Potential Influence of Age and Diabetes Mellitus Type 1 on MSH2 (MutS homolog 2) Expression in a Rat Kidney Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diabetes Mellitus Model
2.2. Tissue Processing and Staining
2.3. Image Acquisition and Quantification
2.4. Statistical Analysis
3. Results
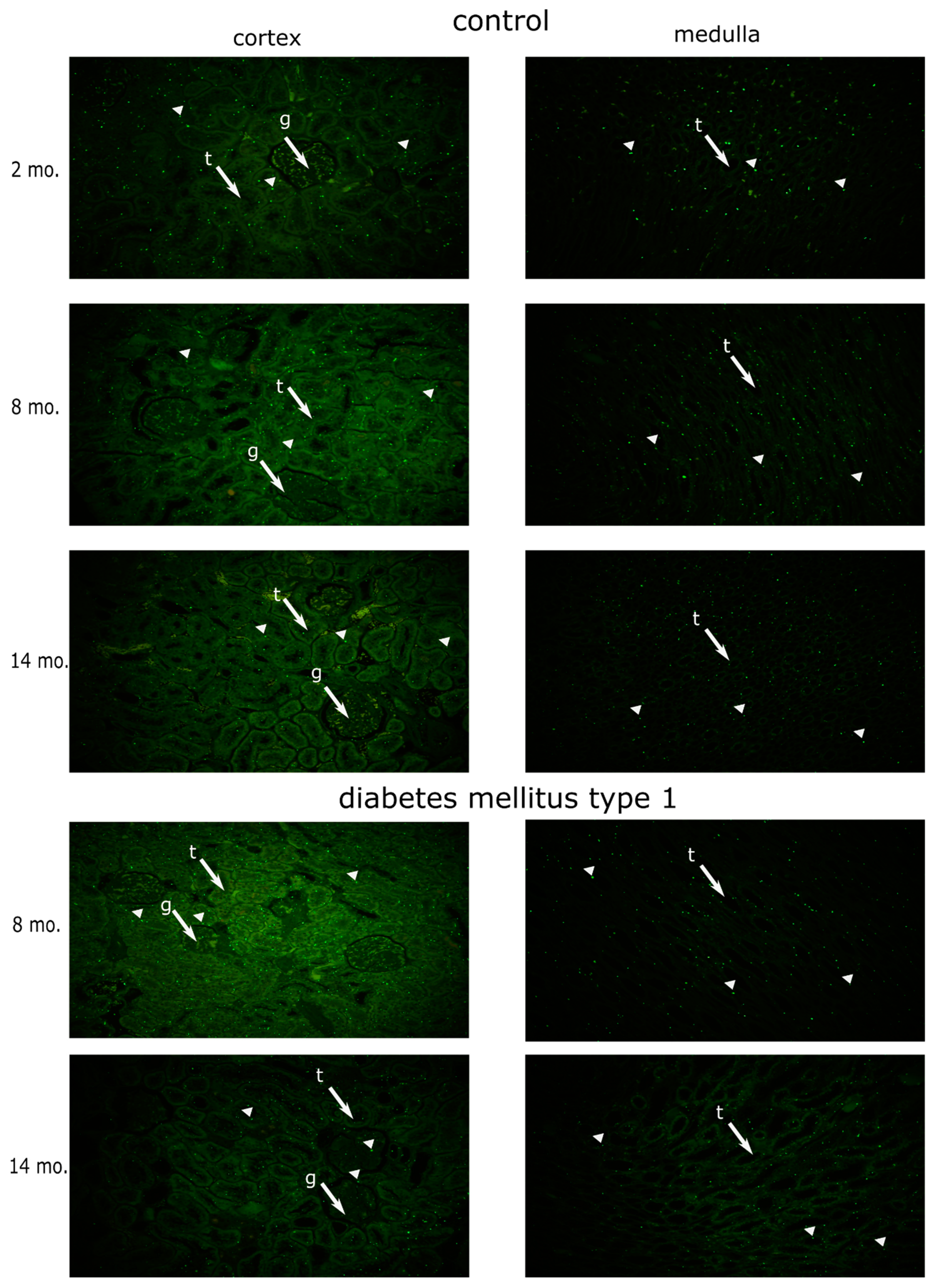
3.1. Dynamics of MSH2 Expression in Ageing Kidney of a Healthy Rat
3.2. Effects of Diabetes Mellitus on MSH2 Expression
4. Discussion
4.1. Limitations of the Current Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zajicek, G. Arber, N. Streaming kidney. Cell Prolif. 1991, 24, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bollain-Y-Goytia, J.J.; Meza-Lamas, E.; Lopez, A.; Avalos-Diaz, E.; Rodriguez-Padilla, C.; Herrera-Esparza, R. Renal cell turnover results in a fine balance between apoptosis and cell proliferation. J. Biol. Res. 2006, 6, 131–138. [Google Scholar]
- Pino, M.S.; Chung, D.C. Microsatellite instability in the management of colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Nakatsu, Y.; Ohno, M.; Taguchi, K.-I.; Tsuzuki, T. Mismatch Repair Deficient Mice Show Susceptibility to Oxidative Stress-Induced Intestinal Carcinogenesis. Int. J. Biol. Sci. 2013, 10, 73–79. [Google Scholar] [CrossRef]
- Christmann, M.; Kaina, B. Nuclear Translocation of Mismatch Repair Proteins MSH2 and MSH6 as a Response of Cells to Alkylating Agents. J. Biol. Chem. 2000, 275, 36256–36262. [Google Scholar] [CrossRef]
- Tennen, R.I.; Haye, J.E.; Wijayatilake, H.D.; Arlow, T.; Ponzio, D.; Gammie, A.E. Cell-cycle and DNA damage regulation of the DNA mismatch repair protein Msh2 occurs at the transcriptional and post-transcriptional level. DNA Repair 2013, 12, 97–109. [Google Scholar] [CrossRef]
- Franchitto, A.; Pichierri, P.; Piergentili, R.; Crescenzi, M.; Bignami, M.; Palitti, F. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 2003, 22, 2110–2120. [Google Scholar] [CrossRef]
- Martin, S.A.; McCarthy, A.; Barber, L.J.; Burgess, D.J.; Parry, S.; Lord, C.J.; Ashworth, A. Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Mol. Med. 2009, 1, 323–337. [Google Scholar] [CrossRef]
- Iwanaga, R.; Komori, H.; Ohtani, K. Differential regulation of expression of the mammalian DNA repair genes by growth stimulation. Oncogene 2004, 23, 8581–8590. [Google Scholar] [CrossRef][Green Version]
- Campbell, M.R.; Wang, Y.; E Andrew, S.; Liu, Y. Msh2 deficiency leads to chromosomal abnormalities, centrosome amplification, and telomere capping defect. Oncogene 2006, 25, 2531–2536. [Google Scholar] [CrossRef]
- Martinez, P.; Siegl-Cachedenier, I.; Flores, J.M.; Blasco, M.A. MSH2 deficiency abolishes the anticancer and pro-aging activity of short telomeres. Aging Cell 2009, 8, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar] [CrossRef]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. DNA Damage Response and Metabolic Disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet β Cells in Diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Marques, C.; Guo, W.; Pereira, P.; Taylor, A.; Patterson, C.; Evans, P.; Shang, F. The triage of damaged proteins: Degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones. FASEB J. 2006, 20, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Guthrie, R.A.; Guthrie, D.W. Pathophysiology of Diabetes Mellitus. Crit. Care Nurs. Q. 2004, 27, 113–125. [Google Scholar] [CrossRef]
- Chang, C.L.; Marra, G.; Chauhan, D.P.; Ha, H.T.; Chang, D.K.; Ricciardiello, L.; Randolph, A.; Carethers, J.M.; Boland, C.R. Oxidative stress inactivates the human DNA mismatch repair system. Am. J. Physiol. Physiol. 2002, 283, C148–C154. [Google Scholar] [CrossRef]
- Yoo, K.H.; Won, K.Y.; Lim, S.-J.; Park, Y.-K.; Chang, S.-G. Deficiency of MSH2 expression is associated with clear cell renal cell carcinoma. Oncol. Lett. 2014, 8, 2135–2139. [Google Scholar] [CrossRef]
- Kostic, S.; Hauke, T.; Ghahramani, N.; Filipovic, N.; Vukojevic, K. Expression pattern of apoptosis-inducing factor in the kidneys of streptozotocin-induced diabetic rats. Acta Histochem. 2020, 122, 151655. [Google Scholar] [CrossRef] [PubMed]
- Dragun, M.; Filipović, N.; Racetin, A.; Kostić, S.; Vukojević, K. Immunohistochemical Expression Pattern of Mismatch Repair Genes in the Short-term Streptozotocin-induced Diabetic Rat Kidneys. Appl. Immunohistochem. Mol. Morphol. 2021, 29, e83–e91. [Google Scholar] [CrossRef] [PubMed]
- Junod, A.; Lambert, A.E.; Stauffacher, W.; Renold, A.E. Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J. Clin. Investig. 1969, 48, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Courteix, C.; Eschalier, A.; Lavarenne, J. Streptozocin-induced diabetic rats: Behavioural evidence for a model of chronic pain. Pain 1993, 53, 81–88. [Google Scholar] [CrossRef]
- Mark, S.; Claire, H.F.; Wilson, R.; Foley, P. (Eds.) The Laboratory Rat, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Kim Suvarna, S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bankhead, P. Analyzing Fluorescence Microscopy Images with ImageJ. 2014. Available online: https://www.researchgate.net/publication/260261544_Analyzing_fluorescence_microscopy_images_with_ImageJ (accessed on 8 June 2022).
- Kapushesky, M.; Adamusiak, T.; Burdett, T.; Culhane, A.; Farne, A.; Filippov, A.; Holloway, E.; Klebanov, A.; Kryvych, N.; Kurbatova, N.; et al. Gene Expression Atlas update—A value-added database of microarray and sequencing-based functional genomics experiments. Nucleic Acids Res. 2012, 40, D1077–D1081. [Google Scholar] [CrossRef]
- Anderson, D.R. Quantifying the Evidence About Science Hypotheses. In Model Based Inference in the Life Sciences: A Primer on Evidence, 1st ed.; Springer: New York, NY, USA, 2008; pp. 83–103. [Google Scholar]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Lee, C.-J.; Gardiner, B.; Evans, R.G.; Smith, D.W. A model of oxygen transport in the rat renal medulla. Am. J. Physiol. Physiol. 2018, 315, F1787–F1811. [Google Scholar] [CrossRef]
- Sands, J.M.; Layton, H.E. The Physiology of Urinary Concentration: An Update. Semin. Nephrol. 2009, 29, 178–195. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Melk, A.; Kittikowit, W.; Sandhu, I.; Halloran, K.M.; Grimm, P.; Schmidt, B.M.; Halloran, P. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003, 63, 2134–2143. [Google Scholar] [CrossRef]
- Feld, S.; Hirschberg, R. Growth Hormone, the Insulin-Like Growth Factor System, and the Kideny. Endocr. Rev. 1996, 17, 423–480. [Google Scholar] [PubMed]
- Sonntag, W.; Steger, R.W.; Forman, L.J.; Meites, J. Decreased Pulsatile Release of Growth Hormone in Old Male Rats. Endocrinology 1980, 107, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kawashima, S.; Seo, H.; Matsui, N. Age-Related Changes in Growth Hormone and Prolactin Messenger RNA Levels in the Rat. Endocrinol. Jpn. 1990, 37, 827–840. [Google Scholar] [CrossRef][Green Version]
- McElroy, G.; Chandel, N. Mitochondria control acute and chronic responses to hypoxia. Exp. Cell Res. 2017, 356, 217–222. [Google Scholar] [CrossRef]
- Nyengaard, J.R.; Flyvbjerg, A.; Rasch, R. The impact of renal growth, regression and regrowth in experimental diabetes mellitus on number and size of proximal and distal tubular cells in the rat kidney. Diabetologia 1993, 36, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Obineche, E.N.; Mensah-Brown, E.; Chandranath, S.I.; Ahmed, I.; Naseer, O.; Adem, A. Morphological Changes in the Rat Kidney Following Long-Term Diabetes. Arch. Physiol. Biochem. 2001, 109, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Vogetseder, A.; Picard, N.; Gaspert, A.; Walch, M.; Kaissling, B.; Le Hir, M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am. J. Physiol. Physiol. 2008, 294, C22–C28. [Google Scholar] [CrossRef] [PubMed]
- Obineche, E.N.; Mensah-Brown, E.; Chandranath, S.I.; Arafar, K.; Adem, A. Loss of Kidney IGF-1 Receptors in Experimental Long-Term Diabetic Rats. Endocr. Res. 2001, 27, 293–302. [Google Scholar] [CrossRef]
- Inoue, M.; Inoue, K.; Akimoto, K. Effects of Age and Sex in the Diagnosis of Type 2 Diabetes Using Glycated Haemoglobin in Japan: The Yuport Medical Checkup Centre Study. PLoS ONE 2012, 7, e40375. [Google Scholar] [CrossRef]
- Meyer, M.R.; Clegg, D.J.; Prossnitz, E.R.; Barton, M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. 2011, 203, 259–269. [Google Scholar] [CrossRef]
- Washio, M.; Mori, M.; Khan, M.; Sakauchi, F.; Watanabe, Y.; Ozasa, K.; Hayashi, K.; Miki, T.; Nakao, M.; Mikami, K.; et al. Diabetes mellitus and kidney cancer risk: The results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Int. J. Urol. 2007, 14, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, R.; Klaassen, Z.; Terris, M.K. Renal cell carcinoma: Links and risks. Int. J. Nephrol. Renov. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef]

| Factor | % of Total Variation Explained * | p-Value * |
|---|---|---|
| age | 57.07 | <0.0001 |
| diabetes mellitus | 0.7612 | 0.2514 |
| tissue compartment | 13.91 | <0.0001 |
| Interactions | ||
| age × DM | 6.581 | 0.0015 |
| age × tissue compartment | 1.193 | 0.1531 |
| DM × tissue compartment | 0.9100 | 0.2107 |
| age × DM × tissue compartment | 0.5807 | 0.3154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić, P.; Filipović, N.; Hamzić, L.F.; Puljak, L.; Vukojević, K.; Benzon, B. Potential Influence of Age and Diabetes Mellitus Type 1 on MSH2 (MutS homolog 2) Expression in a Rat Kidney Tissue. Genes 2022, 13, 1053. https://doi.org/10.3390/genes13061053
Babić P, Filipović N, Hamzić LF, Puljak L, Vukojević K, Benzon B. Potential Influence of Age and Diabetes Mellitus Type 1 on MSH2 (MutS homolog 2) Expression in a Rat Kidney Tissue. Genes. 2022; 13(6):1053. https://doi.org/10.3390/genes13061053
Chicago/Turabian StyleBabić, Paško, Natalija Filipović, Lejla Ferhatović Hamzić, Livia Puljak, Katarina Vukojević, and Benjamin Benzon. 2022. "Potential Influence of Age and Diabetes Mellitus Type 1 on MSH2 (MutS homolog 2) Expression in a Rat Kidney Tissue" Genes 13, no. 6: 1053. https://doi.org/10.3390/genes13061053
APA StyleBabić, P., Filipović, N., Hamzić, L. F., Puljak, L., Vukojević, K., & Benzon, B. (2022). Potential Influence of Age and Diabetes Mellitus Type 1 on MSH2 (MutS homolog 2) Expression in a Rat Kidney Tissue. Genes, 13(6), 1053. https://doi.org/10.3390/genes13061053








