Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The BSs of miRNAs in mRNAs of Genes Having Dinucleotide Repeats Associated with Neurodegenerative Disorders
3.2. The BSs of miRNAs in mRNAs of Genes Having Dinucleotide Repeats Associated with Oncological Diseases
3.3. The BSs of miRNAs in mRNAs of Genes Having Dinucleotide Repeats Associated with Diabetes
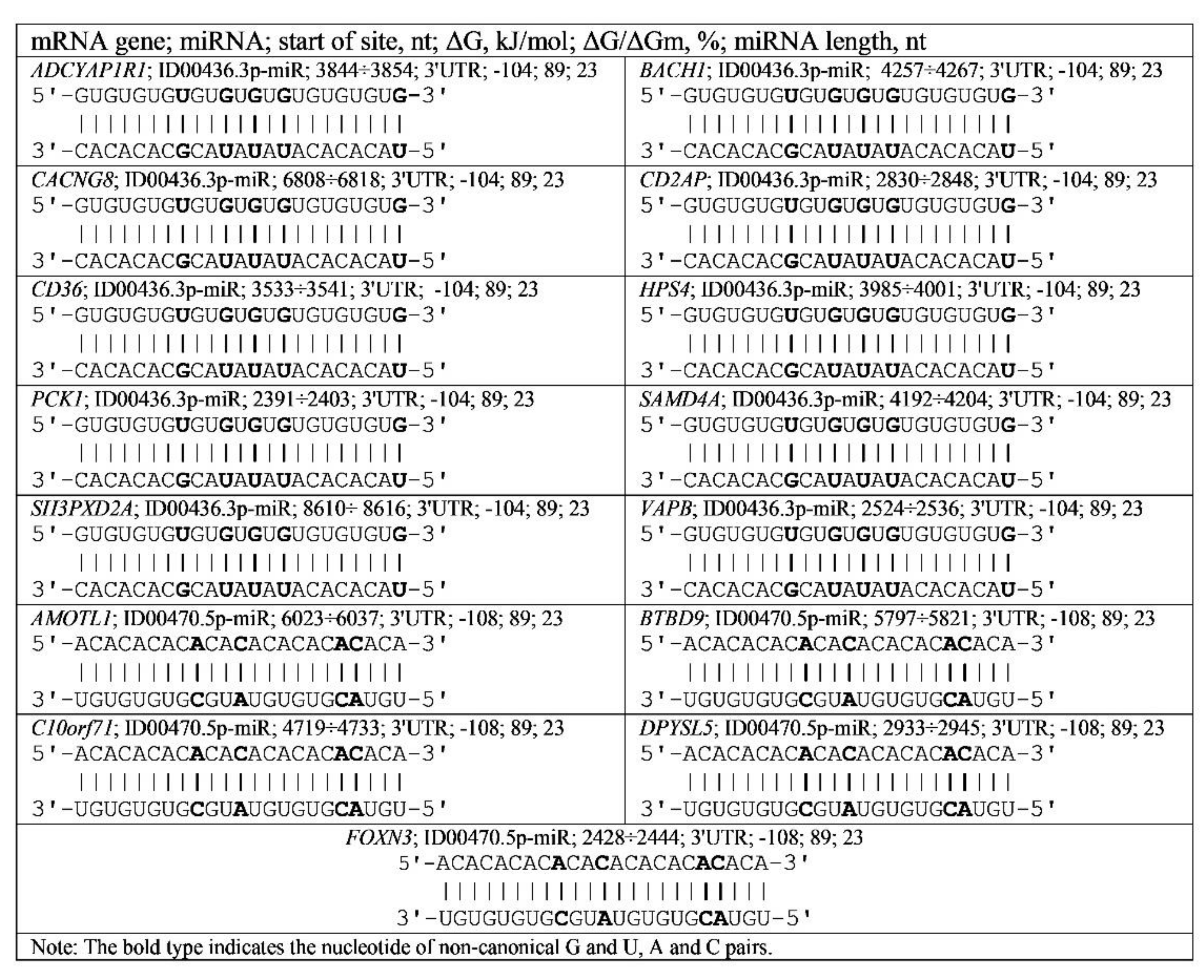
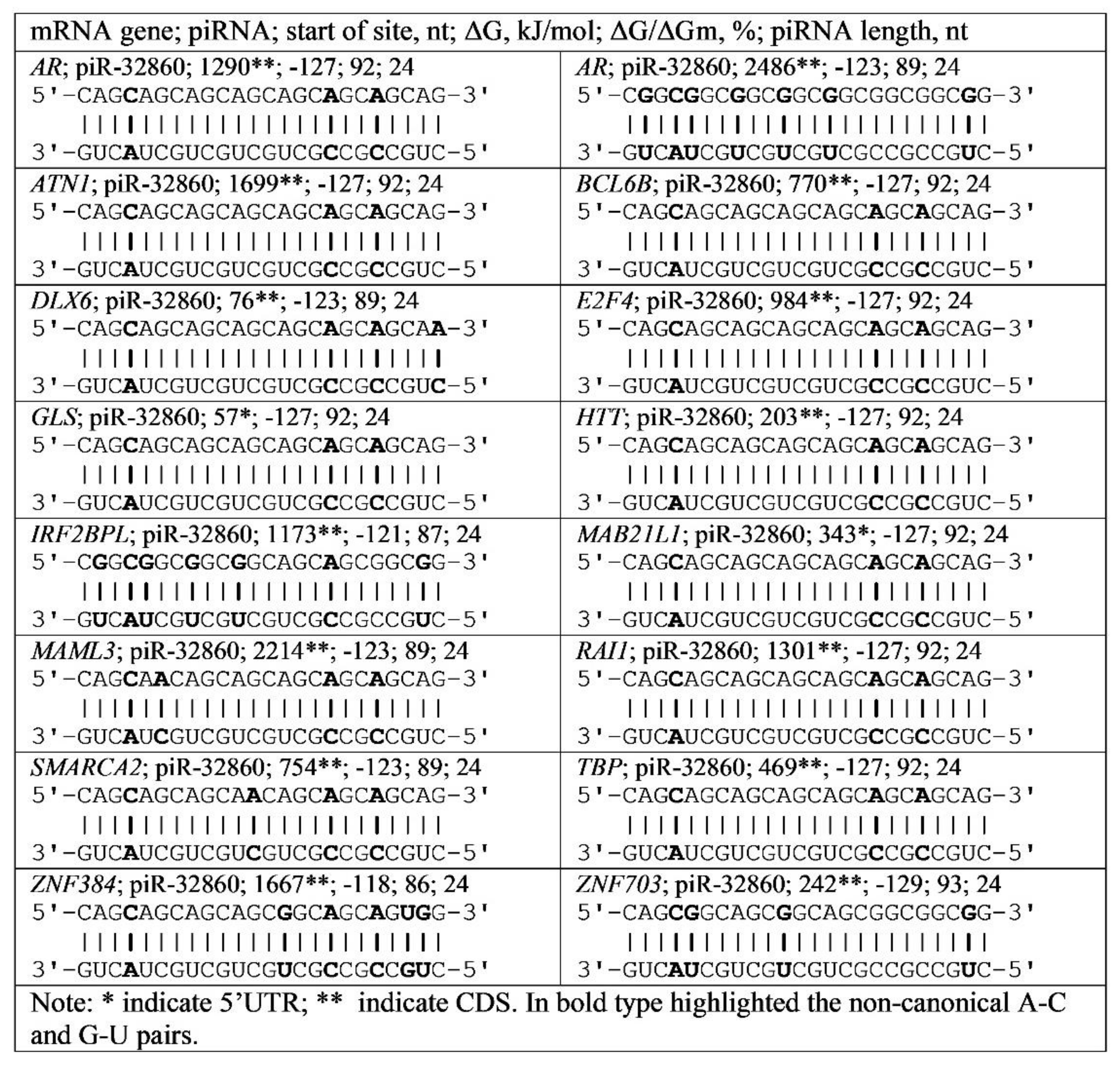
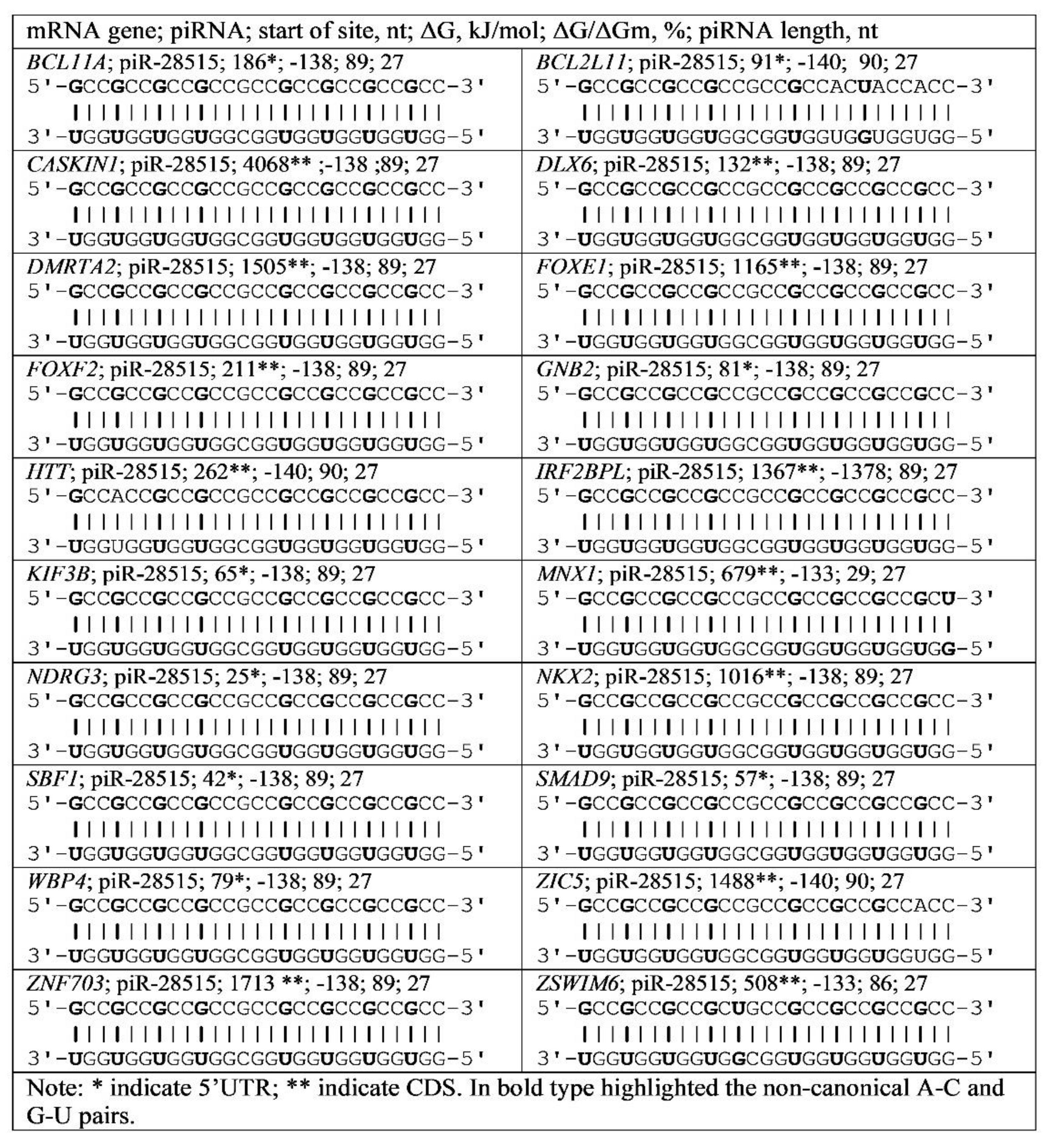
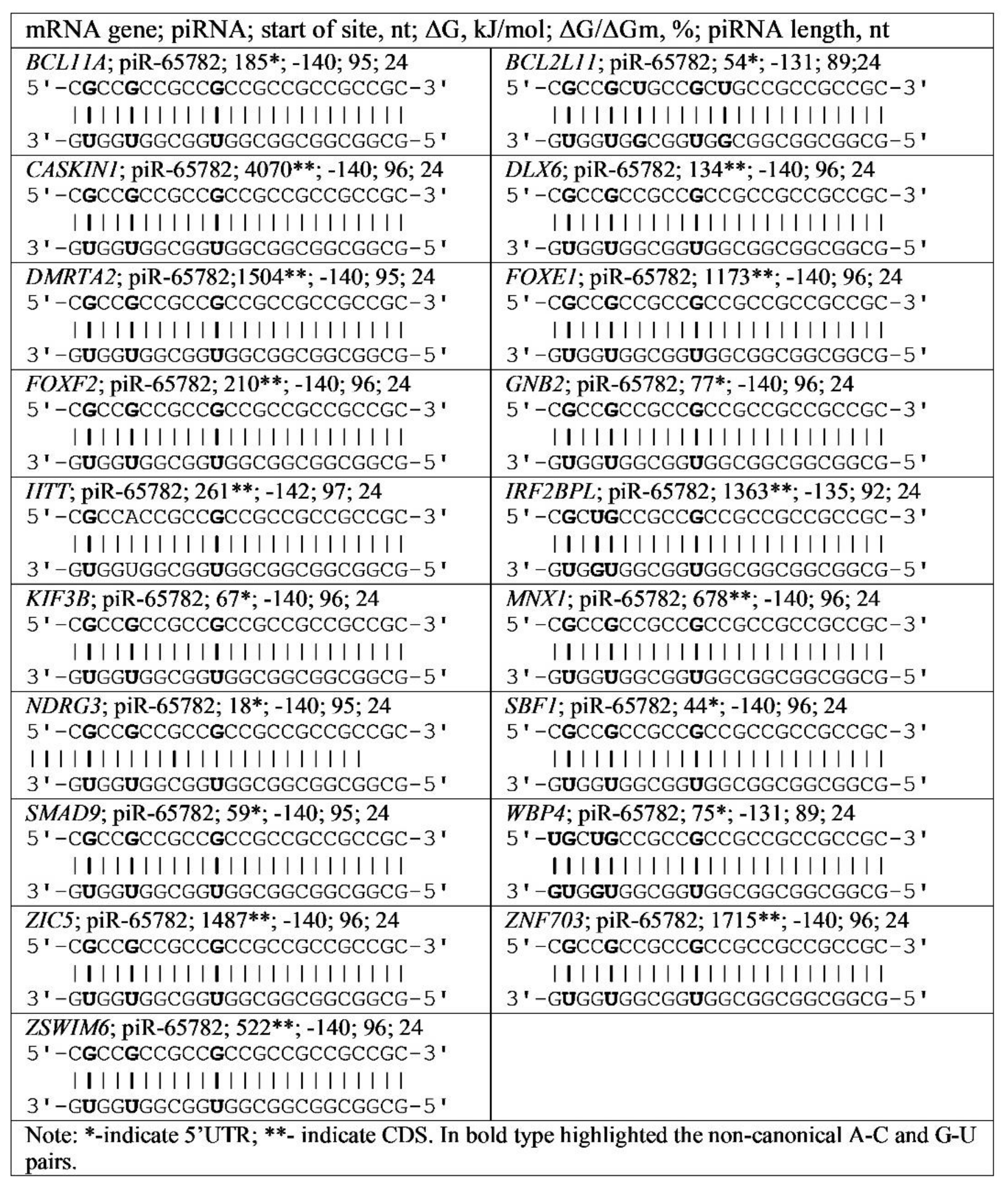
3.4. Binding of miRNA and piRNA to mRNA of Genes with Trinucleotide Repeats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordick, K.; Khalili, Y. Genetics, Trinucleotide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545250/ (accessed on 5 January 2022).
- Kozlowski, P.; de Mezer, M.; Krzyzosiak, W.J. Trinucleotide repeats in human genome and exome. Nucleic Acids Res. 2010, 38, 4027–4039. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5′UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Schuster, S.L.; Hsieh, A.C. The Untranslated Regions of mRNAs in Cancer. Trends Cancer 2019, 5, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [Green Version]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [Green Version]
- Oliveto, S.; Mancino, M.; Manfrini, N.; Biffo, S. Role of microRNAs in translation regulation and cancer. World. J. Biol. Chem. 2017, 8, 45–56. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules 2020, 10, 1285. [Google Scholar] [CrossRef]
- Ivashchenko, A.; Berillo, O.; Pyrkova, A.; Niyazova, R. Binding sites of miR-1273 family on the mRNA of target genes. BioMed. Res. Int. 2014, 2014, 620530. [Google Scholar] [CrossRef] [Green Version]
- Aisina, D.; Niyazova, R.; Atambayeva, S.; Ivashchenko, A. Prediction of clusters of miRNA binding sites in mRNA candidate genes of breast cancer subtypes. PeerJ 2019, 7, e8049. [Google Scholar] [CrossRef]
- Kamenova, S.; Aralbayeva, A.; Kondybayeva, A.; Akimniyazova, A.; Pyrkova, A.; Ivashchenko, A. Evolutionary Changes in the Interaction of miRNA with mRNA of Candidate Genes for Parkinson’s Disease. Front. Genet. 2021, 12, 647288. [Google Scholar] [CrossRef]
- Mukushkina, D.; Aisina, D.; Pyrkova, A.; Ryskulova, A.; Labeit, S.; Ivashchenko, A. In silico Prediction of miRNA Interactions with Candidate Atherosclerosis Gene mRNAs. Front. Genet. 2020, 11, 605054. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Cong, S. MicroRNAs in Huntington’s Disease: Diagnostic Biomarkers or Therapeutic Agents? Front. Cell Neurosci. 2021, 15, 705348. [Google Scholar] [CrossRef] [PubMed]
- Rayford, K.J.; Cooley, A.; Rumph, J.T.; Arun, A.; Rachakonda, G.; Villalta, F.; Lima, M.F.; Pratap, S.; Misra, S.; Nde, P.N. piRNAs as Modulators of Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 2373. [Google Scholar] [CrossRef]
- Barreñada, O.; Larriba, E.; Brieño-Enriquez, M.A.; Mazo, J.D. piRNA-IPdb: A PIWI-bound piRNAs database to mining NGS sncRNA data and beyond. BMC Genom. 2021, 22, 765. [Google Scholar] [CrossRef]
- Huang, S.; Yoshitake, K.; Asakawa, S. A Review of Discovery Profiling of PIWI-Interacting RNAs and Their Diverse Functions in Metazoans. Int. J. Mol. Sci. 2021, 22, 11166. [Google Scholar] [CrossRef]
- Chetta, M.; Di Pietro, L.; Bukvic, N.; Lattanzi, W. Rising Roles of Small Noncoding RNAs in Cotranscriptional Regulation: In Silico Study of miRNA and piRNA Regulatory Network in Humans. Genes 2020, 11, 482. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, M.; Song, X.; Dong, Y.; Liu, S.; Liu, H.; Tao, J.; Li, W.; Yin, X.; Xu, W. The emerging role of the piRNA/piwi complex in cancer. Mol. Cancer 2019, 18, 123. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Han, X. The disease-related biological functions of PIWI-interacting RNAs (piRNAs) and underlying molecular mechanisms. ExRNA 2019, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Liu, Y.; Zhang, T.; Cong, S. Integrated analysis of differentially expressed genes and construction of a competing endogenous RNA network in human Huntington neural progenitor cells. BMC Med. Genom. 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Massey, T.H.; Jones, L. The central role of DNA damage and repair in CAG repeat diseases. Dis. Model Mech. 2018, 11, dmm031930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.; Marks, L.; May, G.; Wilson, J.B. The genetic basis of disease. Essays Biochem. 2018, 62, 643–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashchenko, A.T.; Pyrkova, A.Y.; Niyazova, R.Y.; Alybayeva, A.; Baskakov, K. Prediction of miRNA Minding Sites in mRNA. Bioinformation 2016, 12, 237–240. [Google Scholar] [CrossRef]
- Friedman, R.A.; Honig, B.A. Free Energy Analysis of Nucleic Acid Base Stacking in Aqueous Solution. Biophys. J. 1995, 69, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Heinemann, U. A Novel Form of RNA Double Helix Based on G·U and C·A+ Wobble Base Pairing. RNA 2018, 24, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Kanoria, S.; Rennie, W.; Liu, S.; Carmack, C.S.; Lu, J.; Ding, Y. STarMir Tools for Prediction of microRNA Binding Sites. Methods Mol. Biol. 2016, 1490, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Leontis, N.B.; Stombaugh, J.; Westhof, E. The Non-watson-crick Base Pairs and Their Associated Isostericity Matrices. Nucleic Acids Res. 2002, 30, 3497–3531. [Google Scholar] [CrossRef]
- Davis, E.; Caiment, F.; Tordoir, X.; Cavaillé, J.; Ferguson-Smith, A.; Cockett, N.; Georges, M.; Charlier, C. RNAi-Mediated Allelic Trans-interaction at the Imprinted Rtl1/ Peg11 Locus. Curr. Biol. 2005, 15, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Z.; Liu, B.; Chen, G.; Shao, N.; Ying, X.; Wang, Y. Systematic Study of Cis-Antisense miRNAs in Animal Species Reveals miR-3661 to Target PPP2CA in Human Cells. RNA 2016, 22, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurikova, O.Y.; Aisina, D.E.; Niyazova, R.E.; Atambayeva, S.A.; Labeit, S.; Ivashchenko, A.T. The Interactions of miRNA-5p and miRNA-3p with the mRNAs of Ortolologous Genes. Mol. Biol. 2019, 53, 692–704. [Google Scholar] [CrossRef]
- Paulson, H. Repeat expansion diseases. Handb. Clin. Neurol. 2018, 147, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Gil, P.; Caballero, C.J.; Catalan-Moreno, A.; Irurzun, N.; Barrio-Hernandez, I.; Caldelari, I.; Toledo-Arana, A. Di ferential evolution in 3′UTRs leads to specific gene expression in Staphylococcus. Nucleic Acids Res. 2020, 48, 2544–2563. [Google Scholar] [CrossRef] [Green Version]
- Ajjugal, Y.; Kolimi, N.; Rathinavelan, T. Secondary structural choice of DNA and RNA associated with CGG/CCG trinucleotide repeat expansion rationalizes the RNA misprocessing in FXTAS. Sci. Rep. 2021, 11, 8163. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, I.; McMurray, C. Features of trinucleotide repeat instability in vivo. Cell Res. 2008, 18, 198–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproviero, W.; Shatunov, A.; Stahl, D.; Shoai, M.; van Rheenen, W.; Jones, A.R.; Al-Sarraj, S.; Andersen, P.M.; Bonini, N.M.; Conforti, F.L.; et al. ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol. Aging 2017, 51, 178.e1–178.e9. [Google Scholar] [CrossRef]
- Bae, B.; Miura, P. Emerging Roles for 3′ UTRs in Neurons. Int. J. Mol. Sci. 2020, 21, 3413. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, R.H.; Du, K.; Zhu, J.; Zheng, J.; Xie, L.H.; Pereira, A.A.; Zhang, C.; Ricci, E.P.; Li, X.Z. Coupled protein synthesis and ribosome-guided piRNA processing on mRNAs. Nat. Commun. 2021, 12, 5970. [Google Scholar] [CrossRef]
- Du, W.; Yang, W.; Xuan, J.; Gupta, S.; Krylov, S.N.; Ma, X.; Yang, Q.; Yang, B.B. Reciprocal regulation of miRNAs and piRNAs in embryonic development. Cell Death Differ. 2016, 23, 1458–1470. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkozhayev, A.; Niyazova, R.; Wilson, C.; Jainakbayev, N.; Pyrkova, A.; Ashirbekov, Y.; Akimniyazova, A.; Sharipov, K.; Ivashchenko, A. Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats. Genes 2022, 13, 800. https://doi.org/10.3390/genes13050800
Belkozhayev A, Niyazova R, Wilson C, Jainakbayev N, Pyrkova A, Ashirbekov Y, Akimniyazova A, Sharipov K, Ivashchenko A. Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats. Genes. 2022; 13(5):800. https://doi.org/10.3390/genes13050800
Chicago/Turabian StyleBelkozhayev, Ayaz, Raigul Niyazova, Cornelia Wilson, Nurlan Jainakbayev, Anna Pyrkova, Yeldar Ashirbekov, Aigul Akimniyazova, Kamalidin Sharipov, and Anatoliy Ivashchenko. 2022. "Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats" Genes 13, no. 5: 800. https://doi.org/10.3390/genes13050800
APA StyleBelkozhayev, A., Niyazova, R., Wilson, C., Jainakbayev, N., Pyrkova, A., Ashirbekov, Y., Akimniyazova, A., Sharipov, K., & Ivashchenko, A. (2022). Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats. Genes, 13(5), 800. https://doi.org/10.3390/genes13050800







