Multi-Objective Artificial Bee Colony Algorithm Based on Scale-Free Network for Epistasis Detection
Abstract
1. Introduction
2. Methods
2.1. Scale-Free Network
- (1)
- Growth: Start with a small fully connected network which has nodes, and gradually add new nodes one at a time.
- (2)
- Connection: Assume that the original network already has nodes . When a new node is added, it connects links to the original nodes, where .
- (3)
- Priority connection: The connection strategy gives priority to the nodes with a higher degree. For an original node , the probability that the new node is connected to it can be described as
2.2. Artificial Bee Colony Algorithm
2.3. Multi-Objective Artificial Bee Colony Algorithm Based on the Scale-Free Network
2.3.1. Objective Function
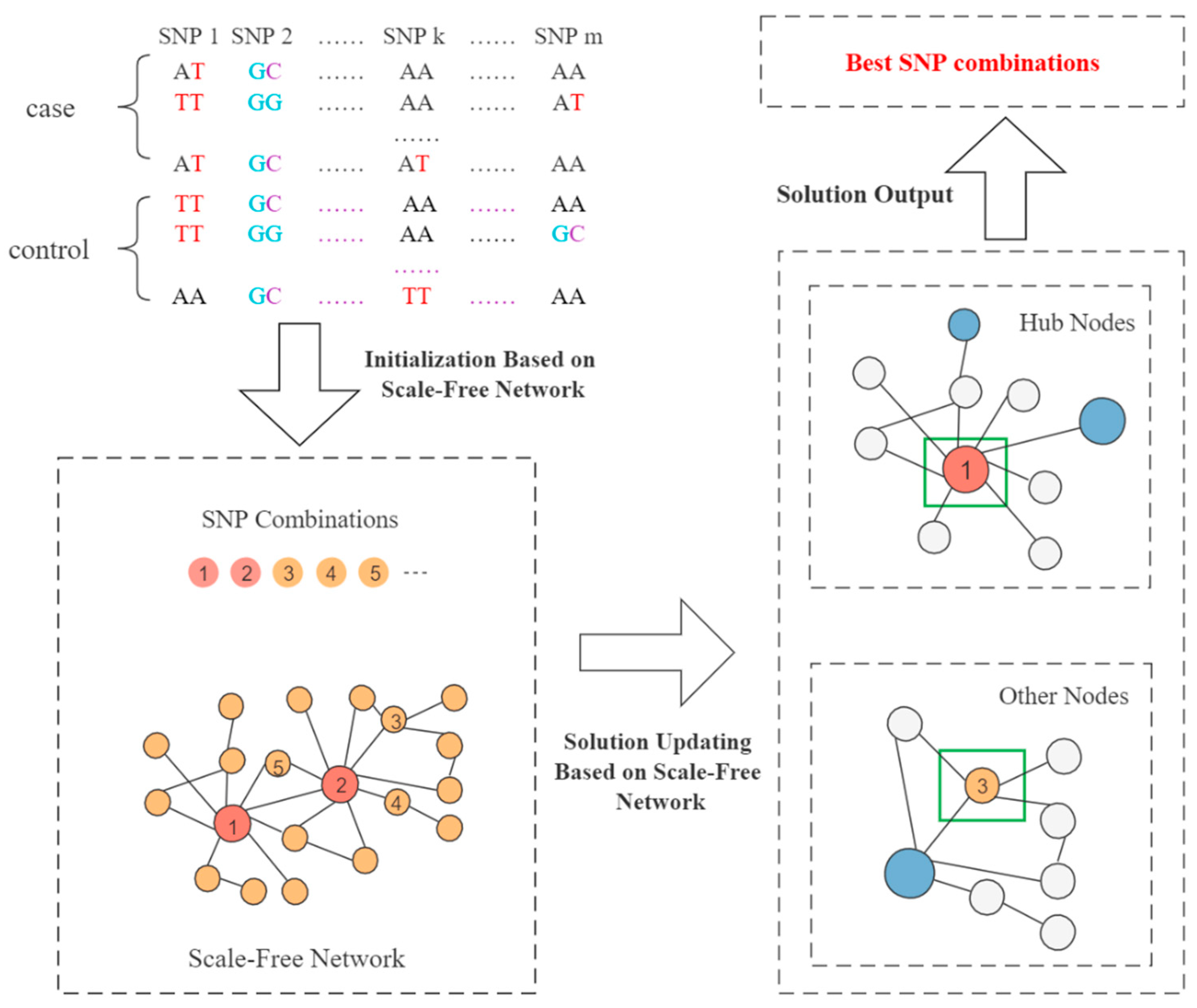
2.3.2. Initialization Based on the Scale-Free Network
2.3.3. Solution Updating Based on the Scale-Free Network
2.3.4. Time Complexity Analysis
2.3.5. Overall Framework
3. Experiments
3.1. Evaluation Measures
| Algorithm 1: SFMOABC |
| Input: the number of the food sources ; the dimension of problems ; a count parameter representing the number of times the current solution has not been improved ; the maximum number of not be improved ; the number of hub nodes in the scale-free network ; the number of edges when the node joins the network . Output: the optimal solution . 01. Initialize food sources to form a population ; 02. Calculate the fitness value of each solution ; 03. Build a scale-free network with node, and number each node in the network. 04. While the stopping criteria is not satisfied do 05. for do 06. if then 07. ; 08. Find the neighbor with the largest degree of ; 09. 10. ; 11. Calculate the fitness value of the . 12. ; 13. Calculate the fitness value of ; 14. if then 15. ; ; 16. else 17. ; ; 18. end 19. if then 20. ; ; 21. else 22. ; 23. end 24. else 25. ; 26. Find the neighbor with the largest degree of ; 27. ; 28. Repeat (Steps 10–23) 29. end 30. end 31. Calculate the probability of ; 32. ; ; 33. while do 34. if then 35. repeat (Steps 06–30) 36. end 37. ; 38. if then 39. ; 40. end 41. end 42. Find the individual with the maximum trail value; 43. if then 44. Randomly generated a new food source to replace the -th food source; 45. end end |
| Return the optimal solution with the largest fitness value. |
3.2. Simulation Data
3.3. Parameter Settings
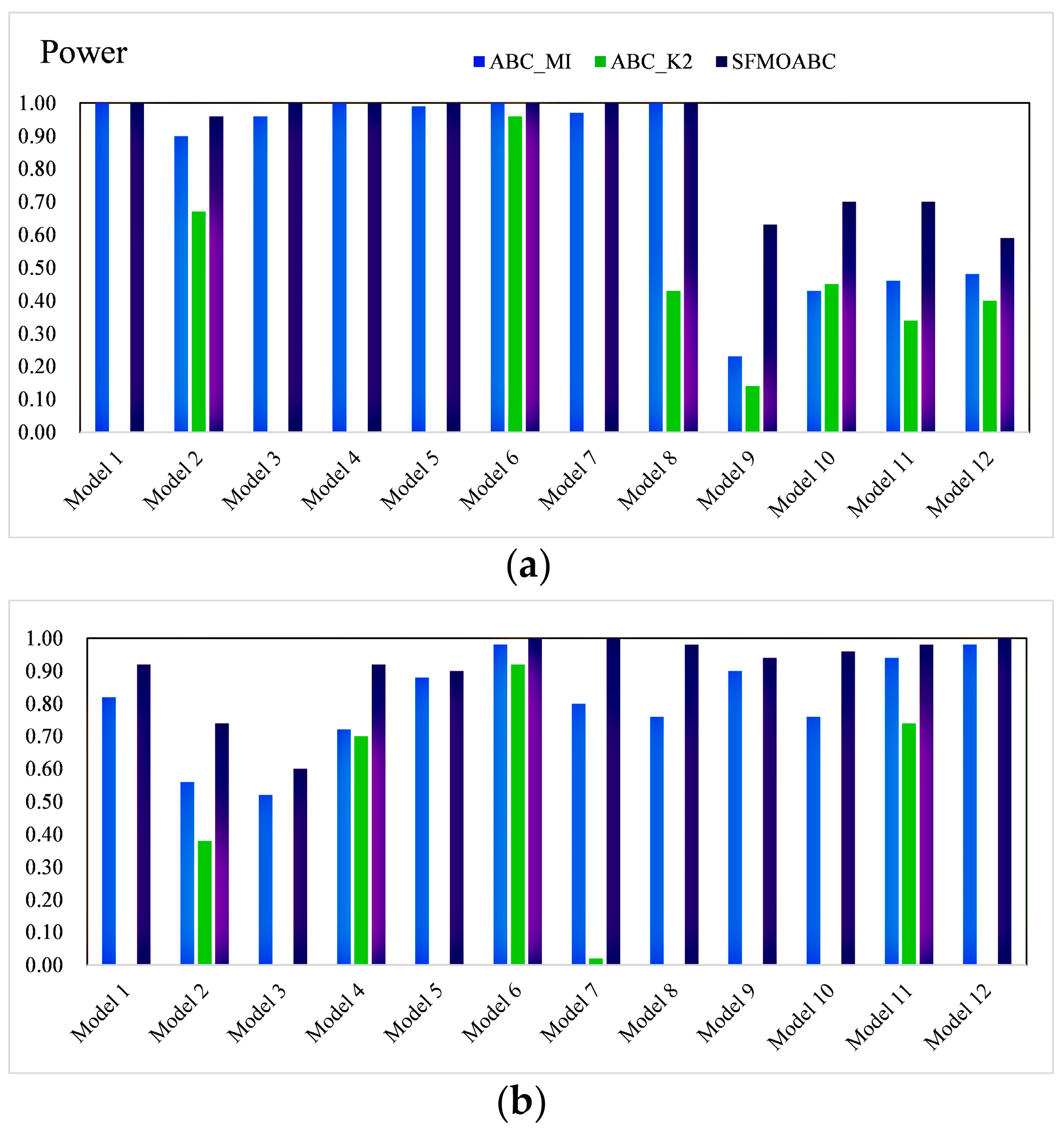
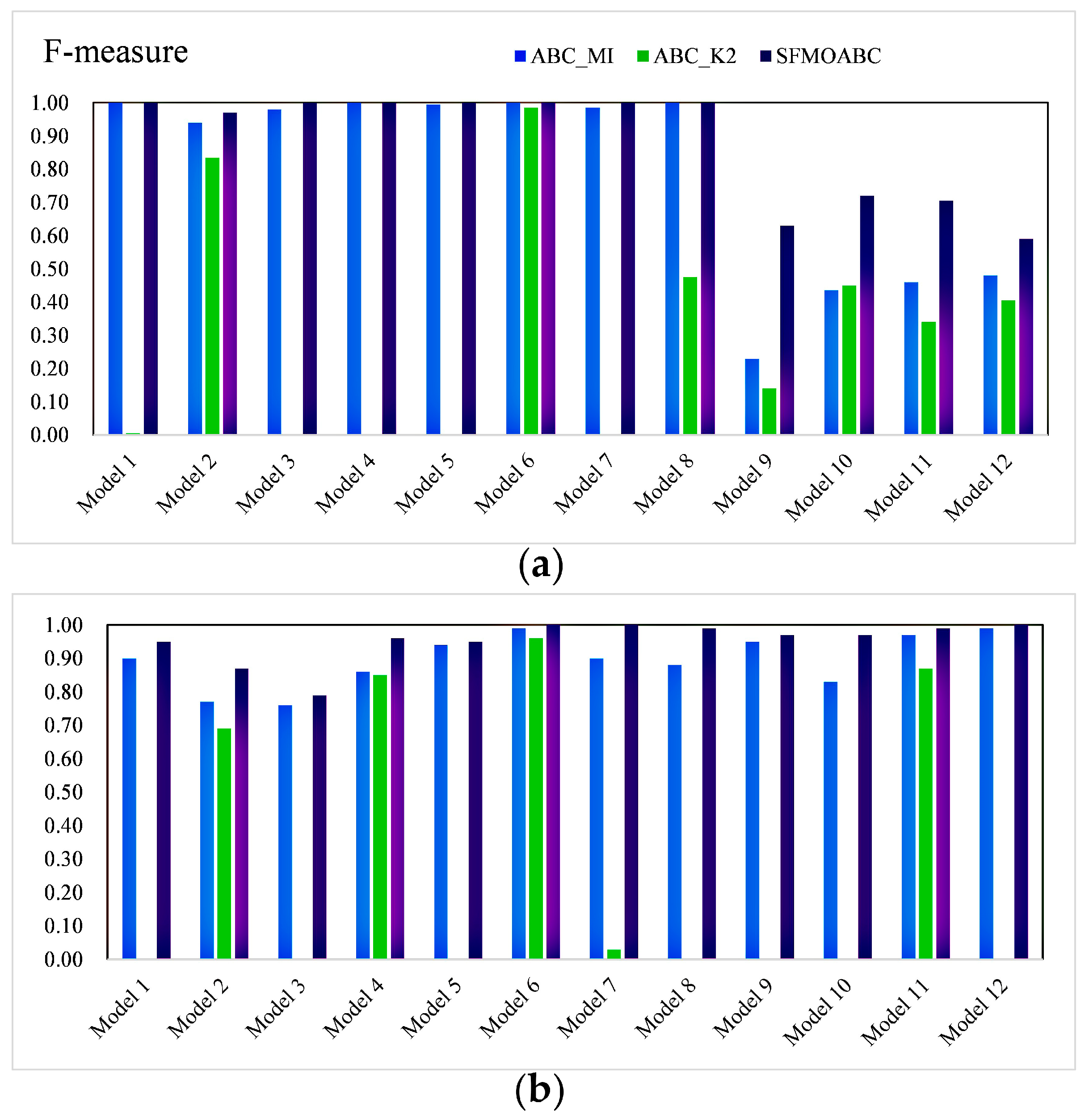
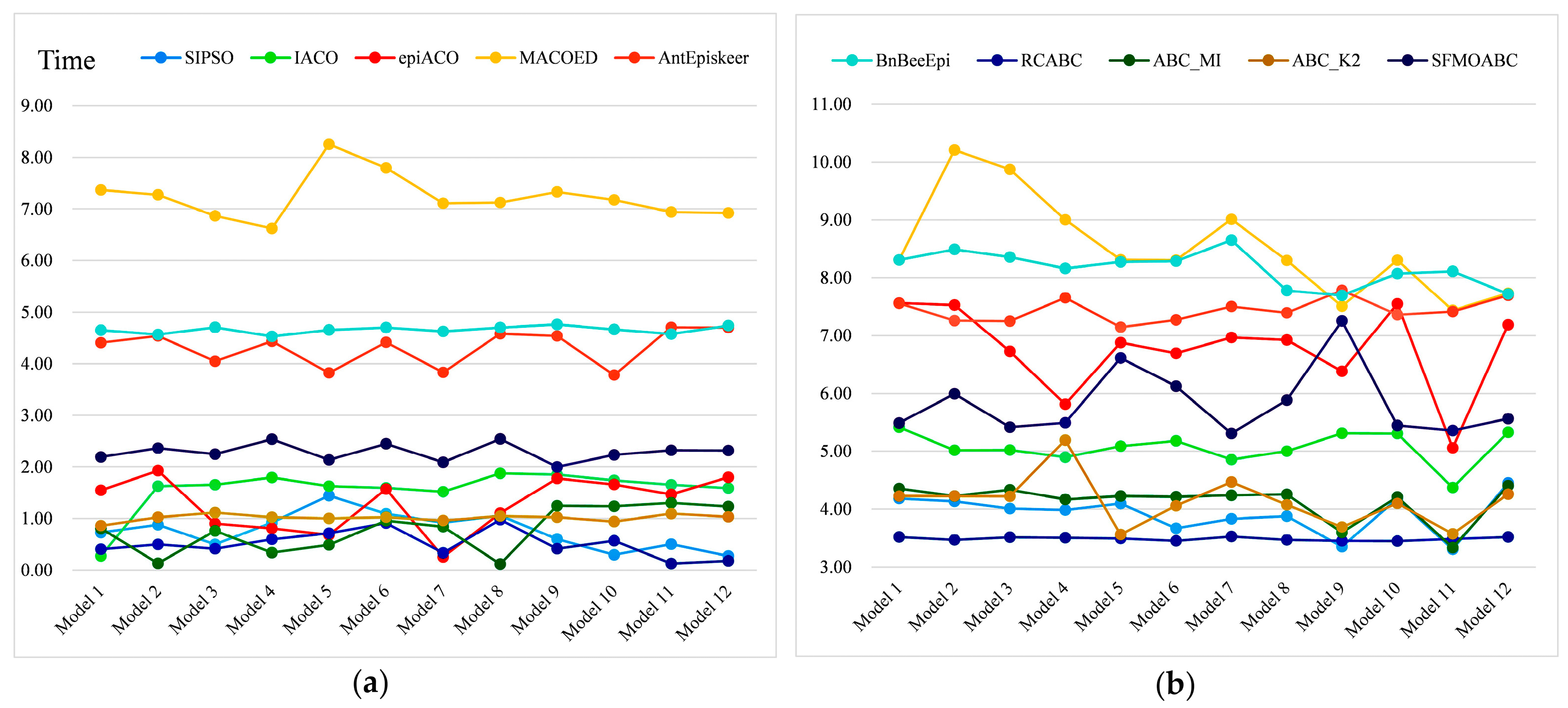
3.4. Experimental Results on Simulation Data
3.5. Experiment Results for Real AMD Data
4. Discussion
5. Conclusions
5.1. Advantages
5.2. Limitations
5.3. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, J.H.; Asselbergs, F.W.; Williams, S.M. Bioinformatics challenges for genome-wide association studies. Bioinformatics 2010, 26, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shang, J.; Liu, J.-X.; Li, S.; Zheng, C.-H. Epiaco—A method for identifying epistasis based on ant colony optimization algorithm. BioData Min. 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhang, J.; Lei, X.; Zhang, Y.; Chen, B. Incorporating heuristic information into ant colony optimization for epistasis detection. Genes Genom. 2012, 34, 321–327. [Google Scholar] [CrossRef]
- Shang, J.; Sun, Y.; Liu, J.-X.; Xia, J.; Zhang, J.; Zheng, C.-H. Cinoedv: A co-information based method for detecting and visualizing n-order epistatic interactions. BMC Bioinform. 2016, 17, 214. [Google Scholar] [CrossRef]
- Ding, X.; Wang, J.; Zelikovsky, A.; Guo, X.; Xie, M.; Pan, Y. Searching high-order snp combinations for complex diseases based on energy distribution difference. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 12, 695–704. [Google Scholar] [CrossRef]
- Jiang, X.; Neapolitan, R.E.; Barmada, M.M.; Visweswaran, S. Learning genetic epistasis using bayesian network scoring criteria. BMC Bioinform. 2011, 12, 89. [Google Scholar] [CrossRef]
- Han, B.; Chen, X.-W. In Bneat: A bayesian network method for detecting epistatic interactions in genome-wide association studies. In BMC Genomics; BioMed Central: Hong Kong, China, 2011; pp. 1–8. [Google Scholar]
- Upstill-Goddard, R.; Eccles, D.; Fliege, J.; Collins, A. Machine learning approaches for the discovery of gene–gene interactions in disease data. Brief. Bioinform. 2013, 14, 251–260. [Google Scholar] [CrossRef]
- Wan, X.; Yang, C.; Yang, Q.; Xue, H.; Tang, N.L.; Yu, W. Predictive rule inference for epistatic interaction detection in genome-wide association studies. Bioinformatics 2010, 26, 30–37. [Google Scholar] [CrossRef]
- Wan, X.; Yang, C.; Yang, Q.; Xue, H.; Fan, X.; Tang, N.L.; Yu, W. Boost: A fast approach to detecting gene-gene interactions in genome-wide case-control studies. Am. J. Hum. Genet. 2010, 87, 325–340. [Google Scholar] [CrossRef]
- Ponte-Fernández, C.; González-Domínguez, J.; Carvajal-Rodríguez, A.; Martin, M.J. Evaluation of existing methods for high-order epistasis detection. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 19, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, X.; Wu, X.; Sun, Y.; Ding, Q.; Liu, J.-X.; Zhang, H. A review of ant colony optimization based methods for detecting epistatic interactions. IEEE Access 2019, 7, 13497–13509. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Robbins, K.; Rekaya, R. Antepiseeker: Detecting epistatic interactions for case-control studies using a two-stage ant colony optimization algorithm. BMC Res. Notes 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Shang, J.; Liu, J.-X.; Zheng, C.-H.; Lei, X. Introducing heuristic information into ant colony optimization algorithm for identifying epistasis. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 17, 1253–1261. [Google Scholar] [CrossRef]
- Zhang, W.; Shang, J.; Li, H.; Sun, Y.; Liu, J.-X. SIPSO: Selectively informed particle swarm optimization based on mutual information to determine snp-snp interactions. In International Conference on Intelligent Computing; Springer: Cham, Switzerland, 2016; pp. 112–121. [Google Scholar]
- Tuo, S. Fdhe-iw: A fast approach for detecting high-order epistasis in genome-wide case-control studies. Genes 2018, 9, 435. [Google Scholar] [CrossRef]
- Aflakparast, M.; Salimi, H.; Gerami, A.; Dubé, M.; Visweswaran, S.; Masoudi-Nejad, A. Cuckoo search epistasis: A new method for exploring significant genetic interactions. Heredity 2014, 112, 666–674. [Google Scholar] [CrossRef]
- Tuo, S.; Liu, H.; Chen, H. Multipopulation harmony search algorithm for the detection of high-order snp interactions. Bioinformatics 2020, 36, 4389–4398. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Pian, C.; Xu, M.; Kong, L.; Fang, J.; Li, Z.; Zhang, L. Epimoga: An epistasis detection method based on a multi-objective genetic algorithm. Genes 2021, 12, 191. [Google Scholar] [CrossRef]
- Pashaei, E.; Pashaei, E. Gene selection using hybrid dragonfly black hole algorithm: A case study on rna-seq covid-19 data. Anal. Biochem. 2021, 627, 114242. [Google Scholar] [CrossRef]
- Karaboga, D.; Basturk, B. A powerful and efficient algorithm for numerical function optimization: Artificial bee colony (abc) algorithm. J. Glob. Optim. 2007, 39, 459–471. [Google Scholar] [CrossRef]
- Yang, C.; Gao, H.; Yang, X.; Huang, S.; Kan, Y.; Liu, J. BnBeeEpi: An approach of epistasis mining based on artificial bee colony algorithm optimizing bayesian network. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 232–239. [Google Scholar]
- Guan, B.; Xu, T.; Zhao, Y.; Li, Y.; Dong, X. A random grouping-based self-regulating artificial bee colony algorithm for interactive feature detection. Knowl. Based Syst. 2022, 243, 108434. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wong, K.-C. Nature-inspired multiobjective epistasis elucidation from genome-wide association studies. IEEE/ACM Trans. Comput. Biol. Bioinf. 2018, 17, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Karaboga, D.; Akay, B. A comparative study of artificial bee colony algorithm. Appl. Math. Comput. 2009, 214, 108–132. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Albert, R. Emergence of scaling in random networks. Science 1999, 286, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Karaboga, D.; Basturk, B. On the performance of artificial bee colony (abc) algorithm. Appl. Soft Somput. 2008, 8, 687–697. [Google Scholar] [CrossRef]
- Rao, R.S.; Narasimham, S.; Ramalingaraju, M. Optimization of distribution network configuration for loss reduction using artificial bee colony algorithm. Int. J. Electr. Power Energy Syst. 2008, 1, 116–122. [Google Scholar]
- Ma, C.; Shang, J.; Li, S.; Sun, Y. Detection of SNP-SNP interaction based on the generalized particle swarm optimization algorithm. In Proceedings of the 2014 8th International Conference on Systems Biology (ISB), Qingdao, China, 24–27 October 2014; pp. 151–155. [Google Scholar]
- Shang, J.; Sun, Y.; Fang, Y.; Li, S.; Liu, J.-X.; Zhang, Y. Hypergraph supervised search for inferring multiple epistatic interactions with different orders. In Proceedings of the International Conference on Intelligent Computing, Fuzhou, China, 20–23 August 2015; pp. 623–633. [Google Scholar]
- Zhang, Y.; Liu, J.S. Bayesian inference of epistatic interactions in case-control studies. Nat. Genet. 2007, 39, 1167–1173. [Google Scholar] [CrossRef]
- Han, B.; Chen, X.-W.; Talebizadeh, Z.; Xu, H. Genetic studies of complex human diseases: Characterizing snp-disease associations using bayesian networks. BMC Syst. Biol. 2012, 6, S14. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, J.; Liu, J.; Li, S. An improved ant colony optimization algorithm for the detection of SNP-SNP interactions. In Proceedings of the International Conference on Intelligent Computing, Lanzhou, China, 2–5 August 2016; pp. 21–32. [Google Scholar]
- Shang, J.; Sun, Y.; Li, S.; Liu, J.-X.; Zheng, C.-H.; Zhang, J. An improved opposition-based learning particle swarm optimization for the detection of snp-snp interactions. BioMed Res. Int. 2015, 2015, 524821. [Google Scholar] [CrossRef]
- Niel, C.; Sinoquet, C.; Dina, C.; Rocheleau, G. Smmb: A stochastic markov blanket framework strategy for epistasis detection in gwas. Bioinformatics 2018, 34, 2773–2780. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, J.; Lei, X.; Zhao, W.; Dong, Y. Episim: Simulation of multiple epistasis, linkage disequilibrium patterns and haplotype blocks for genome-wide interaction analysis. Genes Genom. 2013, 35, 305–316. [Google Scholar] [CrossRef]
- Jing, P.-J.; Shen, H.-B. Macoed: A multi-objective ant colony optimization algorithm for snp epistasis detection in genome-wide association studies. Bioinformatics 2014, 31, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, S. A steady-state and generational evolutionary algorithm for dynamic multiobjective optimization. IEEE Trans. Evol. Comput. 2016, 21, 65–82. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T. Complement factor h polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Tutz, G.; Ramzan, S. Improved methods for the imputation of missing data by nearest neighbor methods. Comput. Stat. Data Anal. 2015, 90, 84–99. [Google Scholar] [CrossRef]
- Gili, P.; Lloreda Martín, L.; Martín-Rodrigo, J.-C.; Kim-Yeon, N.; Modamio-Gardeta, L.; Fernández-García, J.L.; Rebolledo-Poves, A.B.; Gómez-Blazquez, E.; Pazos-Rodriguez, R.; Pérez-Fernández, E.; et al. Gene polymorphisms associated with an increased risk of exudative age-related macular degeneration in a spanish population. Eur. J. Ophthalmol. 2021, 32, 11206721211002698. [Google Scholar] [CrossRef]
- Tuo, S.; Zhang, J.; Yuan, X.; He, Z.; Liu, Y.; Liu, Z. Niche harmony search algorithm for detecting complex disease associated high-order snp combinations. Sci. Rep. 2017, 7, 11529. [Google Scholar] [CrossRef]
- Tuo, S.; Zhang, J.; Yuan, X.; Zhang, Y.; Liu, Z. Fhsa-sed: Two-locus model detection for genome-wide association study with harmony search algorithm. PLoS ONE 2016, 11, e0150669. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Dai, H.; Dorajoo, R.; Liu, J.; Kong, J.; Yin, X.; Ren, Y. Discovery of novel genetic risk loci for acute central serous chorioretinopathy and genetic pleiotropic effect with age-related macular degeneration. Front. Cell Dev. Biol. 2021, 9, 696885. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, M.; Chen, A.; Liu, Z.; Young, C.A.; Wang, S.b.; Zheng, D.; Jin, G. Genetic associations of anti-vascular endothelial growth factor therapy response in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2021, 100, e669–e680. [Google Scholar] [CrossRef]
- Tang, R.; Xu, X.; Yang, W.; Yu, W.; Hou, S.; Xuan, Y.; Tang, Z.; Zhao, S.; Chen, Y.; Xiao, X. Med27 promotes melanoma growth by targeting akt/mapk and nf-κb/inos signaling pathways. Cancer Lett. 2016, 373, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Meng, Y.; Yu, N.; Pan, Y. Cloud computing for detecting high-order genome-wide epistatic interaction via dynamic clustering. BMC Bioinform. 2014, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Fan, L.; Li, M.; Deng, H.; Yang, A.; Liu, F. Mpp7 promotes the migration and invasion of breast cancer cells via egfr/akt signaling. Cell Biol. Int. 2021, 45, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Pieri, K.; Nanni, P.; Tica, J.; Barratt, J.; Didangelos, A. Phosphatidylethanolamine binding protein-4 (pebp4) is increased in iga nephropathy and is associated with iga-positive b-cells in affected kidneys. J. Autoimmun. 2019, 105, 102309. [Google Scholar] [CrossRef] [PubMed]
- Markus-Koch, A.; Schmitt, O.; Seemann, S.; Lukas, J.; Koczan, D.; Ernst, M.; Fuellen, G.; Wree, A.; Rolfs, A.; Luo, J. Adam23 promotes neuronal differentiation of human neural progenitor cells. Cell. Mol. Biol. Lett. 2017, 22, 1–13. [Google Scholar] [CrossRef]
- Takada, H.; Imoto, I.; Tsuda, H.; Nakanishi, Y.; Ichikura, T.; Mochizuki, H.; Mitsufuji, S.; Hosoda, F.; Hirohashi, S.; Ohki, M. Adam23, a possible tumor suppressor gene, is frequently silenced in gastric cancers by homozygous deletion or aberrant promoter hypermethylation. Oncogene 2005, 24, 8051–8060. [Google Scholar] [CrossRef][Green Version]




| Model | AABB | AABb | AAbb | AaBB | AaBb | Aabb | aaBB | aaBb | aabb |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | 0.087 | 0.087 | 0.087 | 0.087 | 0.146 | 0.190 | 0.087 | 0.190 | 0.247 |
| Model 2 | 0.078 | 0.078 | 0.078 | 0.078 | 0.105 | 0.122 | 0.078 | 0.122 | 0.142 |
| Model 3 | 0.009 | 0.009 | 0.009 | 0.013 | 0.006 | 0.006 | 0.013 | 0.006 | 0.006 |
| Model 4 | 0.092 | 0.092 | 0.092 | 0.092 | 0.319 | 0.319 | 0.092 | 0.319 | 0.319 |
| Model 5 | 0.084 | 0.084 | 0.084 | 0.084 | 0.210 | 0.210 | 0.084 | 0.210 | 0.210 |
| Model 6 | 0.052 | 0.052 | 0.052 | 0.052 | 0.137 | 0.137 | 0.052 | 0.137 | 0.137 |
| Model 7 | 0.072 | 0.164 | 0.164 | 0.164 | 0.072 | 0.072 | 0.164 | 0.072 | 0.072 |
| Model 8 | 0.067 | 0.155 | 0.155 | 0.155 | 0.067 | 0.067 | 0.155 | 0.067 | 0.067 |
| Model 9 | 0.486 | 0.960 | 0.538 | 0.947 | 0.004 | 0.811 | 0.640 | 0.606 | 0.909 |
| Model 10 | 0.103 | 0.063 | 0.124 | 0.098 | 0.086 | 0.069 | 0.021 | 0.147 | 0.059 |
| Model 11 | 0.000 | 0.000 | 0.000 | 0.000 | 0.050 | 0.000 | 0.100 | 0.000 | 0.000 |
| Model 12 | 0.000 | 0.020 | 0.000 | 0.020 | 0.000 | 0.020 | 0.000 | 0.020 | 0.000 |
| SNP1 | SNP2 | Fitness Value | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Name | Gene | Chr | Name | Gene | Chr | ||
| rs380390 | CFH | 1 | rs1363688 | N/A | 5 | 39.16 | 1.5453 × 10−9 |
| rs380390 | CFH | 1 | rs2402053 | N/A | 7 | 37.72 | 1.4679 × 10−8 |
| rs380390 | CFH | 1 | rs1374431 | LOC107985962 | 2 | 37.19 | 2.6240 × 10−8 |
| rs1329428 | CFH | 1 | rs9328536 | MED27 | 9 | 36.81 | 3.0901 × 10−8 |
| rs380390 | CFH | 1 | rs2380684 | N/A | 2 | 36.00 | 3.9086 × 10−8 |
| rs380390 | CFH | 1 | rs3009336 | N/A | 1 | 34.59 | 5.3535 × 10−8 |
| rs380390 | CFH | 1 | rs555174 | N/A | 21 | 34.14 | 5.7995 × 10−8 |
| rs380390 | CFH | 1 | rs2794520 | N/A | 1 | 33.56 | 6.7417 × 10−8 |
| rs380390 | CFH | 1 | rs10508731 | MPP7 | 10 | 33.56 | 7.2188 × 10−8 |
| rs380390 | CFH | 1 | rs1740752 | N/A | 10 | 33.50 | 1.0917 × 10−7 |
| rs1329428 | CFH | 1 | rs10489076 | N/A | 4 | 33.43 | 1.9228 × 10−7 |
| rs1329428 | CFH | 1 | rs3913094 | N/A | 12 | 33.16 | 4.2460 × 10−7 |
| rs380390 | CFH | 1 | rs724972 | N/A | 3 | 33.02 | 4.4484 × 10−7 |
| rs1329428 | CFH | 1 | rs724972 | N/A | 3 | 33.02 | 1.3223 × 10−6 |
| rs1329428 | CFH | 1 | rs2466215 | PEBP4 | 8 | 32.35 | 1.7865 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Sun, Y.; Shang, J.; Li, F.; Guan, B.; Liu, J.-X. Multi-Objective Artificial Bee Colony Algorithm Based on Scale-Free Network for Epistasis Detection. Genes 2022, 13, 871. https://doi.org/10.3390/genes13050871
Gu Y, Sun Y, Shang J, Li F, Guan B, Liu J-X. Multi-Objective Artificial Bee Colony Algorithm Based on Scale-Free Network for Epistasis Detection. Genes. 2022; 13(5):871. https://doi.org/10.3390/genes13050871
Chicago/Turabian StyleGu, Yijun, Yan Sun, Junliang Shang, Feng Li, Boxin Guan, and Jin-Xing Liu. 2022. "Multi-Objective Artificial Bee Colony Algorithm Based on Scale-Free Network for Epistasis Detection" Genes 13, no. 5: 871. https://doi.org/10.3390/genes13050871
APA StyleGu, Y., Sun, Y., Shang, J., Li, F., Guan, B., & Liu, J.-X. (2022). Multi-Objective Artificial Bee Colony Algorithm Based on Scale-Free Network for Epistasis Detection. Genes, 13(5), 871. https://doi.org/10.3390/genes13050871







