MAPK1 Is Regulated by LOC102188416/miR-143-3p Axis in Dairy Goat Mammary Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Statement
2.2. Cell Culture
2.3. RNA Isolation, Library Construction, Sequencing, and Data Analysis
2.4. Real-Time Fluorescence Quantitative PCR
2.5. Vector Construction and Dual Luciferase Reporter Assay
2.6. Statistics
3. Results
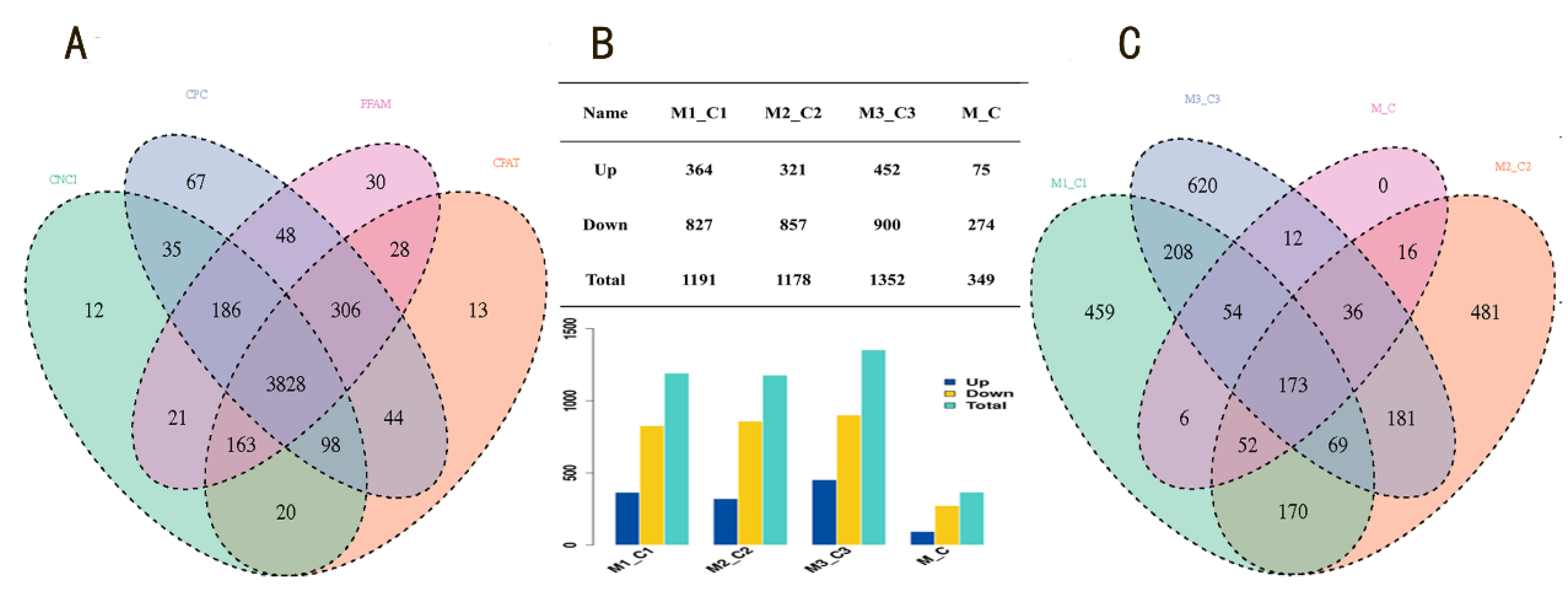
3.1. Screening of Differentially Expressed LncRNAs Induced by miR-143-3p
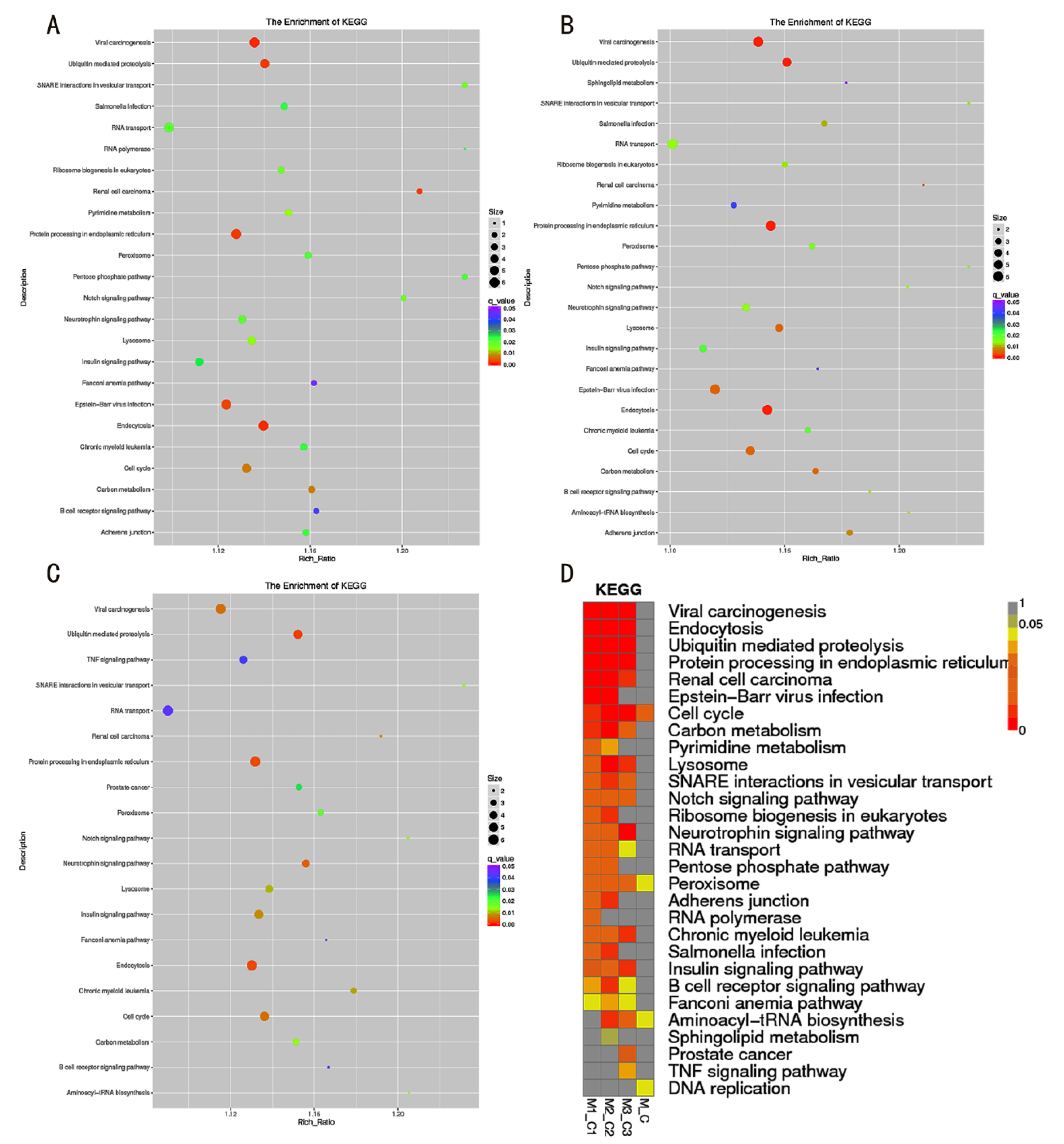
3.2. GO and KEGG Function Analysis of DE-LncRNAs
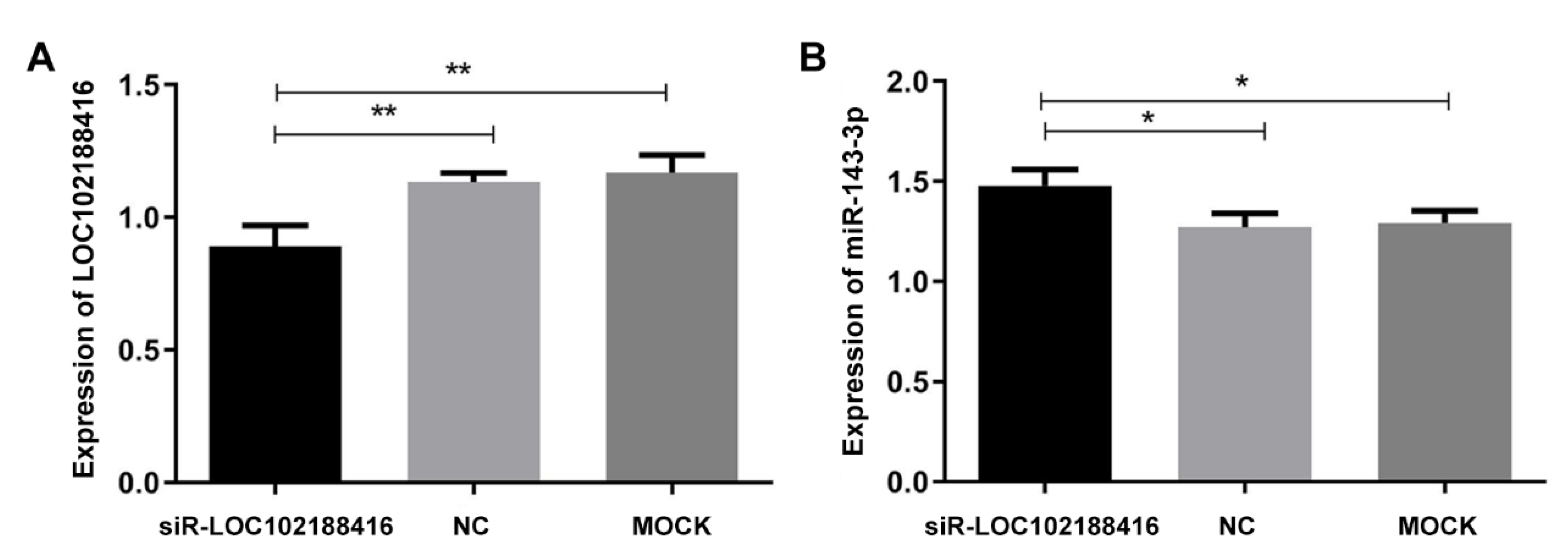
3.3. The Prediction of Target Genes of LncRNA LOC102188416/miR-143-3p
3.4. The Construction of LncRNA LOC102188416/miR-143-3p/MAPK1 Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Z.; Liu, Z.; Chao, T.; Hou, L.; Fan, R.; He, R.; Wang, G.; Wang, J. Screening of miRNA profiles and construction of regulation networks in early and late lactation of dairy goat mammary glands. Sci. Rep. 2017, 7, 11933. [Google Scholar] [CrossRef] [PubMed]
- Xuan, R.; Chao, T.; Wang, A.; Zhang, F.; Sun, P.; Liu, S.; Guo, M.; Wang, G.; Ji, Z.; Wang, J.; et al. Characterization of microRNA profiles in the mammary gland tissue of dairy goats at the late lactation, dry period and late gestation stages. PLoS ONE 2020, 15, e0234427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Li, W.; Cao, F.; Niu, G.; Ji, S.; Du, X.; Cao, B.; An, X. A Regulatory Circuit Orchestrated by Novel-miR-3880 Modulates Mammary Gland Development. Front. Cell Dev. Biol. 2020, 8, 383. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Liu, J.; An, X.; Cao, B. Circ-140/chi-miR-8516/STC1-MMP1 Regulates αs1-/β-Casein Secretion and Lipid Formation in Goat Mammary Epithelial Cells. Genes 2021, 12, 671. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Niu, G.; Liu, J.; Cao, F.; An, X.; Cao, B. EGF-Induced miR-223 Modulates Goat Mammary Epithelial Cell Apoptosis and Inflammation via ISG15. Front. Cell Dev. Biol. 2021, 9, 660933. [Google Scholar] [CrossRef]
- Ji, Z.; He, R.; Chao, T.; Xuan, R.; Liu, S.; Wang, G.; Wang, J. chi-miR-143-3p Promotes Apoptosis of Mammary Gland Epithelial Cells from Dairy Goats by Targeting Ndfip1. DNA Cell Biol. 2019, 38, 1188–1196. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, G.; Hou, L.; Liu, Z.; Wang, J.; Chao, T. miR-143 inhibits proliferation and induces apoptosis of mammary epithelial cells in dairy goat. Anim. Cells Syst. 2016, 20, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wu, Z.-Q.; Wang, Y.-J.; Wang, M.; Yang, W.-C. MiR-143 Regulates Milk Fat Synthesis by Targeting Smad3 in Bovine Mammary Epithelial Cells. Animals 2020, 10, 1453. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA–LncRNA Interactions. In Long Non-Coding RNAs: Methods and Protocols; Feng, Y., Zhang, L., Eds.; Springer: New York, NY, USA, 2016; pp. 271–286. [Google Scholar]
- Ang, C.E.; Trevino, A.E.; Chang, H.Y. Diverse lncRNA mechanisms in brain development and disease. Curr. Opin. Genet. Dev. 2020, 65, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.K.; Covarrubias, S.; Carpenter, S. The how and why of lncRNA function: An innate immune perspective. Biochim. Et Biophys. Acta. Gene Regul. Mech. 2020, 1863, 194419. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Lu, L.M.; Li, Q.Z.; Huang, J.G.; Gao, X.J. Proteomic and functional analyses reveal MAPK1 regulates milk protein synthesis. Molecules 2013, 18, 263–275. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, D.; Shi, H.; Bian, Y.; Guo, R. MiR-143 inhibits endometrial cancer cell proliferation and metastasis by targeting MAPK1. Oncotarget 2017, 8, 84384–84395. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–d419. [Google Scholar] [CrossRef]
- Simonis, S.A.; de Kok, B.M.; Korving, J.C.; Kopp, W.H.; Baranski, A.G.; Huurman, V.; Wasser, M.; van der Boog, P.; Braat, A.E. Applicability and reproducibility of the CPAT-grading system for pancreas allograft thrombosis. Eur. J. Radiol. 2021, 134, 109462. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhao, Y.; Lai, F.; Chu, M.; Hao, Y.; Feng, Y.; Zhang, H.; Liu, J.; Cheng, M.; Li, L.; et al. LncRNA as ceRNAs may be involved in lactation process. Oncotarget 2017, 8, 98014–98028. [Google Scholar] [CrossRef] [Green Version]
- Swami, M. Pseudogenes act as microRNA decoys. Nat. Rev. Cancer 2010, 10, 535. [Google Scholar] [CrossRef]
- Sun, D.; Chen, L.; Lv, H.; Gao, Y.; Liu, X.; Zhang, X. Circ_0058124 upregulates MAPK1 expression to promote proliferation, metastasis and metabolic abilities in thyroid cancer through sponging miR-940. OncoTargets Ther. 2020, 13, 1569. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Wang, D.; Ma, L.; Zhu, H. MicroRNA-362 inhibits cell growth and metastasis in glioblastoma by targeting MAPK1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8931–8939. [Google Scholar]
- Xu, Y.; Dong, M.; Wang, J.; Zhao, W.; Jiao, M. LINC01436 inhibited miR-585-3p expression and upregulated MAPK1 expression to promote gastric cancer progression. Dig. Dis. Sci. 2021, 66, 1885–1894. [Google Scholar] [CrossRef]
- Fei, B.; Wu, H. MiR-378 inhibits progression of human gastric cancer MGC-803 cells by targeting MAPK1 in vitro. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2013, 20, 557–564. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.J.; Li, P.; Guo, X.T. MicroRNA-145 regulates the proliferation, migration and invasion of human primary colon adenocarcinoma cells by targeting MAPK1. Int. J. Mol. Med. 2018, 42, 3171–3180. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Fan, Y.; Zhao, G.; Zhang, Q.; Bao, Y.; Cui, Y.; Ye, Z.; Chen, G.; Piao, X.; Guo, F. Novel lncRNA PSMG3-AS1 functions as a miR-143-3p sponge to increase the proliferation and migration of breast cancer cells. Oncol. Rep. 2020, 43, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Hu, J.; Song, H.; Xu, H.; Wu, C.; Zhao, B.; Xie, D.; Wu, T.; Zhao, J.; Fang, L. miR-143-3p targeting LIM domain kinase 1 suppresses the progression of triple-negative breast cancer cells. Am. J. Transl. Res. 2017, 9, 2276. [Google Scholar]
- Xia, C.; Yang, Y.; Kong, F.; Kong, Q.; Shan, C. MiR-143-3p inhibits the proliferation, cell migration and invasion of human breast cancer cells by modulating the expression of MAPK7. Biochimie 2018, 147, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shen, H.; Xu, J.; Zhao, S.; Yao, S.; Jiang, N. MiR-143-3p suppresses the progression of ovarian cancer. Am. J. Transl. Res. 2018, 10, 866. [Google Scholar] [PubMed]
- Liu, M.; Jia, J.; Wang, X.; Liu, Y.; Wang, C.; Fan, R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 2018, 19, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Q. Deng, Z. Lv, Y. Ling, X. Hou, Z. Chen, X. Dinglin, S. Ma, D. Li and Y. Wu. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Molecular cancer. 2019, 18, 181. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Ge, Y.; Ma, X.; Li, Z.; Shi, L.; Lin, L.; Xiao, J.; Chen, W.; Ni, P.; Yang, L. Long non-coding RNA CCDC144NL-AS1 sponges miR-143-3p and regulates MAP3K7 by acting as a competing endogenous RNA in gastric cancer. Cell Death Dis. 2020, 11, 521. [Google Scholar] [CrossRef]
- Huang, F.; Wen, C.; Zhuansun, Y.; Huang, L.; Chen, W.; Yang, X.; Liu, H. A novel long noncoding RNA OECC promotes colorectal cancer development and is negatively regulated by miR-143-3p. Biochem. Biophys. Res. Commun. 2018, 503, 2949–2955. [Google Scholar] [CrossRef]



| Primer | Sequences (5′-3′) |
|---|---|
| lncRNA LOC102188416 forward | TGGGTTGAGGCACACTGGTCACC |
| lncRNA LOC102188416 reverse | CTCGCTTCGGCAGCACA |
| MAPK1 forward | ACTGCCAGAGGACGCTGAGAG |
| MAPK1 reverse | ATGTGGTCGTTGCTGAGGTGTTG |
| GAPDH forward | CACCCTCAAGATTGTCAGC |
| GAPDH reverse | CAGTGGTCATAAGTCCCTCC |
| miR-143-3p forward | TGAGATGAAGCACTGTAGCTCG |
| U6 forward | CTCGCTTCGGCAGCACA |
| U6 reverse | AACGCTTCACGAATTTGCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, J.; Liu, S.; Ji, Z. MAPK1 Is Regulated by LOC102188416/miR-143-3p Axis in Dairy Goat Mammary Epithelial Cells. Genes 2022, 13, 1013. https://doi.org/10.3390/genes13061013
Zhang Y, Zhou J, Liu S, Ji Z. MAPK1 Is Regulated by LOC102188416/miR-143-3p Axis in Dairy Goat Mammary Epithelial Cells. Genes. 2022; 13(6):1013. https://doi.org/10.3390/genes13061013
Chicago/Turabian StyleZhang, Yue, Jie Zhou, Shuang Liu, and Zhibin Ji. 2022. "MAPK1 Is Regulated by LOC102188416/miR-143-3p Axis in Dairy Goat Mammary Epithelial Cells" Genes 13, no. 6: 1013. https://doi.org/10.3390/genes13061013
APA StyleZhang, Y., Zhou, J., Liu, S., & Ji, Z. (2022). MAPK1 Is Regulated by LOC102188416/miR-143-3p Axis in Dairy Goat Mammary Epithelial Cells. Genes, 13(6), 1013. https://doi.org/10.3390/genes13061013





