpals in the forelimbs (Figure 2G) [27]. Additional deformities are also reported to be part of SD5, such as the irregular derivation of the fifth finger in both hands and unusual interphalangeal distortions [26]. Deformity of the feet involves defective metatarsals, for example, abnormal growth of the first metatarsal and the lesser small size of the remaining metatarsals, which severely affects the shape and function of the feet [8,26]. Correspondingly, syndactyly type VI (SD6) can be recognized by webbing of the four fingers (2nd to 5th) in the right hand, integration of the phalanges, and webbing of the 2nd and 3rd toes in the affected feet (Figure 2H) [7].
Syndactyly type VII (SD7) is the most severe form of SD, whereby the whole hand is distorted by the bony webbing of all fingers in the affected hand. The skeletal structure of the affected hand is fully disordered, to a degree that the phalanges cannot be distinguished as separate entities (Figure 2I) [38]. Furthermore, the carpals, metacarpals, and phalanges also have an uneven shape. Sometimes, other bones, such as the radius and ulna, also get affected, causing the length of the whole arm to shorten [7,23]. Two different phenotypic features of SD7 have been proposed, i.e., the spoon-head and oligodactyly types [39].
The main phenotypic feature of syndactyly type VIII (SD8) is the skeletal fusion of the 4th and 5th metacarpals, the shortness of the 4/5 metacarpals, and a few other small deformities in the skeletal structure of the affected hand (Figure 2J) [20]. Syndactyly type IX (SD9) can usually be recognized by phalangeal lessening, the osseous fusion of the metacarpals, 5th-finger clinodactyly, hypoplasia of the thumb and phalanges in the hand, and webbing of the toes (Figure 2K) [31,32].
4. Genetic Factors Underlying the Differential Phenotypes of Syndactyly
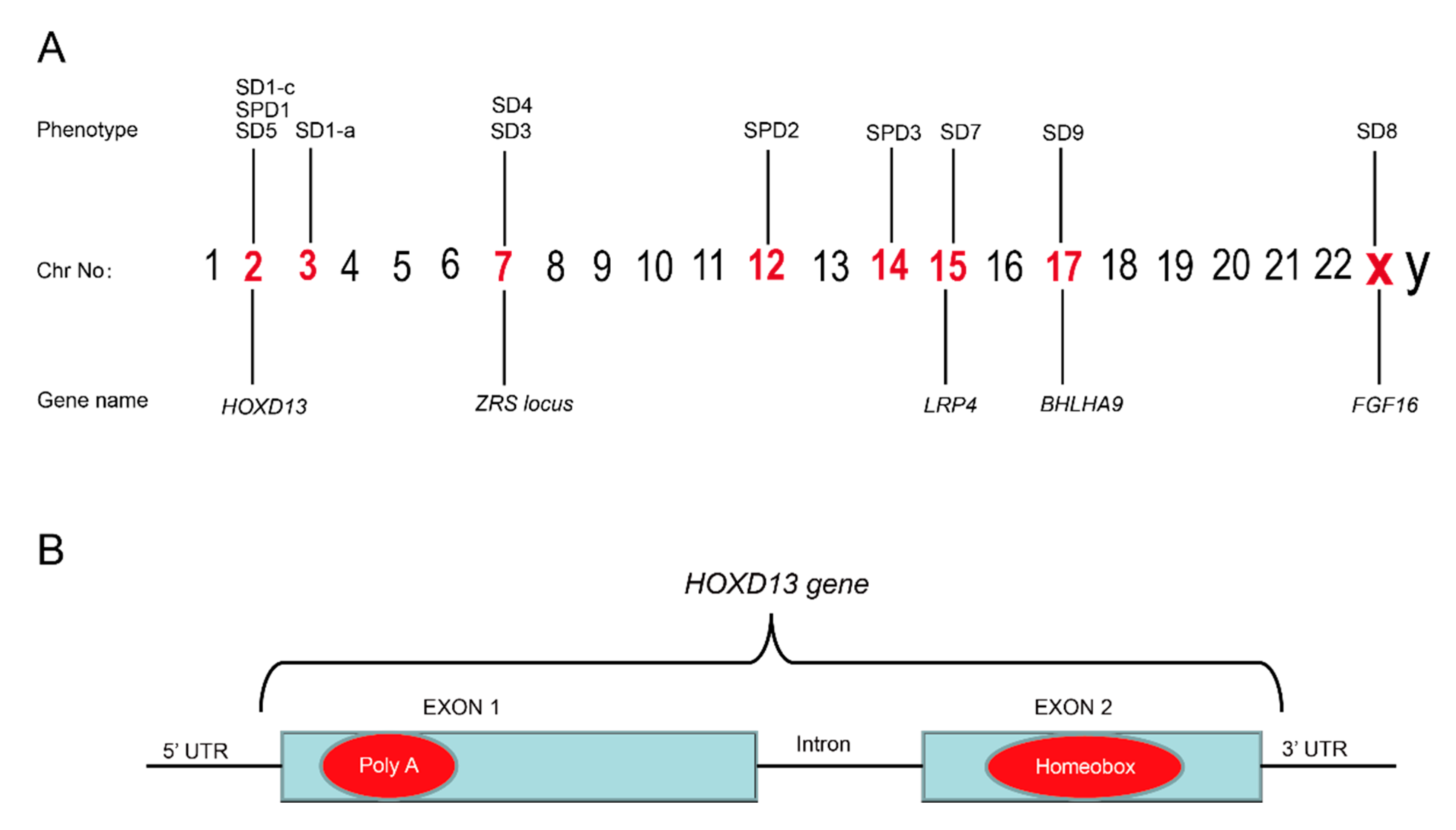
Mutations associated with the pathogenesis of SD have been recognized in numerous genes due to recent advancements in molecular genetics [40] (Figure 3). In 2000, SD1-b was mapped to chromosomal 2q34-q36 in the members of large families of German and Iranian kindred, but no specific gene has yet been identified [4,12,41], although recently a missense mutation (c.500A>G;p.Y167C) in the HOXD13 gene has been reported to cause SD1-b [42]. Mutation in the HOXD13, present on chromosome 2, has also been reported to be associated with SD1-c. A study concerning two Chinese families affected with the Montagu type reported mutations in HOXD13 [13]. Recently, missense variants (c.961A>C;p.T321P, c.917G>A;p.R306Q) in the HOXD13 have been linked with SD1-c in families [42,43].
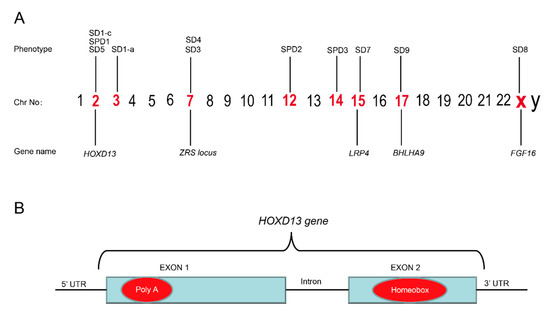
Figure 3.
(A) Illustration of chromosomes and the syndactyly types belonging to different chromosomes. (B) The mutational hot spots in the poly(A) region in exon 1 and homeobox region in exon 2 of the HOXD13 gene are marked in red.
Different types of duplication, as well as missense and deletion variants in HOXD13, cause typical SPD disease [44]. Missense mutations in HOXD13 have been linked with SPD1, which possibly affects the stability of the HOXD13 protein [45]. Recently, a missense variant (c.1157C>T;p.A375V) in the TTC30B has been reported in a Chinese family with SPD1 phenotypic features [46]. The FBLN1 has been linked with SPD2, as mutations in this gene have been reported to result in a complex type of SPD [47]. The most common mutation involved polyalanine expansion or contraction in the N-terminal region of the HOXD13 protein [17].
Molecular evidence for SD3 has been confirmed in a family with SD3 and was linked to a locus at chr.7q36.3 [10]. Although SD3 is described in families as an isolated anomaly, it also occurs as a part of other diseases or syndromes [21,48,49].
Likewise, the duplication of 115.3 kb at a locus called ZRS (limb-specific cis regulator) on chromosome 7 has been linked with SD4 [22,23,24,25]. Recently, in two different studies, large duplications that involve several exons in the LMBR1, present on the same locus at chromosome 7, were associated with SD4 deformity in two large Chinese families [50,51].
A missense mutation (c.950A>G;p.Q317R) in the HOXD13 has been confirmed to cause SD5 in a large Chinese family [27].
SD7 has been linked with the LRP4 gene in several studies. For example, two brothers affected with SD7 deformity had a missense mutation (c.4910G>A;p.C1637T) in the LRP4 [52]. In a large Pakistani family, a mutation in LRP4 (c.316+1G>A) has been reported to cause SD7 [53]. Similarly, another study reported a missense mutation (c.1151A>G;p.T384C) in the LRP4 in a family affected with SD7 [54]. A deleterious variant (c.1348A>G;p.I450V) in LRP4 was also associated with SD7 in two affected members of a Sri Lankan family [55].
In the case of SD8, a mutation in FGF16 on the locus chrXq21.1 is the main cause, as two nonsense mutations (p.R179X and p.S157X) in FGF16 have been linked with SD8 [56].
Likewise, a mutation in BHLHA9 present on chromosome 17q13.3 has been linked with the SD9 [32]. Several other studies have reported missense (c.311T>C;p.I104T), frameshift (c.74delG;p.G25Afs*55), and deletion (c.252_270delinsGCA;p.F85Qfs*108) variants in the BHLHA9 (Reference sequence: NM_001164405.2) in families affected with SD9 [33,57,58].
5. Some Excluded Types of Syndactyly and Underlying Genetic Factors
According to the current classification system, syndactyly can be classified into nine types, but this classification system does not consider numerous other syndromic and non-syndromic forms of SD. For a better understanding of the genetic factors behind all SD types, these excluded types of SD must be considered because several genes in combination are involved in limb development at the embryonic stage. For example, Saudi-type familial SD has been linked to the hammer-toe locus in mice [10], while Cenani–Lenz SD is associated with APC variations [59], missense alterations in FIBULIN1 are associated with brain atrophy-syndactyly syndrome [60], and genomic replications of the SHH enhancer ZRS lead to triphalangeal thumb polysyndactyly syndrome [61], Greig syndrome, acrocephalosyndactyly syndromes and other SD phenotypes linked with the GLI3 variants [62].
A large family affected with polydactyly and SD was shown to have a disease-linked variant (c.739C>T;p.Q247X) in the GLI3 gene that was co-segregated in all affected family members [63]. Furthermore, a heterozygous mutation in the NSDHL (c.713C>A;p.T238N) gene has been reported in a nine-month-old female affected with a CHILD syndrome phenotype and SD, who has non-consanguineous parents [64]. In addition, the TP63 gene has been found to be associated with SD in the presence of other abnormalities [65,66]. Recently, autosomal dominant SD has been associated with a microdeletion of 2.79 Mb at chr14q22-q22.2 in four affected members of a three-generation family with limb defects (syndactyly and polydactyly) along with other disorders, such as developmental delay and facial defects [67]. Recently, it has been reported that children with SD and prolonged heart-rate-corrected QT (QTc) interval have more multisystem diseases and electrocardiographic abnormalities [68]. Heterozygous missense alterations in GLI3 (c.1622C>T;p.T541M) and GJA1 (c.274T>C;p.Y92H) were identified in patients with the phenotypic features of SD type I [69], and two variants (p.D1403H, p.Q1564K) of LRP4 have been reported in a child affected with isolated SD of both hands, although the LRP4 gene has been reported to cause SD7 [70]. In recent studies, the combinations of SD, cleft hand, and polydactyly in a single patient suggested that some common genetic factors are behind these deformities [71,72]. Similarly, a missense variant (c.1622C>T;p.T541M) in GLI3 has been reported in a patient with isolated postaxial synpolydactyly [73]. In another study, mutations in the GJA1 gene, i.e., that located on chromosome 6q22-q23, have been reported to be linked with oculodentodigital dysplasia syndrome, and the SD3 phenotype has also been reported in some cases [74]. Recently, SD1-a has been reported to be associated with other diseases, e.g., diabetes [75].
We have listed the genes linked with these deformities in Table 2.

Table 2.
Genes linked with the excluded types of syndactyly.
6. HOXD13 and Its Role in Causing Syndactyly
HOXD13 belongs to a group of evolutionarily conserved HOX gene-family, which encode a group of transcription factors that regulate morphogenesis at an early embryonic stage [76]. Germline mutation in HOXD13 is known to cause the deformity of limbs in humans. The phenomena of variable expressivity and incomplete penetrance are common with HOXD13 mutations [77]. Mutations in HOXD13 have also been linked with brachydactyly-syndactyly syndrome and VACTERL association [27,78]. HOXD13 is known to cause different types of SD, e.g., SD1, SD5, and SPD1, which shows that the HOXD13 gene has an important function in limb development (Table 3). The most common variation is a polyalanine expansion in the N-terminal domain of HOXD13, which is widely reported in families of different kindred (Figure 3B). For example, nine extra alanine residues that are added to the same region of HOXD13, due to the duplication of 27 bases, have been found in Turkish families with SPD1 [14]. In 2005, a polyalanine extension in HOXD13 has been reported to cause SPD1 in four Danish families [79], while the duplication of 27 base pairs (c.184_210dup) has been reported to cause the addition of nine extra alanines to HOXD13 in a large Chinese family with SPD1 [80]. In 2009, a mutation within the N-terminal transcription-regulating domain of HOXD13 (c.659G>T;p.G220V) was reported in a Greek family with a variant form of SPD [81]. In our own study, with the help of whole-genome sequencing (WGS), we identified a 24-base pair duplication variant, c.183_206dupGCGGCGGCTGCGGCGGCGGCGGC (Reference sequence: NM_000523.3) in HOXD13 that results in the addition of eight extra alanines in four generations of a family in northern China [82]. Similarly, missense and nonsense mutations in HOXD13 have also been reported in large families affected with SPD1 [83,84,85].
Previously, it has been reported that families inheriting a homozygous mutation in the HOXD13 have a severe form of SPD [86] but, recently, this has been demonstrated not to be true in all cases of SPD1 patients with homozygous mutations [87]. Studies have also reported HOXD13 mutations in families with different syndactyly types, e.g., SD1-a, SD1-c, and SD5 (Table 3), which gives a clear indication that HOXD13 has a critical role in limb formation and that it may also interact with other limb-formation genes during the process. Recently, a study demonstrated how a missense mutation in the homeodomain of HOXD13 leads to impaired transcriptional activity of EPHA7 (one of the downstream genes of HOXD13) [88]. EPHA7 is known to play a crucial role in limb development [89]. Hence, variations in the normal sequence of HOXD13 can negatively affect other gene’s normal functions that could possibly results in differential phenotypes of SD.

Table 3.
List of HOXD13 gene mutations reported for different types of non-syndromic syndactyly in the literature.
Table 3.
List of HOXD13 gene mutations reported for different types of non-syndromic syndactyly in the literature.
| Mutation Type | cDNA Change | AA Change | NCBI Ref. Sequence | Allele | Phenotype | Ref. |
|---|---|---|---|---|---|---|
| Missense | c.917G>A | p.R306Q | NM_000523.4 | Heterozygous | SD1-c | [13] |
| Missense | c.500A>G | p.Y167C | NM_000523.4 | Heterozygous | SD1-b | [42] |
| Missense | c.961A>C | p.T321P | NM_000523.4 | Heterozygous | SD1-c | [42] |
| Missense | c.917G>A | p.R306Q | NM_000523.3 | Heterozygous | SD1-c | [43] |
| Duplication | c.183_206dup | p.A64_A71dup | NM_000523.3 | Heterozygous | SPD1 | [82] |
| Duplication | c.184_210dup | p.A63_A71dup | NM_000523.3 | Heterozygous | SPD1 | [80] |
| Duplication | c.183_206dup | p.A64_A71dup | NM_000523.4 | Heterozygous | SPD1 | [90] |
| Duplication | c.186-212dup | p.A63_A71dup | NM_000523.4 | Heterozygous | SPD1 | [91] |
| Missense | c.859C>T | p.G287X | NM_000523.3 | Heterozygous | SPD1 | [83] |
| Missense | c.556C>T | p.R186X | NM_000523.4 | Heterozygous | SPD1 | [84] |
| Missense | c.938C>G | p.T313R | NM_000523.4 | Homozygous | SPD1 | [85] |
| Missense | c.892C>T | p.R298W | NM_000523.2 | Heterozygous | SPD1 | [45] |
| Missense | c.659G>T | p.G220V | NM_000523.2 | Heterozygous | SPD1 | [81] |
| Missense | c.938C>G | p.T313R | NM_000523.3 | Homozygous | SPD1 | [86] |
| Missense | c.893G>A | p.A298G | NM_000523.3 | Heterozygous | SPD1 | [44] |
| Deletion | c.708delC | p.A236Lfs*30 | NM_000523.4 | Heterozygous | SPD1 | [92] |
| Missense | c.925A>T | p.I309F | NM_000523.4 | Heterozygous | SPD1 | [88] |
| Splice donor site | c.781+1G>A | - | NC_000002.12 NM_000523.3 | Heterozygous | SPD1 | [93] |
| Missense | c.950A>G | p.Q317R | NM_000523.3 | Heterozygous | SD5 | [27] |
7. Diagnosis and Surgical Treatment of Syndactyly
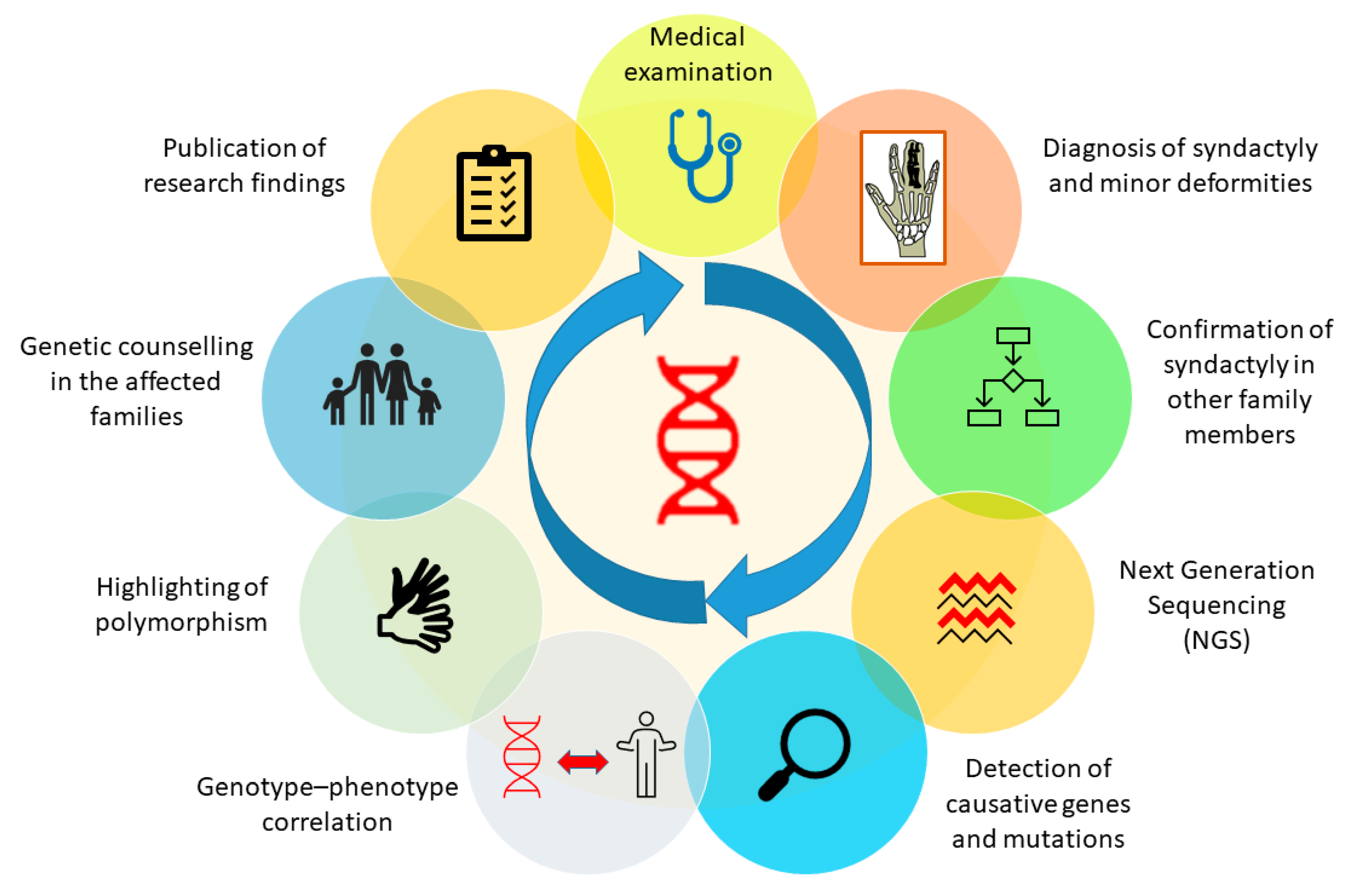
SD is basically a limb malformation that belongs to congenital anomalies affecting bone or skeletal structure or function. It is caused when the digits of the fetus in the womb do not separate successfully, resulting in a webbed hand or feet. As an apparent deformity of the hands and feet, the characteristic is so obvious that it attracts instant attention or concern soon after birth, especially when it occurs in the hands. SD can be managed using different diagnostic tools, plus a genetic background of the patient’s family history and clinical data regarding deformities in affected family members. Genetic screening of the affected person and affected family members can easily reveal information about the genetic background, which can make it easy for a clinician to diagnose the deformity. Furthermore, other tools, such as X-rays and ultrasound, can also make the deformity clearer to the clinician and, therefore, more easily diagnosed. In the presence of all this information, a clinician will be able to diagnose the problem immediately and perform treatment effectively and efficiently [80,94]. Furthermore, after successfully diagnosing the deformity, genetic analysis of the patient and his family members will be helpful in establishing a clearer genotype–phenotype correlation. We have proposed a genetic analysis procedure in the form of a schematic diagram to obtain clearer genotype–phenotype correlation in future (Figure 4).
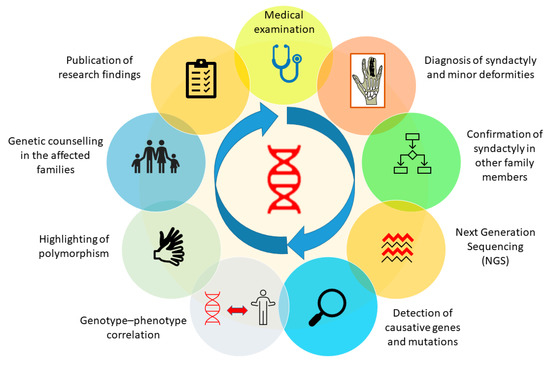
Figure 4.
Genetic analysis procedure to attain a clearer genotype-phenotype correlation in all types of syndactyly.
The most important aim and objective of surgical treatment for SD is to minimize possible complications, reinstate the space between the digits, and detach the limbs by using minimal medical techniques and avoiding problems that are likely to happen, such as recurrence and post-surgery complications, until a useful hand is obtained [95]. Skin implantation, open treatment, and the zigzag method of surgery techniques are usually conducted in corrective SD patient surgeries. Surgical outcomes in SD are more positive in simple-type SD compared to complex-type SD. In the United States, a recent study reported that the occurrence of SD is roughly 7 for every 10,000 babies born and that almost every affected child receives surgery before reaching two years of age. The study also pointed out that there may be some genuine problems in getting immediate health care, which includes accessibility to specialized surgeons for correcting limb deformity, failure to get to well-equipped hospitals, especially for people living in remote areas, and poor financial status [96].
The most significant and simple way to eliminate the deformity is early treatment of new-borns by surgery. For new-borns with simple SD, the best age to receive surgery ranges from 6 to 18 months old, whereas in case of complex syndactyly, surgery should be performed prior to 6 months of age [95]. It is very difficult to predict the effectiveness of the surgery because of the tremendous variety and phenotypic range of SD types. The simpler the SD, the higher the chance of achieving useful and fully recovered hand movement [97]. In case of simple SD, corrective and operational outcomes are typically excellent, with fewer chances of recurrence or the possibility of re-arising hand-related problems, whereas in case of complex SD, the chances of post-surgery complications are higher and involve difficulty in normal hand movement and nail deformities [23,97,98]. Complex-SD patients who have received surgery always require revisiting the clinician or surgeon to diagnose post-operative complications.
Surgeons specializing in pediatrics often admit children with rare limb deformities. Closely associated deformities and syndromes should be always taken into consideration, because if not diagnosed accurately, surgery in that case can lead the patient into a worsen situation [99]. The main principle of the surgical treatment of SD and other associated limb deformities is to gain functional and useful limbs with less chance of recurrence. Skin grafts are the operational procedures most commonly used for corrective purposes in limbs affected with SD [100], although open-treatment methods for SD avoid leftover postoperative marks on the skin and are comparatively useful, with the best end results [101]. Several modern surgical techniques have been successfully practiced in the clinics with the aim of achieving useful limbs with no post-operative scars, smooth mobility of the digits, and fewer chances of recurrence. The free skin graft (full-thickness) surgical technique produces the best results when practiced in combination with the Z-method of incision, which can successfully diminish the scars usually obtained at the end of surgery and, as a result, can attain fully functional and useful limbs [102]. Recently, a technique called the improved flap technique was successfully implemented and involves the use of skin grafts with full thickness and different types of flaps to provide sufficient soft tissue cover. The results involved no post-operative complications, provided full recovery of the affected hand, and no discomfort to the child who received surgery [103]. Furthermore, the use of a dermal fat graft surgical technique specifically intended for treating the complex type of SD has recently been introduced [104]. In recent years, the use of abdominal flaps for complex SD release has also proved to be successful [105]. The part of the donor skin used for the corrective surgery of simple or complicated SD must have the features of both dorsal and palm skin as it can leave post-operative skin flaws in the digits, which can eventually affect mobility. Recently, it has been demonstrated that for the surgical purposes of SD, a gradation skin graft is far better cosmetically, compared to skin grafts from the sub-malleolar part, as it has been used traditionally; however, proper alignment with other parts is critical [106].
In case of the treatment of webbed toes in SD, it is slightly more complicated to perform surgery in children because of its high recurrence rate and post-surgery complications, especially in those children who are older than 24 months (the younger the age of the child at the time of surgery, lower the risk of recurrence) [107]. Recently, it has been demonstrated that the most effective surgical procedure suitable for both simple and complex SD involves the interdigitating of rectangular flaps because of its simple design, flexibility in alteration during surgery, and inclusive flap tips [108]. Another study suggested that the dorsal hexagon flap can be a useful substitute technique in treating syndactyly [109]. Furthermore, for skin grafting in which the donor site of the patient also gets disturbed, dorsal rectangular flaps can be very useful, with appealing results for the patient [110]. Based on the condition of the deformity, an individualized treatment plan should be made that can better restore the shape and function of the thumb, especially in SD5 [111]. Moreover, the use of methotrexate can reduce keloid formation just after the dissection of the webbed digits [112].
Post-operative check-ups of the patients should be frequently arranged prior to complete recovery to avoid any difficulties due to surgery [95]. Overall, treatment and surgery for SD are carried out by surgeons of other specialties, which shows that SD treatment is a harmless and effective process with few postoperative complications, but it does need to be followed up by clinicians to ensure fully recovered limbs [113].
8. Future Perspectives
Due to modern techniques, more of the genetic factors behind SD are being revealed as research proceeds on inborn limb deformities. It is, therefore, of considerable importance to further elucidate the genetic etiologic factors that contribute to the differential phenotype and incomplete penetrance of all types of non-syndromic SD. Next-generation sequencing can play a crucial role in identifying new pathogenic genes and provide a better understanding of this deformity in the future [114]. More in vitro and in vivo studies should be conducted to investigate the interaction of HOXD13 with other closely related genes that are involved in limb deformities. A stronger phenotype–genotype correlation needs to be established using the modern technologies of genetic engineering and biotechnology to investigate the factors involved in causing differential phenotype of SD.
Author Contributions
Writing—original draft preparation, T.Z.; writing—review and editing, H.R. and H.K.; conceptualization, T.Z. and X.Z.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81571994, No. 81870432 and 81570567); the Natural Science Foundation of Guangdong Province, China (No. 2020A1515010054); and the Li Ka Shing Shantou University Foundation (No. L1111 2008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Stanley Lin from Shantou University Medical College for his useful advice.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Hay, S. Incidence of selected congenital malformations in Iowa. Am. J. Epidemiol. 1971, 94, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Castilla, E.E.; Paz, J.E.; Orioli-Parreiras, I.M. Syndactyly: Frequency of specific types. Am. J. Med. Genet. 1980, 5, 357–364. [Google Scholar] [CrossRef]
- Jordan, D.; Hindocha, S.; Dhital, M.; Saleh, M.; Khan, W. The epidemiology, genetics and future management of syndactyly. Open Orthop. J. 2012, 6, 14–27. [Google Scholar] [CrossRef]
- Malik, S.; Schott, J.; Ali, S.W.; Oeffner, F.; Amin-ud-Din, M.; Ahmad, W.; Grzeschik, K.H.; Koch, M.C. Evidence for clinical and genetic heterogeneity of syndactyly type I: The phenotype of second and third toe syndactyly maps to chromosome 3p21.31. Eur. J. Hum. Genet. 2005, 13, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Giele, H.; Giele, C.; Bower, C.; Allison, M. The incidence and epidemiology of congenital upper limb anomalies: A total population study. J. Hand Surg. Am. 2001, 26, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Yabe, K.; Kimura, Y.; Ito, Y.; Rokukawa, M.; Furukawa, M.; Ito, K.; Matsuura, M.; Kiguchi, M. Syndactyly lethal: New mutation with multiple malformations occurring in Sprague Dawley rats. Congenit. Anom. 2009, 49, 262–268. [Google Scholar] [CrossRef]
- Temtamy, S.A.; McKusick, V.A. The genetics of hand malformations. Birth Defects Orig. Artic. Ser. 1978, 14, 1–619. [Google Scholar]
- Malik, S. Syndactyly: Phenotypes, genetics and current classification. Eur. J. Hum. Genet. 2012, 20, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sobreira, N.L.; Cernach, M.C.; Brunoni, D.; Perez, A.B. Complex toe syndactyly with characteristic facial phenotype: A new syndrome? Am. J. Med. Genet. A 2008, 146A, 1725–1728. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Shamseldin, H.E.; Al Mazyad, M.; Al Deghaither, S.; Alkuraya, F.S. Genetic heterogeneity in type III familial cutaneous syndactyly and linkage to chromosome 7q36. Am. J. Med. Genet. A 2013, 161A, 1579–1584. [Google Scholar] [CrossRef]
- Andersen, H.J.; Hansen, A.K. Tibial hypo-/aplasia with preaxial syn- and polydactyly. Arch. Orthop. Trauma Surg. 1990, 109, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Bosse, K.; Betz, R.C.; Lee, Y.A.; Wienker, T.F.; Reis, A.; Kleen, H.; Propping, P.; Cichon, S.; Nothen, M.M. Localization of a gene for syndactyly type 1 to chromosome 2q34-q36. Am. J. Hum. Genet. 2000, 67, 492–497. [Google Scholar] [CrossRef][Green Version]
- Dai, L.; Liu, D.; Song, M.; Xu, X.; Xiong, G.; Yang, K.; Zhang, K.; Meng, H.; Guo, H.; Bai, Y. Mutations in the homeodomain of HOXD13 cause syndactyly type 1-c in two Chinese families. PLoS ONE 2014, 9, e96192. [Google Scholar] [CrossRef]
- Akarsu, A.N.; Stoilov, I.; Yilmaz, E.; Sayli, B.S.; Sarfarazi, M. Genomic structure of HOXD13 gene: A nine polyalanine duplication causes synpolydactyly in two unrelated families. Hum. Mol. Genet. 1996, 5, 945–952. [Google Scholar] [CrossRef]
- Malik, S.; Grzeschik, K.H. Synpolydactyly: Clinical and molecular advances. Clin. Genet. 2008, 73, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sarfarazi, M.; Akarsu, A.N.; Sayli, B.S. Localization of the syndactyly type II (synpolydactyly) locus to 2q31 region and identification of tight linkage to HOXD8 intragenic marker. Hum. Mol. Genet. 1995, 4, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Mundlos, S.; Upton, J.; Olsen, B.R. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science 1996, 272, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Debeer, P.; Schoenmakers, E.F.; Thoelen, R.; Holvoet, M.; Kuittinen, T.; Fabry, G.; Fryns, J.P.; Goodman, F.R.; Van de Ven, W.J. Physical map of a 1.5 mb region on 12p11.2 harbouring a synpolydactyly associated chromosomal breakpoint. Eur. J. Hum. Genet. 2000, 8, 561–570. [Google Scholar] [CrossRef][Green Version]
- Malik, S.; Abbasi, A.A.; Ansar, M.; Ahmad, W.; Koch, M.C.; Grzeschik, K.H. Genetic heterogeneity of synpolydactyly: A novel locus SPD3 maps to chromosome 14q11.2-q12. Clin. Genet. 2006, 69, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, F.; Della Monica, M.; Riccardi, G.; Riccio, I.; Riccio, V.; Scarano, G. A family with X-linked recessive fusion of metacarpals IV and V. Am. J. Med. Genet. A 2004, 124A, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, A.; Donnai, D.; Metcalfe, K.; Schrander-Stumpel, C.; Brueton, L.; Verloes, A.; Aylsworth, A.; Toriello, H.; Winter, R.; Dixon, M. Localization of a gene for oculodentodigital syndrome to human chromosome 6q22-q24. Hum. Mol. Genet. 1997, 6, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, D.; Pawlik, B.; Li, Y.; Akarsu, N.A.; Caliebe, A.; May, K.J.; Schweiger, B.; Vargas, F.R.; Balci, S.; Gillessen-Kaesbach, G.; et al. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum. Mutat. 2010, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Samson, P.; Salazard, B. Syndactyly. Chir. Main 2008, 27 (Suppl. 1), S100–S114. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Guo, H.; Meng, H.; Zhang, K.; Hu, H.; Yao, H.; Bai, Y. Confirmation of genetic homogeneity of syndactyly type IV and triphalangeal thumb-polysyndactyly syndrome in a Chinese family and review of the literature. Eur. J. Pediatr. 2013, 172, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.; Spielmann, M.; Doelken, S.C.; Flottmann, R.; Muhammad, F.; Baig, S.M.; Wajid, M.; Hulsemann, W.; Habenicht, R.; Kjaer, K.W.; et al. Microduplications encompassing the Sonic hedgehog limb enhancer ZRS are associated with Haas-type polysyndactyly and Laurin-Sandrow syndrome. Clin. Genet. 2014, 86, 318–325. [Google Scholar] [CrossRef]
- Robinow, M.; Johnson, G.F.; Broock, G.J. Syndactyly type V. Am. J. Med. Genet. 1982, 11, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, M.; Zhao, J.; Leyva, J.A.; Zhu, H.; Yang, W.; Zeng, X.; Ao, Y.; Liu, Q.; Liu, G.; et al. Mutations in HOXD13 underlie syndactyly type V and a novel brachydactyly-syndactyly syndrome. Am. J. Hum. Genet. 2007, 80, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.N.; Klar, J.; Ali, Z.; Khan, F.; Baig, S.M.; Dahl, N. Cenani-Lenz syndrome restricted to limb and kidney anomalies associated with a novel LRP4 missense mutation. Eur. J. Med. Genet. 2013, 56, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pawlik, B.; Elcioglu, N.; Aglan, M.; Kayserili, H.; Yigit, G.; Percin, F.; Goodman, F.; Nurnberg, G.; Cenani, A.; et al. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am. J. Hum. Genet. 2010, 86, 696–706. [Google Scholar] [CrossRef]
- Dimitrov, B.I.; Voet, T.; De Smet, L.; Vermeesch, J.R.; Devriendt, K.; Fryns, J.P.; Debeer, P. Genomic rearrangements of the GREM1-FMN1 locus cause oligosyndactyly, radio-ulnar synostosis, hearing loss, renal defects syndrome and Cenani--Lenz-like non-syndromic oligosyndactyly. J. Med. Genet. 2010, 47, 569–574. [Google Scholar] [CrossRef]
- Percin, E.F.; Percin, S.; Egilmez, H.; Sezgin, I.; Ozbas, F.; Akarsu, A.N. Mesoaxial complete syndactyly and synostosis with hypoplastic thumbs: An unusual combination or homozygous expression of syndactyly type I? J. Med. Genet. 1998, 35, 868–874. [Google Scholar] [CrossRef][Green Version]
- Malik, S.; Percin, F.E.; Ahmad, W.; Percin, S.; Akarsu, N.A.; Koch, M.C.; Grzeschik, K.H. Autosomal recessive mesoaxial synostotic syndactyly with phalangeal reduction maps to chromosome 17p13.3. Am. J. Med. Genet. A 2005, 134, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, R.; Han, S.; Ahmad, W.; Zhang, X. A novel homozygous missense mutation in BHLHA9 causes mesoaxial synostotic syndactyly with phalangeal reduction in a Pakistani family. Hum. Genome Var. 2017, 4, 17054. [Google Scholar] [CrossRef] [PubMed]
- Deunk, J.; Nicolai, J.P.; Hamburg, S.M. Long-term results of syndactyly correction: Full-thickness versus split-thickness skin grafts. J. Hand Surg. Br. 2003, 28, 125–130. [Google Scholar] [CrossRef]
- Hsu, C.K. Hereditary syndactylia in a Chinese family. Chin. Med. J. 1965, 84, 482–485. [Google Scholar]
- Cross, H.E.; Lerberg, D.B.; McKusick, V.A. Type II syndactyly. Am. J. Hum. Genet. 1968, 20, 368–380. [Google Scholar]
- Malik, S.; Girisha, K.M.; Wajid, M.; Roy, A.K.; Phadke, S.R.; Haque, S.; Ahmad, W.; Koch, M.C.; Grzeschik, K.H. Synpolydactyly and HOXD13 polyalanine repeat: Addition of 2 alanine residues is without clinical consequences. BMC Med. Genet. 2007, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Cenani, A.; Lenz, W. Total syndactylia and total radioulnar synostosis in 2 brothers. A contribution on the genetics of syndactylia. Z. Kinderheilkd. 1967, 101, 181–190. [Google Scholar] [CrossRef]
- Harpf, C.; Pavelka, M.; Hussl, H. A variant of Cenani-Lenz syndactyly (CLS): Review of the literature and attempt of classification. Br. J. Plast. Surg. 2005, 58, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Philip-Sarles, N. Genetics of congenital hand malformations. Chir. Main 2008, 27 (Suppl. 1), S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, M.; Majidzadeh, A.K.; Haerian, B.S.; Damavandi, E.; Yamada, K.; Pasallar, P.; Najafi, M.T.; Nishimura, G.; Tomita, H.A.; Yoshiura, K.I.; et al. Confirmation of genetic homogeneity of syndactyly type 1 in an Iranian family. Am. J. Med. Genet. 2001, 104, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Singh, S.K.; Bhattacharya, V.; Ali, A. Novel HOXD13 variants in syndactyly type 1b and type 1c, and a new spectrum of TP63-related disorders. J. Hum. Genet. 2022, 67, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tan, T.; He, Q.; Lin, Q.; Yang, Z.; Zhu, A.; Guan, L.; Xiao, J.; Song, Z.; Guo, Y. Identification of a missense HOXD13 mutation in a Chinese family with syndactyly type I-c using exome sequencing. Mol. Med. Rep. 2017, 16, 473–477. [Google Scholar] [CrossRef]
- Wang, B.; Xu, B.; Cheng, Z.; Zhou, X.; Wang, J.; Yang, G.; Cheng, L.; Yang, J.; Ma, X. A novel non-synonymous mutation in the homeodomain of HOXD13 causes synpolydactyly in a Chinese family. Clin. Chim. Acta 2012, 413, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Debeer, P.; Bacchelli, C.; Scambler, P.J.; De Smet, L.; Fryns, J.P.; Goodman, F.R. Severe digital abnormalities in a patient heterozygous for both a novel missense mutation in HOXD13 and a polyalanine tract expansion in HOXA13. J. Med. Genet. 2002, 39, 852–856. [Google Scholar] [CrossRef]
- Du, Y.; Chen, F.; Zhang, J.; Lin, Z.; Ma, Q.; Xu, G.; Xiao, D.; Gui, Y.; Yang, J.; Wan, S. A rare TTC30B variant is identified as a candidate for synpolydactyly in a Chinese pedigree. Bone 2019, 127, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Debeer, P.; Schoenmakers, E.F.; Twal, W.O.; Argraves, W.S.; De Smet, L.; Fryns, J.P.; Van De Ven, W.J. The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet. 2002, 39, 98–104. [Google Scholar] [CrossRef]
- Nishat, S.; Mansoor, Q.; Javaid, A.; Ismail, M. Oculodentodigital Syndrome with Syndactyly Type III in a Pakistani consanguineous family. J. Dermatol. Case Rep. 2012, 6, 43–48. [Google Scholar] [CrossRef][Green Version]
- Schrander-Stumpel, C.T.; De Groot-Wijnands, J.B.; De Die-Smulders, C.; Fryns, J.P. Type III syndactyly and oculodentodigital dysplasia: A clinical spectrum. Genet. Couns. 1993, 4, 271–276. [Google Scholar]
- Xu, J.; Wu, J.; Teng, X.; Cai, L.; Yuan, H.; Chen, X.; Hu, M.; Wang, X.; Jiang, N.; Chen, H. Large duplication in LMBR1 gene in a large Chinese pedigree with triphalangeal thumb polysyndactyly syndrome. Am. J. Med. Genet. A 2020, 182, 2117–2123. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Jiang, Q.; Huang, R.; Fu, W.; Mao, L.; Wei, X.; Cui, H.; Lin, K.; Cai, L.; et al. Sub-Exome Target Sequencing in a Family With Syndactyly Type IV Due to a Novel Partial Duplication of the LMBR1 Gene: First Case Report in Fujian Province of China. Front. Genet. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Steel, E.; Hurst, J.A.; Cullup, T.; Calder, A.; Sivakumar, B.; Shah, P.; Wilson, L.C. Cenani-Lenz syndactyly in siblings with a novel homozygous LRP4 mutation and recurrent hypoglycaemia. Clin. Dysmorphol. 2020, 29, 73–80. [Google Scholar] [CrossRef]
- Afzal, M.; Zaman, Q.; Kornak, U.; Mundlos, S.; Malik, S.; Flottmann, R. Novel splice mutation in LRP4 causes severe type of Cenani-Lenz syndactyly syndrome with oro-facial and skeletal symptoms. Eur. J. Med. Genet. 2017, 60, 421–425. [Google Scholar] [CrossRef]
- Alrayes, N.; Aziz, A.; Ullah, F.; Ishfaq, M.; Jelani, M.; Wali, A. Novel missense alteration in LRP4 gene underlies Cenani-Lenz syndactyly syndrome in a consanguineous family. J. Gene Med. 2020, 22, e3143. [Google Scholar] [CrossRef] [PubMed]
- Hettiaracchchi, D.; Bonnard, C.; Jayawardana, S.M.A.; Ng, A.Y.J.; Tohari, S.; Venkatesh, B.; Reversade, B.; Singaraja, R.; Dissanayake, V.H.W. Cenani-Lenz syndactyly syndrome—A case report of a family with isolated syndactyly. BMC Med. Genet. 2018, 19, 125. [Google Scholar] [CrossRef]
- Jamsheer, A.; Zemojtel, T.; Kolanczyk, M.; Stricker, S.; Hecht, J.; Krawitz, P.; Doelken, S.C.; Glazar, R.; Socha, M.; Mundlos, S. Whole exome sequencing identifies FGF16 nonsense mutations as the cause of X-linked recessive metacarpal 4/5 fusion. J. Med. Genet. 2013, 50, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Sedighzadeh, S.S.; Sedaghat, A.; Zamani, M.; Seifi, T.; Shariati, G.; Zeighami, J.; Mazaheri, N.; Galehdari, H. Whole exome sequencing identified a novel frameshift variant in the BHLHA9 in an Iranian family with mesoaxial synostotic syndactyly. Congenit. Anom. 2021, 61, 220–225. [Google Scholar] [CrossRef]
- Ullah, A.; Ali, R.H.; Majeed, A.I.; Liaqat, K.; Shah, P.W.; Khan, B.; Bilal, M.; Umair, M.; Ahmad, W. A novel insertion and deletion mutation in the BHLHA9 underlies polydactyly and mesoaxial synostotic syndactyly with phalangeal reduction. Eur. J. Med. Genet. 2019, 62, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.M.; Alkuraya, F.S. Cenani-Lenz syndrome and other related syndactyly disorders due to variants in LRP4, GREM1/FMN1, and APC: Insight into the pathogenesis and the relationship to polyposis through the WNT and BMP antagonistic pathways. Am. J. Med. Genet. A 2019, 179, 266–279. [Google Scholar] [CrossRef]
- Bohlega, S.; Al-Ajlan, H.; Al-Saif, A. Mutation of fibulin-1 causes a novel syndrome involving the central nervous system and connective tissues. Eur. J. Hum. Genet. 2014, 22, 640–643. [Google Scholar] [CrossRef]
- Sun, M.; Ma, F.; Zeng, X.; Liu, Q.; Zhao, X.L.; Wu, F.X.; Wu, G.P.; Zhang, Z.F.; Gu, B.; Zhao, Y.F.; et al. Triphalangeal thumb-polysyndactyly syndrome and syndactyly type IV are caused by genomic duplications involving the long range, limb-specific SHH enhancer. J. Med. Genet. 2008, 45, 589–595. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. A novel frameshift mutation of the GLI3 gene in a family with broad thumbs with/without big toes, postaxial polydactyly and variable syndactyly of the hands/feet. Clin. Genet. 2012, 82, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, X.; Zhan, Z.; Feng, J.; Cai, H.; Li, Y.; Fu, Q.; Xu, Y.; Jiang, H.; Zhang, X. A Novel Nonsense GLI3 Variant Is Associated With Polydactyly and Syndactyly in a Family by Blocking the Sonic Hedgehog Signaling Pathway. Front. Genet. 2020, 11, 542004. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, D.; Panchal, H.; Lai, P.S.; Dissanayake, V.H.W. Novel variant in NSDHL gene associated with CHILD syndrome and syndactyly- a case report. BMC Med. Genet. 2020, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, Y.; Ueda, K.; Nuri, T.; Satoh, C.; Maekawa, R.; Yoshiura, K.I. EEC-LM-ADULT syndrome caused by R319H mutation in TP63 with ectrodactyly, syndactyly, and teeth anomaly: A case report. Medicine 2020, 99, e22816. [Google Scholar] [CrossRef] [PubMed]
- Sutton, V.R.; van Bokhoven, H. TP63-Related Disorders. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Roelandt, M.A.; Devriendt, K.; de Llano-Perula, M.C.; Raes, M.; Willems, G.; Verdonck, A. Dental and Craniofacial Characteristics in Patients With 14Q22.1-Q22.2 Deletion: A Case Series. Cleft Palate Craniofacial J. 2021, 58, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, Y.; Li, S.; Ren, L.; Zhang, J.; Sun, H.; Dong, J.; Zhao, X. Clinical characterization and outcome of prolonged heart rate-corrected QT interval among children with syndactyly. Medicine 2020, 99, e22740. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Duong, N.T.; Quynh, D.H.; Ton, N.D.; Duc, H.H.; Huong, L.T.M.; Anh, L.T.L.; Hai, N.V. Identification of novel missense mutations associated with non-syndromic syndactyly in two vietnamese trios by whole exome sequencing. Clin. Chim. Acta 2020, 506, 16–21. [Google Scholar] [CrossRef]
- Sukenik Halevy, R.; Chien, H.C.; Heinz, B.; Bamshad, M.J.; Nickerson, D.A.; University of Washington Center for Mendelian Genomics; Kircher, M.; Ahituv, N. Mutations in the fourth beta-propeller domain of LRP4 are associated with isolated syndactyly with fusion of the third and fourth fingers. Hum. Mutat. 2018, 39, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Buyukdogan, H. A Case of Nonsyndromic Unilateral Cleft Hand with Central Polydactyly, Syndactyly, and Thumb Hypoplasia: Support for a Common Etiology. J. Hand Microsurg. 2019, 11, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.M. Central and ulnar cleft hands: A review of concurrent deformities in a series of 47 patients and their pathogenesis. J. Hand Surg. 2014, 39, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Tian, Z.; Zheng, J.; Zhi, X.; Du, X.; Shu, J.; Cai, C. A novel missense in GLI3 possibly affecting one of the zinc finger domains may lead to postaxial synpolydactyly: Case report. BMC Med. Genet. 2019, 20, 174. [Google Scholar] [CrossRef]
- Paznekas, W.A.; Boyadjiev, S.A.; Shapiro, R.E.; Daniels, O.; Wollnik, B.; Keegan, C.E.; Innis, J.W.; Dinulos, M.B.; Christian, C.; Hannibal, M.C.; et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 2003, 72, 408–418. [Google Scholar] [CrossRef] [PubMed]
- McConville, D.O.; Archbold, G.P.; Lewis, A.; Morrison, P.J. Zygodactyly (Syndactyly Type A1) Associated With Midfoot Charcot Neuropathy and Diabetes. Diabetes Care 2018, 41, e74–e75. [Google Scholar] [CrossRef]
- Carroll, S.B. Homeotic genes and the evolution of arthropods and chordates. Nature 1995, 376, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Quinonez, S.C.; Innis, J.W. Human HOX gene disorders. Mol. Genet. Metab. 2014, 111, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barcelo, M.M.; Wong, K.K.; Lui, V.C.; Yuan, Z.W.; So, M.T.; Ngan, E.S.; Miao, X.P.; Chung, P.H.; Khong, P.L.; Tam, P.K. Identification of a HOXD13 mutation in a VACTERL patient. Am. J. Med. Genet. A 2008, 146A, 3181–3185. [Google Scholar] [CrossRef]
- Kjaer, K.W.; Hansen, L.; Eiberg, H.; Utkus, A.; Skovgaard, L.T.; Leicht, P.; Opitz, J.M.; Tommerup, N. A 72-year-old Danish puzzle resolved--comparative analysis of phenotypes in families with different-sized HOXD13 polyalanine expansions. Am. J. Med. Genet. A 2005, 138, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lin, P.F.; Wang, Q.M.; Mao, F.; Cai, Y.; Gong, Y.Q. Synpolydactyly in a Chinese kindred: Mutation detection, prenatal ultrasonographic and molecular diagnosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2011, 28, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Vaccari, G.; Brison, N.; Debeer, P.; Tylzanowski, P.; Zappavigna, V. A G220V substitution within the N-terminal transcription regulating domain of HOXD13 causes a variant synpolydactyly phenotype. Hum. Mol. Genet. 2009, 18, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Zaib, T.; Ji, W.; Saleem, K.; Nie, G.; Li, C.; Cao, L.; Xu, B.; Dong, K.; Yu, H.; Hao, X.; et al. A heterozygous duplication variant of the HOXD13 gene caused synpolydactyly type 1 with variable expressivity in a Chinese family. BMC Med. Genet. 2019, 20, 203. [Google Scholar] [CrossRef]
- Guo, X.; Shi, T.; Lin, M.; Zhang, Y. A Nonsense Mutation in HOXD13 Gene from A Chinese Family with Non-Syndromic Synpolydactyly. Tohoku J. Exp. Med. 2019, 249, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, N.; Geng, J.; Wang, Z.; Fu, Q.; Wang, J.; Xu, Y. Exome sequencing identifies a novel nonsense mutation of HOXD13 in a Chinese family with synpolydactyly. Congenit. Anom. 2017, 57, 4–7. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Tayebi, N.; Knaus, A.; Stiege, A.C.; Sahebzamani, A.; Hecht, J.; Mundlos, S.; Spielmann, M. A homozygous HOXD13 missense mutation causes a severe form of synpolydactyly with metacarpal to carpal transformation. Am. J. Med. Genet. A 2016, 170, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kurban, M.; Wajid, M.; Petukhova, L.; Shimomura, Y.; Christiano, A.M. A nonsense mutation in the HOXD13 gene underlies synpolydactyly with incomplete penetrance. J. Hum. Genet. 2011, 56, 701–706. [Google Scholar] [CrossRef][Green Version]
- Al-Qattan, M.M. A Review of the Phenotype of Synpolydactyly Type 1 in Homozygous Patients: Defining the Relatively Long and Medially Deviated Big Toe with/without Cupping of the Forefoot as a Pathognomonic Feature in the Phenotype. Biomed. Res. Int. 2020, 2020, 2067186. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Fang, X.; Mao, H.; Sun, B.; Zhou, J.; An, Y.; Wang, B. A Novel Missense Variant of HOXD13 Caused Atypical Synpolydactyly by Impairing the Downstream Gene Expression and Literature Review for Genotype-Phenotype Correlations. Front. Genet. 2021, 12, 731278. [Google Scholar] [CrossRef] [PubMed]
- Salsi, V.; Zappavigna, V. Hoxd13 and Hoxa13 directly control the expression of the EphA7 Ephrin tyrosine kinase receptor in developing limbs. J. Biol. Chem. 2006, 281, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Zu, B.; Wang, Z.; Xu, Y.; You, G.; Fu, Q. Nonframeshifting indel variations in polyalanine repeat of HOXD13 gene underlies hereditary limb malformation for two Chinese families. Dev. Dyn. 2021, 250, 1220–1228. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Wang, J.; Yu, H.; Ma, X.; Yang, J. Polyalanine repeat expansion mutation of the HOXD13 gene in a Chinese family with unusual clinical manifestations of synpolydactyly. Eur. J. Med. Genet. 2011, 54, 108–111. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Nayak, S.S.; Pai, M.V.; Shukla, A.; Girisha, K.M. Occurrence of Synpolydactyly and Omphalocele in a Fetus with a HOXD13 Mutation. J. Pediatr. Genet. 2017, 6, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ji, C.; Cao, L.; Wu, Y.; Shang, Y.; Wang, W.; Luo, Y. A splice donor site mutation in HOXD13 underlies synpolydactyly with cortical bone thinning. Gene 2013, 532, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.J.; Hansen, S.L.; Jones, N.F. Reconstruction of congenital differences of the hand. Plast. Reconstr. Surg. 2009, 124, 128e–143e. [Google Scholar] [CrossRef]
- Dao, K.D.; Shin, A.Y.; Billings, A.; Oberg, K.C.; Wood, V.E. Surgical treatment of congenital syndactyly of the hand. J. Am. Acad. Orthop. Surg. 2004, 12, 39–48. [Google Scholar] [CrossRef]
- Swarup, I.; Zhang, Y.; Do, H.; Daluiski, A. Epidemiology of syndactyly in New York State. World J. Orthop. 2019, 10, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kvernmo, H.D.; Haugstvedt, J.R. Treatment of congenital syndactyly of the fingers. Tidsskr. Den Nor. Legeforening 2013, 133, 1591–1595. [Google Scholar] [CrossRef]
- Goldfarb, C.A.; Steffen, J.A.; Stutz, C.M. Complex syndactyly: Aesthetic and objective outcomes. J. Hand Surg. Am. 2012, 37, 2068–2073. [Google Scholar] [CrossRef]
- Little, K.J.; Cornwall, R. Congenital Anomalies of the Hand--Principles of Management. Orthop. Clin. N. Am. 2016, 47, 153–168. [Google Scholar] [CrossRef]
- Mandarano-Filho, L.G.; Bezuti, M.T.; Akita, R.; Mazzer, N.; Barbieri, C.H. Congenital syndactyly: Case by case analysis of 47 patients. Acta Ortopédica Bras. 2013, 21, 333–335. [Google Scholar] [CrossRef]
- Hikosaka, M.; Ogata, H.; Nakajima, T.; Kobayashi, H.; Hattori, N.; Onishi, F.; Tamada, I. Advantages of open treatment for syndactyly of the foot: Defining its indications. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Gawlikowska-Sroka, A. Polydactyly and syndactyly as the most common congenital disorders of the limbs. Ann. Acad. Med. Stetin. 2008, 54, 130–133. [Google Scholar]
- Geoghegan, L.; Knowles, B.G.; Nikkhah, D. Syndactyly. J. Surg. Case Rep. 2020, 2020, rjaa517. [Google Scholar] [CrossRef]
- Senda, E.; Ueda, K.; Hirota, Y.; Mitsuno, D.; Nuri, T. Using Dermal Fat Graft to Release Complex Syndactyly: A New Method. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3068. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.; Song, B. The use of abdominal flaps for complex syndactyly release: A case series. J. Hand Surg. 2021, 46, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, M.; Oshima, J.; Aihara, Y.; Sekido, M. Aesthetic reconstruction for syndactyly using the “gradation skin graft” from the plantar instep area. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Langlais, T.; Rougereau, G.; Marty-Diloy, T.; Bachy, M.; Barret, H.; Vialle, R.; Fitoussi, F. Surgical treatment in child’s congenital toe syndactyly: Risk factor of recurrence, complication and poor clinical outcomes. Foot Ankle Surg. 2022, 28, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.P.; Jones, N.F. Interdigitating Rectangular Flaps and Dorsal Pentagonal Island Flap for Syndactyly Release. J. Hand Surg. Am. 2019, 44, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, S.; Li, N.; Feng, Z.; Liu, Q. Dorsal Hexagon Local Flap Without Skin Graft for Web Reconstruction of Congenital Syndactyly. J. Hand Surg. Am. 2020, 45, 63.e61–63.e69. [Google Scholar] [CrossRef]
- Yuan, F.; Zhong, L.; Chung, K.C. Aesthetic Comparison of Two Different Types of Web-Space Reconstruction for Finger Syndactyly. Plast. Reconstr. Surg. 2018, 142, 963–971. [Google Scholar] [CrossRef]
- Tang, H.; Sun, G.; Qi, J.; Nie, K.; Jin, W.; Li, S.; Wei, Z.; Wang, D. Surgical Treatment of Congenital Type V Thumb Syndactyly. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 1127–1129. [Google Scholar] [CrossRef]
- Kong, B.Y.; Baek, G.H.; Gong, H.S. Treatment of keloid formation following syndactyly division: Surgical technique. Hand Surg. 2012, 17, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Chouairi, F.; Mercier, M.R.; Persing, J.S.; Gabrick, K.S.; Clune, J.; Alperovich, M. National Patterns in Surgical Management of Syndactyly: A Review of 956 Cases. Hand 2020, 15, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escrig, A.; Gobernado, I.; Sanchez-Herranz, A. Whole genome sequencing: A qualitative leap forward in genetic studies. Rev. Neurol. 2012, 54, 692–698. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).