Morphological and Molecular Analyses of the Interaction between Rosa multiflora and Podosphaera pannosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Observations and Molecular Identification of Powdery Mildew
2.2. Assessment of R. multiflora Resistance to Powdery Mildew
2.3. Cytological Studies
2.4. De Novo Transcriptome Assembly
2.5. Gene Annotation and Function Analyses
2.6. Expression of Candidate Defense-Related Genes
2.7. Statistical Analysis
3. Results
3.1. Morphological Observations and Molecular Identification of Powdery Mildew
3.2. Evaluation of Resistance in R. multiflora
3.3. Morphological Changes during Rose–Pathogen Interaction
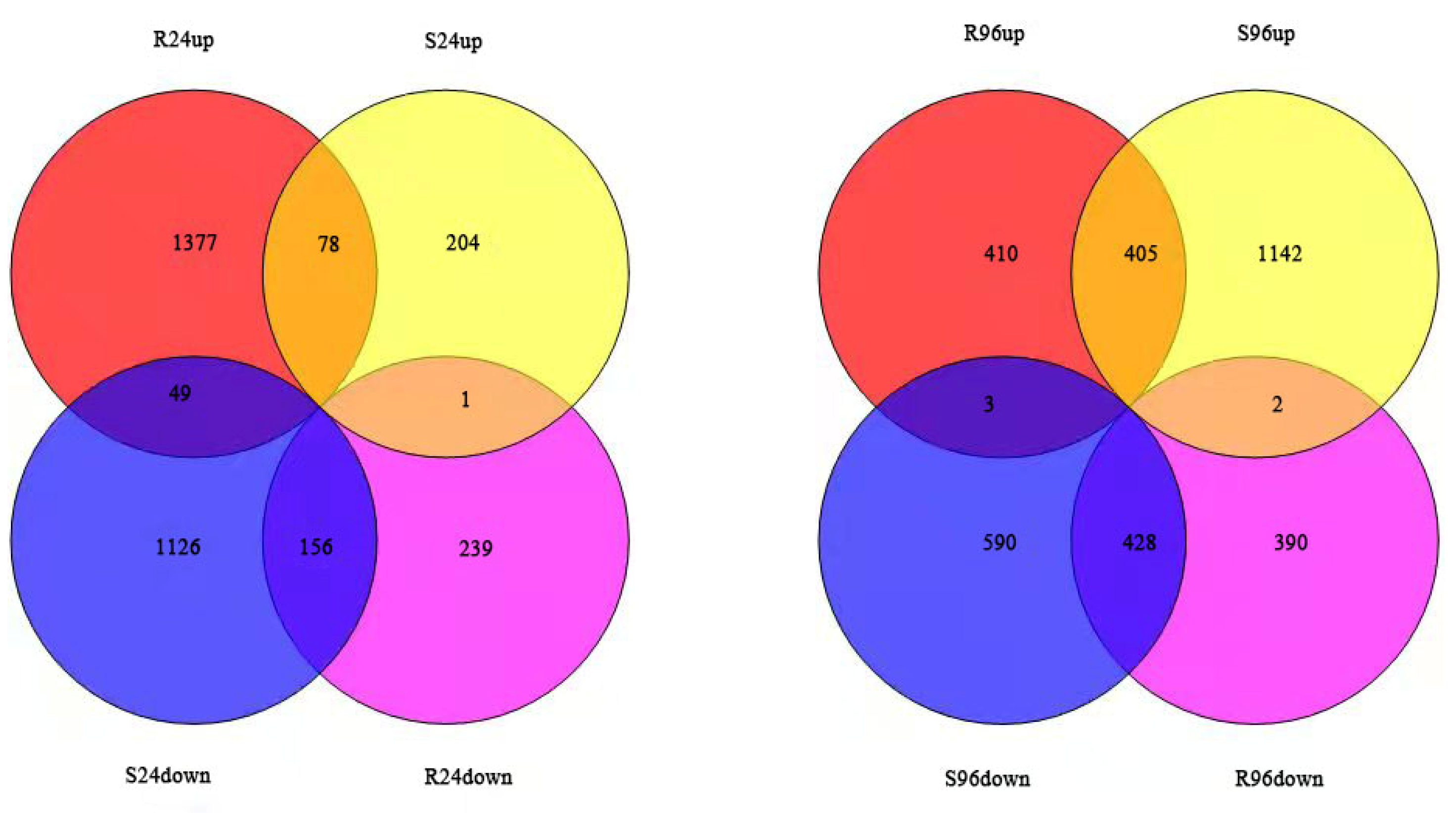
3.4. Statistical Analysis of Transcriptome Sequencing and Gene Expression Profile Results
3.5. GO Enrichment Analysis of Differential Transcripts
3.6. Expression Analysis of Defense-Related Genes during Infection
3.7. Expression Analysis of Phenylpropanoid Pathway Genes
3.8. Expression Analysis of Defense-Related Genes in Response to SA, JA, and ET
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Murashige and Skoog (1962) |
| BA | 6-Benzyladenine |
| NAA | a-Naphthalene acetic acid |
References
- Pemberton, H.B.; Kelly, J.W.; Ferare, J. Pot rose production. In Encyclopedia of Rose Science; Roberts, A., Debener, T., Gudin, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 587–593. [Google Scholar]
- Spencer, D.M. The Powdery Mildews; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1978; p. 429. [Google Scholar]
- Noack, R. Breeding/selection strategies for disease and pest resistance. In Encyclopedia of Rose Science; Elsevier: Oxford, UK, 2003; pp. 49–55. [Google Scholar]
- Mortensen, L.M.; Gislerød, H.R. Effect of air humidity variation on powdery mildew and keeping quality of cut roses. Sci. Hortic. 2005, 104, 49–55. [Google Scholar] [CrossRef]
- Eken, C. A review of biological control of rose powdery mildew (Spaerotheca pannosa var. rosae) by fungal antagonists. Acta Hortic. 2005, 690, 193–196. [Google Scholar] [CrossRef]
- Leus, L.; Dewitte, A.; Van Huylenbroeck, J.; Vanhoutte, N.; Van Bockstaele, E.; Ho¨fte, M. Podosphaera pannosa (syn. Sphaerotheca pannosa) on Rosa and Prunus spp.: Characterization of pathotypes by differential plant reactions and ITS sequences. J. Phytopathol. 2006, 154, 23–28. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, C.H.; Wu, K.; Chen, W.B.; Chen, Y.J.; Hao, X.A.; Wu, Y.F. Advances and prospects in biogenic substances against plant virus: A review. Pestic. Biochem. Physiol. 2017, 135, 15–26. [Google Scholar] [CrossRef]
- Linde, M.; Debener, T. Isolation and identification of eight races of powdery mildew of roses (Podosphaera pannosa) (Wallr.: Fr.) de Bary and the genetic analysis of the resistance gene Rpp1. Theor. Appl. Genet. 2003, 107, 256–262. [Google Scholar] [CrossRef]
- Huckelhoven, R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 2007, 45, 101–127. [Google Scholar] [CrossRef]
- Neu, E.; Domes, H.S.; Menz, I.; Kaufmann, H.; Linde, M.; Debener, T. Interaction of roses with a biotrophic and a hemibiotrophic leaf pathogen leads to differences in defense transcriptome activation. Plant Mol. Biol. 2019, 99, 299–316. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence: A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Maor, R.; Shirasu, K. The arms race continues: Battle strategies between plants and fungal pathogens. Curr. Opin. Microbiol. 2005, 8, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Niwa, Y.; Goto, S.; Ogawa, T.; Shimizu, M.; Suzuki, A.; Kobayashi, K.; Kobayashi, H. bHLH106 integrates functions of multiple genes through their G-Box to confer salt tolerance on Arabidopsis. PLoS ONE 2015, 10, e0126872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, C.J.; Lawton, M.A.; Dron, M.; Dixon, R.A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell 1989, 56, 215–224. [Google Scholar] [CrossRef]
- Zhang, S.; Moyne, A.N.; Reddy, M.S.; Kloepper, J.W. The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol. Control 2002, 25, 288–296. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.Q.; Dong, X.N. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.Y.; Zhou, M.; Yoo, H.J.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X.N. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 9166–9173. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Wang, Y.Y.; Penniket, C.; Lu, Z.X.; Bakkeren, G.; Laroche, A. Morphological and molecular analyses of host and nonhost interactions involving barley and wheat and the covered smut pathogen Ustiago hordei. Mol. Plant-Microbe Interact. 2010, 23, 1619–1634. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.P.; Wei, B.; Li, G.L.; Gong, C.Y.; Fan, R.C.; Zhang, X.Q. TaEDS1 genes positively regulate resistance to powdery mildew in wheat. Plant Mol. Biol. 2018, 96, 607–625. [Google Scholar] [CrossRef]
- Yan, J.X.; Deng, Y.N.; Yu, J.; Zhang, Y.Q.; Chi, D.F. A study on JA-and BTH-induced resistance of Rosa rugosa ‘Plena’ to powdery mildew (Sphaerotheca pannosa). J. Foraminifer. Res. 2018, 29, 823–831. [Google Scholar] [CrossRef]
- Mao, S.; Kenji, K.; Eiji, M.; Hiroki, Y.; Takashi, M.; Susumu, T.; Shuichi, T.; Hiroshi, S.; Kenichi, M.; Masaki, A. Identification of medicinal atractylodes based on ITS sequences of nrDNA. Biol. Pharm. Bull. 2006, 29, 315–320. [Google Scholar]
- Li, H.P. Plant Microscopy; Science Press: Beijing, China, 2009; p. 258. [Google Scholar]
- Turner, J.G.; Elli, C.; Devoto, A. The Jasmonate signal pathway. Plant Cell 2002, 14, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardham, A.R.; Jones, D.A.; Takemoto, D. Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 2007, 10, 342–348. [Google Scholar] [CrossRef]
- Yang, Y.L.; Fan, P.; Liu, J.X.; Xie, W.J.; Liu, N.; Niu, Z.B.; Li, Q.Q.; Song, J.; Tian, Q.J.; Bao, Y.G.; et al. Thinopyrum intermedium TiAP1 interacts with a chitin deacetylase from Blumeria graminis f. sp. tritici and increases the resistance to Bgt in wheat. Plant Biotechnol. J. 2022, 20, 454–467. [Google Scholar] [CrossRef]
- Hauck, P.; Thilmony, R.; He, S.Y. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc. Natl. Acad. Sci. USA 2003, 100, 8577–8582. [Google Scholar] [CrossRef] [Green Version]
- Bartetzko, V.; Sonnewald, S.; Vogel, F.; Hartner, K.; Stadler, R.; Hammes, U.-Z.; Bornke, F. The Xanthomonas campestris pv. vesicatoria type III effector protein XopJ inhibits protein secretion: Evidence for interference with cell wall-associated defense responses. Mol. Plant-Microbe Interact. 2009, 22, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 2012, 356, 279–290. [Google Scholar] [CrossRef]
- Yang, C.C.; Wu, P.F.; Yao, X.H.; Sheng, Y.; Zhang, C.C.; Lin, P.; Wang, K.L. Integrated Transcriptome and Metabolome Analysis Reveals Key Metabolites Involved in Camellia oleifera Defense against Anthracnose. Int. J. Mol. Sci. 2022, 23, 536. [Google Scholar] [CrossRef]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jørgensen, H.G.L.; Newman, M.; Christensen, L.P. Silicon-Induced Changes in Antifungal Phenolic Acids, Flavonoids and Key Phenylpropanoid Pathway Genes during the Interaction between Miniature Roses and the Biotrophic Pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef] [Green Version]
- Shetty, R.; Jensen, B.; Shetty, N.P.; Hansen, M.; Hansen, C.W.; Starkey, K.R.; Jørgensen, H.G. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 2012, 61, 120–131. [Google Scholar] [CrossRef]
- Zhang, J.T.; Duan, G.M.; Yu, Z.Y. Relationship between phenylalanine ammonia-Lysae (PAL) activity and resistance to rice blast. Plant Physiol. Commun. 1987, 6, 34–37. (In Chinese) [Google Scholar]
- Collinge, D.B.; Slusarenko, A.J. Plant gene expression in response to pathogens. Plant Mol. Biol. 1987, 9, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Kirk, T.K.; Sherwood, R.T. Lignifigation as a mechanism of disease resistance. Annu. Rev. Phytopathol. 1980, 18, 259–288. [Google Scholar] [CrossRef]
- Hematy, K.; Sado, P.E.; VanTuinen, A.; Rochange, S.; Desnos, T.; Balzergue, S.; Pelletier, S.; Renou, J.P.; Hofte, H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007, 17, 922–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.D.; King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 2009, 60, 509–521. [Google Scholar] [CrossRef]
- Gallego, L.; Jikumaru, Y.; Kamiya, Y.; Tang, Y.; Dixon, R.A. Selective lignin downregulation leads to constitutive defense response expression in alfalfa (Medicago sativa L.). New Phytol. 2011, 90, 627–639. [Google Scholar] [CrossRef]
- Walter, M.H.; Grima Pettenati, J.; Grand, C.; Boudet, A.M.; Lamb, C.J. Cinnamyl-alcohol dehydrogenase, a molecular marker specific for lignin synthesis: cDNA cloning and mRNA induction by fungal elicitor. Proc. Natl. Acad. Sci. USA 1988, 85, 5546–5550. [Google Scholar] [CrossRef] [Green Version]
- Tronchet, M.; Balague, C.; Kroj, T.; Jouanin, L.; Roby, D. Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol. Plant Pathol. 2010, 11, 83–92. [Google Scholar] [CrossRef]
- Yanti, Y. Peroxidase enzyme activity of rhizobacteria-introduced shallots bulbs to induce resistance of shallot towards bacterial leaf blight (Xanthomonas axonopodis pv. allii). Prog. Chem. 2015, 14, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Laurent, Z.; Mónica, S.; Volker, L.; Paul, S.L.; Shauna, S. Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J. 2004, 40, 633–646. [Google Scholar]
- Achuo, E.A.; Audenaert, K.; Meziane, H. The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol. 2004, 53, 65–72. [Google Scholar] [CrossRef]
- Gaffney, T.; Friedrich, L. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Cho, B.H.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz Ordenana, K.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Krinke, O.; Ruelland, E.; Valentová, O.; Vergnolle, C.; Renou, J.P.; Taconnat, L.; Flemr, M.; Burketová, L.; Zachowski, A. Phosphatidylinositol 4-kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiol. 2007, 144, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Maity, A.; Sharma, J.; Sarkar, A.; More, A.K.; Pal, R.K.; Nagane, V.P.; Maity, A. Salicylic acid mediated multi-pronged strategy to combat bacterial blight disease (Xanthomonas axonopodis pv. punicae) in pomegranate. Eur. J. Plant Pathol. 2018, 150, 923–937. [Google Scholar] [CrossRef]
- Stein, M.; Dittgen, J.; Sánchez, C.; Hou, B.H.; Molina, A.; Schulze Lefert, P.; Lipka, V.; Somerville, S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to non-host resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 2006, 18, 731–746. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Uehara, Y.; Berberich, T.; Ito, A.; Saitoh, H.; Miyazaki, A.; Terauchi, R.; Kusano, T. A subset of hypersensitive response marker genes, including HSR203J, is the downstream target of a spermine signal transduction pathway in tobacco. Plant J. 2010, 46, 586–595. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [Green Version]
- Siddaiah, C.N.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Gurunathan, S.; Rangappa, S.; Huntrike, S.S.; Srivastava, R.K. Elicitation of resistance and associated defense responses in Trichoderma hamatum induced protection against pearl millet downy mildew pathogen. Sci. Rep. 2017, 7, 43991. [Google Scholar] [CrossRef]
- Xu, L.H.; Liu, F.Q.; Lechner, E. The SCFcol1 Ubiquitin-Lligase complexes are required for Jasmonate response in Arabidopsis. Plant Cell 2002, 14, 1919–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.J.; Shao, S.J.; Zheng, C.F.; Sun, Z.H.; Shi, J.Y.; Yu, J.Q.; Qi, Z.Y.; Shi, K. Induction of systemic resistance in tomato against Botrytis cinerea by N-decanoyl-homoserine lactone via jasmonic acid signaling. Planta 2018, 247, 1217–1227. [Google Scholar] [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.C.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Brown, R.; Kazan, K. NPR1 modulates cross-talk between salicylate-and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomma, B.; Eggermont, K.; Penninckx, I.A.M.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Disease Grade | Incidence Degree |
|---|---|
| Grade 0 | There was no powdery mildew on the leaves of the whole plant |
| Grade 1 | One to two leaves had thin hyphae |
| Grade 2 | Three to four leaves had medium mycelium and some spores |
| Grade 3 | Five to six leaves had thick hyphae and more spores |
| Grade 4 | More than seven leaves had large spore piles and a large number of spores |
| Disease Grade | Mycelial Growth Rate and Disease Grade Classification Standard | Classification Criteria of Mycelial Coverage Area and Conidial Stalk Formation |
|---|---|---|
| Grade 0 | Spores did not germinate | No hyphae, some spores did not germinate |
| Grade 1 | Average mycelium length of 0–7 μm | A small amount of mycelium; the hyphae formed by the germination of one spore covered 0–1/20 of the leaves |
| Grade 2 | Average mycelium length of 7–14 μm | Numerous hyphae; the hyphae formed by the germination of one spore covered 1/20–1/10 |
| Grade 3 | Average mycelium length of 14–21 μm | Numerous hyphae; the hyphae formed by one spore germination covered more than 1/10 of the leaves, but no conidiophores were observed |
| Grade 4 | Average mycelium length of more than 21 μm | Numerous hyphae; the mycelium grew fast, and conidiophores were formed |
| Disease Grade | Standard |
|---|---|
| Grade 0 | No pathogens were observed |
| Grade 1 | Leaf area occupied by pathogens <l% |
| Grade 2 | Leaf area occupied by pathogens l–5% |
| Grade 3 | Leaf area occupied by pathogens 6–20% |
| Grade 4 | Leaf area occupied by pathogens 21–40% |
| Grade 5 | Leaf area occupied by pathogens 41–60% |
| Grade 6 | Leaf area occupied by pathogens >60% |
| Materials | Resistance Index | Resistance Level |
|---|---|---|
| R. multiflora ‘13’ | 0.85000 | high resistance |
| R. multiflora ‘1’ | 0.06250 | high susceptibility |
| R. multiflora ‘4’ | 0.08750 | high susceptibility |
| Materials | Resistance Index | Significance of Difference | Resistance Level |
|---|---|---|---|
| R. multiflora ‘13’ | 0.84167 | A | high resistance |
| R. multiflora ‘1’ | 0.39167 | B | moderate susceptibility |
| R. multiflora ‘4’ | 0.04167 | C | high susceptibility |
| Materials | Resistance Index | Resistance Level |
|---|---|---|
| R. multiflora ‘13’ | 0.90500 | high resistance |
| R. multiflora ‘4’ | 0.02400 | high susceptibility |
| Treatment Number of Transcripts | Differential Expression Multiple log2 Value > 5 or <−5 | Differential Expression Multiple log2 Value > 7 or <-7 | Differential Expression Multiple log2 Value > 9 or <−9 |
|---|---|---|---|
| Resistant plants 24 h post-inoculation (hpi) | 62 | 7 | 0 |
| Resistant plants 96 hpi | 531 | 132 | 16 |
| Susceptible plants 24 hpi | 63 | 8 | 0 |
| Susceptible plants 96 hpi | 916 | 189 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Zhang, X.; Sun, X.; Bao, M.; Wang, Y. Morphological and Molecular Analyses of the Interaction between Rosa multiflora and Podosphaera pannosa. Genes 2022, 13, 1003. https://doi.org/10.3390/genes13061003
Bao Y, Zhang X, Sun X, Bao M, Wang Y. Morphological and Molecular Analyses of the Interaction between Rosa multiflora and Podosphaera pannosa. Genes. 2022; 13(6):1003. https://doi.org/10.3390/genes13061003
Chicago/Turabian StyleBao, Ying, Xue Zhang, Xiaoxiang Sun, Manzhu Bao, and Yuanyuan Wang. 2022. "Morphological and Molecular Analyses of the Interaction between Rosa multiflora and Podosphaera pannosa" Genes 13, no. 6: 1003. https://doi.org/10.3390/genes13061003
APA StyleBao, Y., Zhang, X., Sun, X., Bao, M., & Wang, Y. (2022). Morphological and Molecular Analyses of the Interaction between Rosa multiflora and Podosphaera pannosa. Genes, 13(6), 1003. https://doi.org/10.3390/genes13061003






