Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cloning and Expression of the Recombinant Actin Protein
2.3. Generation of Polyclonal Anti-TvACT1r and Anti-TvTUBα1r Antibodies
2.4. Protein Preparation and SDS–PAGE in 1DE and 2DE
2.5. MS Analysis by LC-ESI–HDMS
2.6. Western Blot (WB) Assays
2.7. Indirect Immunofluorescence Assay (IFA)
2.8. Scanning Electron Microscopy
3. Results
3.1. Actin and Tubulin Genes Present in the Genome of T. vaginalis
3.2. In Silico Analysis of the Deduced 29 Actin-like and 31 Tubulin-like Protein Fragments Found in the Genome of T. vaginalis
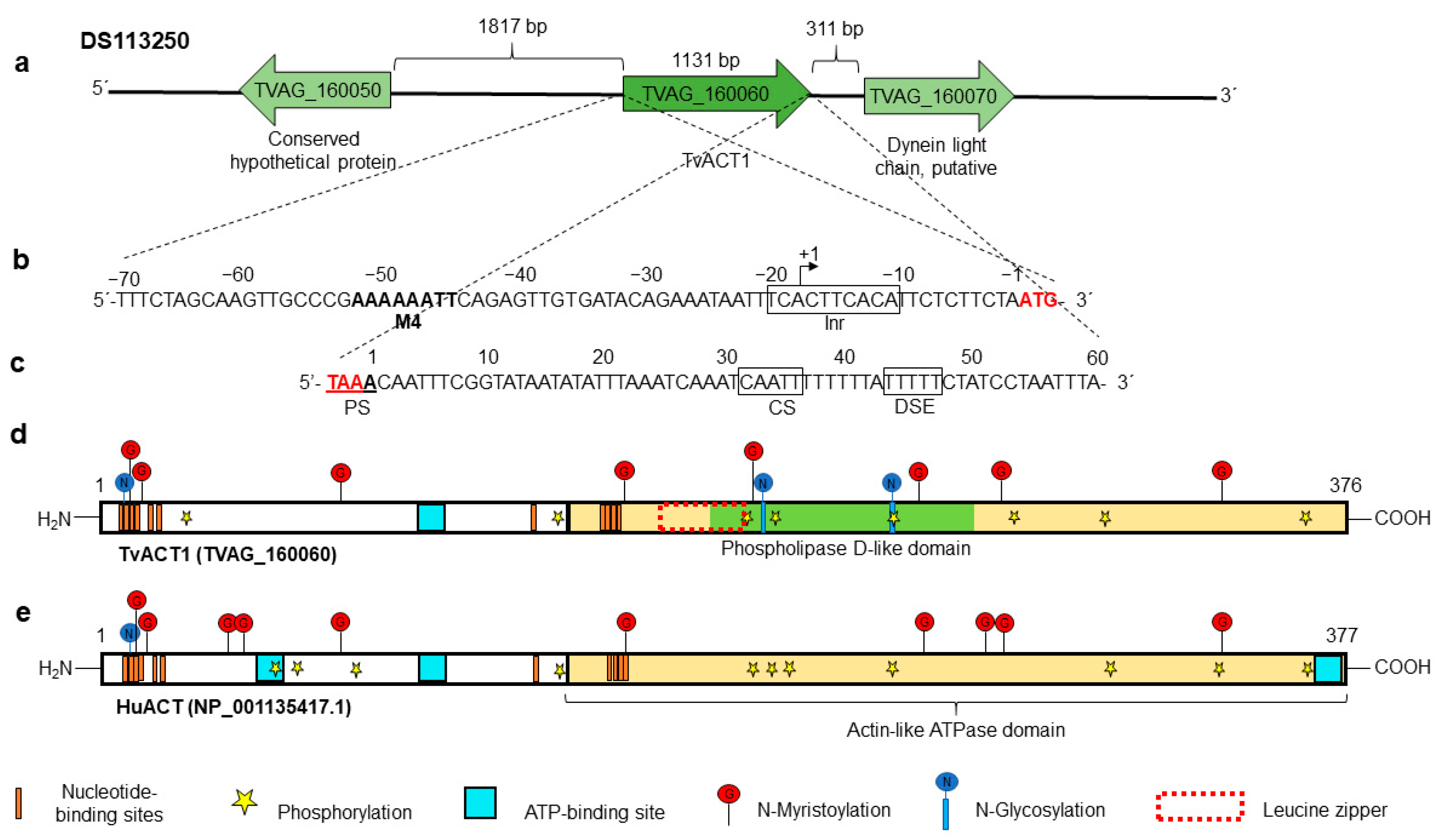
3.3. In Silico Analysis of the tvact1 and tvtubα1 Genes and TvACT1 and TvTUBα1 Proteins
3.4. Expression of Bonafide Actin and Tubulin Genes and Actin-like and Tubulin-like Coding DNA Segments Reported in the Different ESTs and Proteomes of T. vaginalis
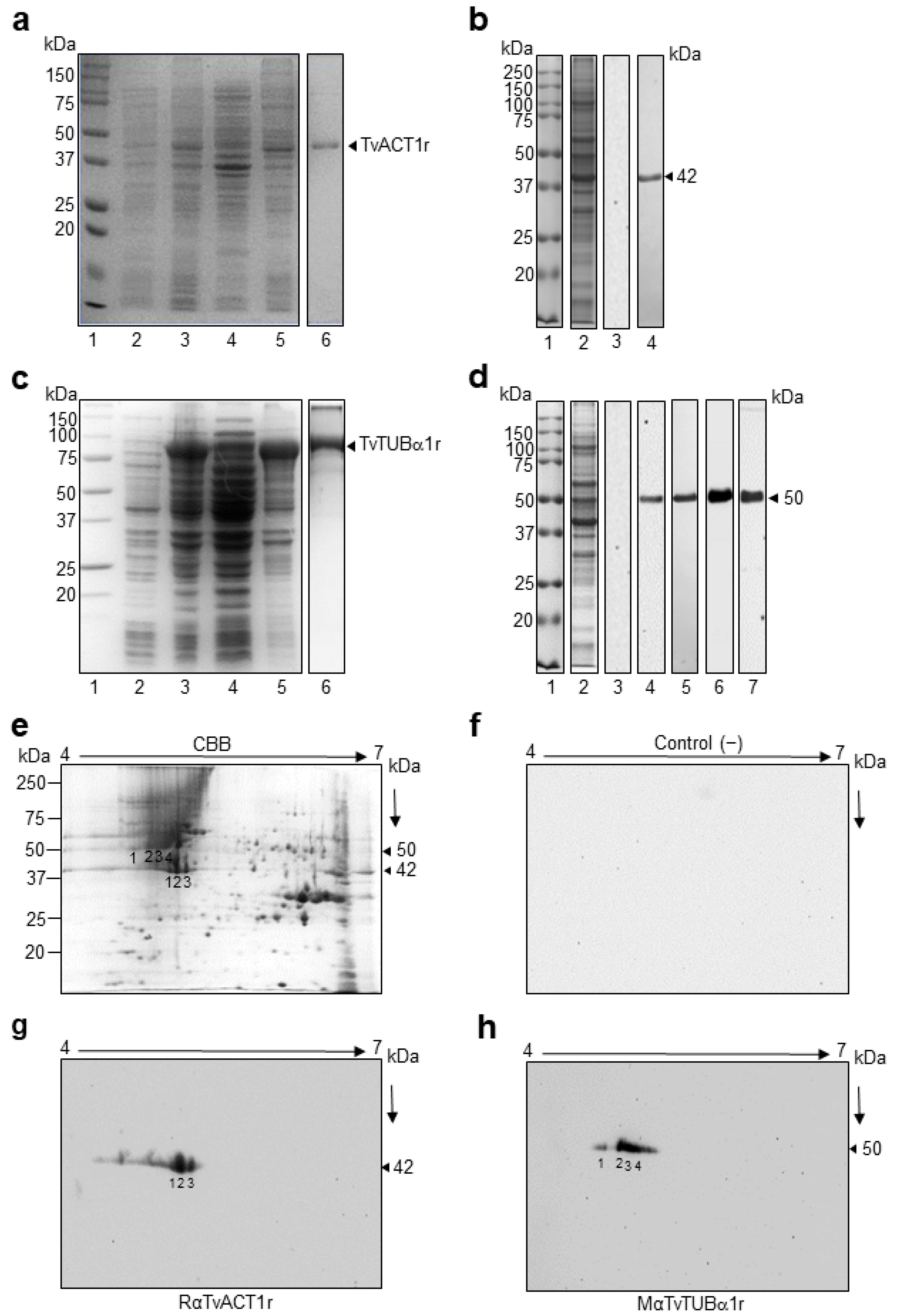
3.5. Cloning and Sequencing of the tvact1 and tvtubα1 Genes and Expression and Purification of the Recombinant TvACT1 and TvTUBα1 Proteins for Antibody Production
3.6. Immunodetection of TvACT and TvTUB in T. vaginalis by 1DE and 2DE WB Assays
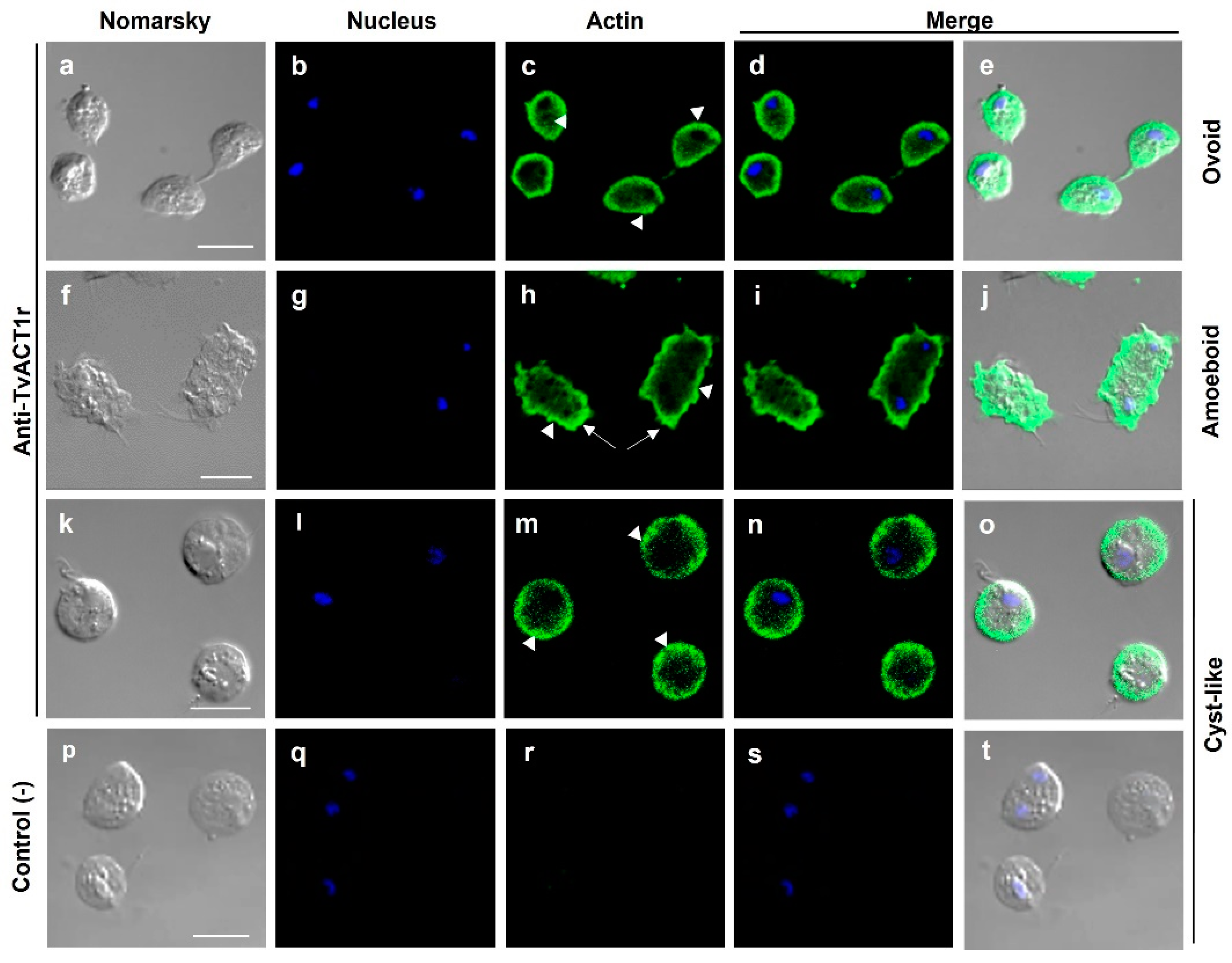
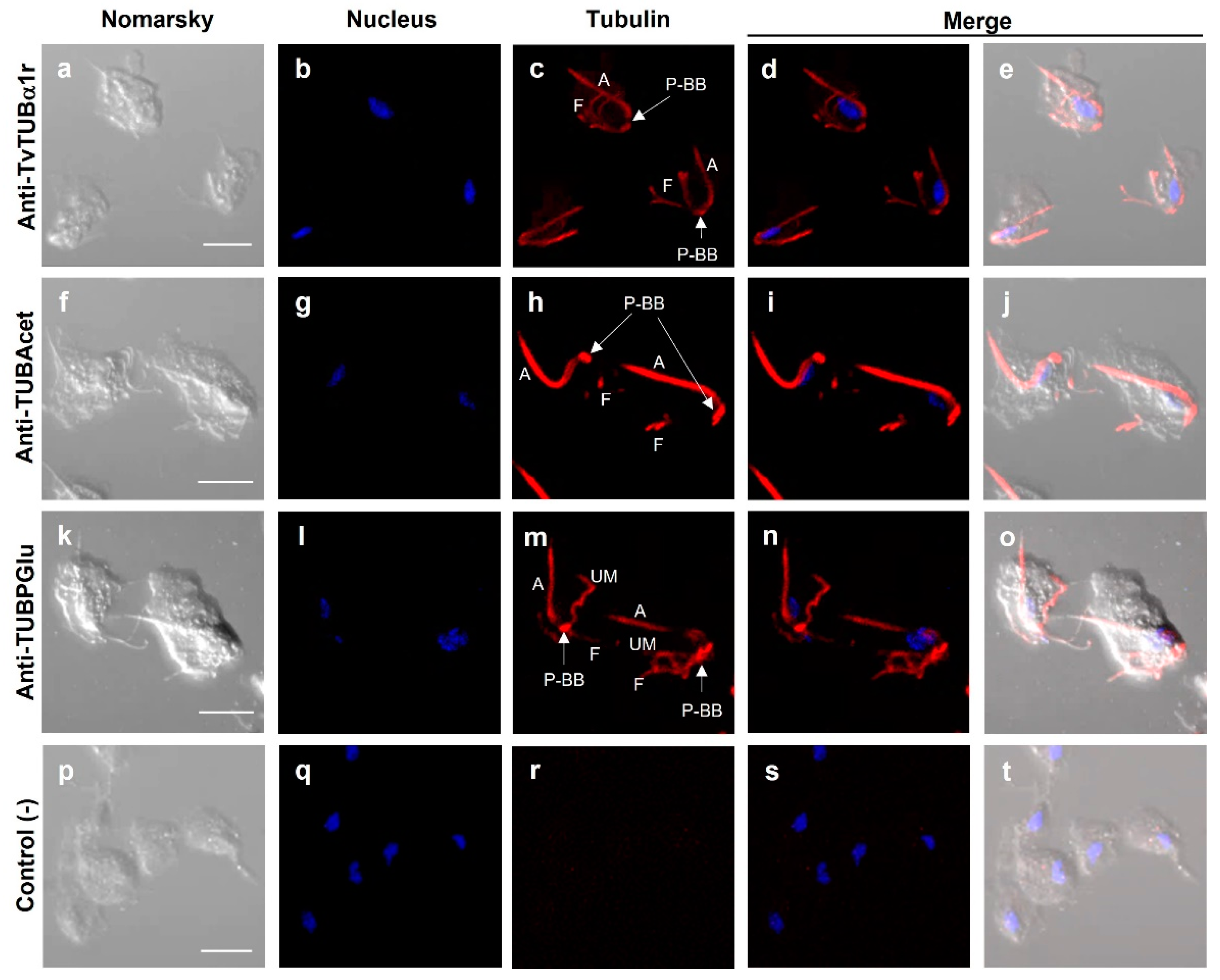
3.7. Immunolocalization of Actin and Tubulin in T. vaginalis
3.8. Different Types of Cell–Cell Interactions Mediated by Actin or Tubulin in T. vaginalis
3.9. Immunolocalization of Both TvACT and TvTUB in T. vaginalis during Karyokinesis and Cytokinesis
3.10. Last Stage of T. vaginalis Cytokinesis Observed by SEM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Sector Strategy of Health Against Infections Sexual Transmission 2016–2021; WHO: Geneva, Switzerland, 2021.
- McClelland, R.S.; Sangare, L.; Hassan, W.M.; Lavreys, L.; Mandaliya, K.; Kiarie, J.; Ndinya-Achola, J.; Jaoko, W.; Baeten, J.M. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 2007, 195, 698–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavedzenge, S.N.; Van Der Pol, B.; Cheng, H.; Montgomery, E.T.; Blanchard, K.; de Bruyn, G.; Ramjee, G.; van der Straten, A. Epidemiological Synergy of Trichomonas vaginalis and in Zimbabwean and South African Women. Sex. Trans. Dis. 2010, 37, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Fastring, D.R.; Amedee, A.; Gatski, M.; Clark, R.A.; Mena, L.A.; Levison, J.; Schmidt, N.; Rice, J.; Gustat, J.; Kissinger, P. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis and vaginal shedding of HIV-1 RNA. Sex. Trans. Dis. 2014, 41, 173–179. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, R.; González-Robles, A.; Martínez-Palomo, A.; Alderete, J.F. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol. Microbiol. 1993, 7, 299–309. [Google Scholar] [CrossRef]
- Pereira-Neves, A.; Ribeiro, K.C.; Benchimol, M. Pseudocysts in Trichomonads-new insights. Protist 2003, 154, 313–329. [Google Scholar] [CrossRef]
- Afzan, M.Y.; Suresh, K. Pseudocyst forms of Trichomonas vaginalis from cervical neoplasia. Parasitol. Res. 2012, 111, 371–381. [Google Scholar] [CrossRef]
- Dias-Lopes, G.; Saboia-Vahia, L.; Margotti, E.T.; de Souza Fernandes, N.; de Faria Castro, C.L.; Oliveira-Junior, F.O.; Figueiredo Peixoto, J.; Britto, C.; Costa e Silva-Filho, F.; Cuervo, P.; et al. Morphologic study of the effect of iron on pseudocyst formation in Trichomonas vaginalis and its interaction with human epithelial cells. Mem. Inst. Oswaldo Cruz 2017, 112, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Kusdian, G.; Woehle, C.; Martin, W.F.; Gould, S.B. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell. Microbiol. 2013, 15, 1707–1721. [Google Scholar] [CrossRef]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, C.M.; Besteiro, S.; Sicheritz-Ponten, T.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomona vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Crucitti, T.; Abdellati, S.; Van Dyck, E.; Buvé, A. Molecular typing of the actin gene of Trichomonas vaginalis isolate by PCR-restriction fragment length polymorphism. Clin. Microbiol. Infect. 2008, 14, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuon-Kyuong, G.; Won-Sik, S.; Hye-Won, Y.; So-Young, J.; Su-Min, S.; Jae-Sook, R.; Hyun-Hee, K.; Won-Ki, L.; Dong-ll, C.; Yeonchul, H. Loop-mediated isothermal amplification targeting actin DNA of Trichomonas vaginalis. Korean J. Parasitol. 2016, 54, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Masha, C.S.; Cools, P.; Crucitti, T.; Sanders, J.E.; Vaneechoutte, M. Molecular typing of Trichomonas vaginalis isolate by actin gene sequence analysis and carriage of T. vaginalis viruses. Parasit. Vectors 2017, 10, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias-Lopes, G.; Wiśniewski, J.R.; Pinho de Souza, N.; Ennes Vidal, V.; Padron, G.; Britto, C.; Cuervo, P.; De Jesus, J.B. In-depth quantitative proteomic analysis of trophozoites and pseudocysts of Trichomonas vaginalis. J. Proteome Res. 2018, 17, 3704–3718. [Google Scholar] [CrossRef]
- De Jesus, J.B.; Cuervo, P.; Junqueira, M.; Britto, C.; Costa Silva-Filho, F.; Sabóia-Vahia, L.; Javier González, L.; Barbosa Domont, G. Application of two-dimensional electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for proteomic analysis of the sexually transmitted parasite Trichomonas vaginalis. J. Mass Spectrom. 2007, 42, 1463–1473. [Google Scholar] [CrossRef]
- Cuervo, P.; Cupolillo, E.; Britto, C.; Javier González, L.; Costa Silva-Filho, F.; Coutinho Lopes, L.; Barbosa Domont, G.; Batista De Jesus, J. Differential soluble protein expression between Trichomonas vaginalis isolates exhibiting low and high virulence phenotypes. J. Proteom. 2008, 7, 109–122. [Google Scholar] [CrossRef]
- De Miguel, N.; Lustig, G.; Twu, O.; Chattopadhyay, A.; Wohlschlegel, J.A.; Johnson, P.J. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol. Cell. Proteom. 2010, 9, 1554–1566. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.E.; Brown, M.T.; Shiflett, A.M.; Dyall, S.D.; Hayes, R.D.; Xie, Y.; Loo, J.A.; Johnson, P.J. The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int. J. Parasitol. 2011, 41, 1421–1434. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.Y.; Huang, P.J.; Ku, F.M.; Lin, R.; Alderete, J.F.; Tang, P. Comparative transcriptomic and proteomic analyses of Trichomonas vaginalis following adherence to fibronectin. Infect. Immun. 2012, 80, 3900–3911. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, N.C.; Horváthová, L.; Jedelský, P.L.; Sedinová, M.; Rada, P.; Marcinčiková, M.; Tachezy, H.I. Iron-induced cha nges in the proteome of Trichomonas vaginalis hydrogenosome. PLoS ONE 2013, 8, e65148. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.B.; Woehle, C.; Kusdian, G.; Landan, G.; Tachezy, J.; Zimorski, V.; Martin, W.F. Deep sequencing of Trichomonas vaginalis during the early infection of vaginal epithelial cells and amoeboid transition. Int. J. Parasitol. 2013, 43, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Granados, L.I.; Villalpando, J.L.; Vázquez-Carrillo, L.I.; Arroyo, R.; Mendoza-Hernández, G.; Alvarez-Sánchez, M.E. TvMP50 is an immunogenic metalloproteinase during male trichomoniasis. Mol. Cell. Proteom. 2013, 12, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, C.G.; Vashisht, A.A.; Wohlschlegel, A.J.; Johnson, J.P. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host: Parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riestra, A.M.; Gandhi, S.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Urban, S.; Johnson, P.J. A Trichomonas vaginalis rhomboid protease and its substrate modulate parasite attachment and cytolysis of host cells. PLoS Pathog 2015, 11, e1005294. [Google Scholar] [CrossRef] [Green Version]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; de Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 2017, 75, 2211–2226. [Google Scholar] [CrossRef]
- Alvarez-Sánchez, M.E.; Quintas-Granados, L.I.; Vázquez-Carrillo, L.I.; Puente-Rivera, J.; Villalobos-Osnaya, A.; Ponce-Regalado, M.D.; Camacho-Nuez, M. Proteomic profile approach od effect of putrescine depletion over Trichomonas vaginalis. Parasitol. Res. 2018, 117, 1371–1380. [Google Scholar] [CrossRef]
- Beri, D.; Yadav, P.; Nandini Devi, H.R.; Narayana, C.; Gadara, D.; Tatu, U. Demonstration and characterization of cyst-like structures in the life cycle of Trichomonas vaginalis. Fromt. Cell. Infect. Microbiol. 2020, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Molgora, B.M.; Kumar Rai, A.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Johnson, P.J. A Novel Trichomonas vaginalis surface protein modulates parasite attachment via protein:host cell proteoglycan interaction. mBio 2021, 12, e03374-20. [Google Scholar] [CrossRef]
- Zimmann, N.; Rada, P.; Žárský, V.; Smutná, T.; Záhonová, K.; Dacks, J.; Harant, K.; Hrdý, I.; Tachezy, J. Proteomic analysis of Trichomonas vaginalis phagolysosome, lysosomal targeting, and unconventional secretion of cysteine peptidases. Mol. Cell. Proteom. 2022, 21, 100174. [Google Scholar] [CrossRef]
- Brugerolle, G.; Bricheux, G.; Coffe, G. Actin cytoskeleton demonstration in Trichomonas vaginalis and other trichomonads. Biol. Cell. 1996, 88, 29–36. [Google Scholar] [CrossRef]
- Juliano, C.; Rubino, S.; Zicconi, D.; Cappuccinelli, P. An immunofluorescent study of the microtubule organization in Trichomonas vaginalis using antitubulin antibodies. J. Protozool. 1986, 33, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, J.D.; Krug, E.C. Detection of microtubules of the flagellate Trichomonas vaginalis by monoclonal antibodies specific for beta-tubulin. J. Protozool. 1986, 33, 576–578. [Google Scholar] [CrossRef]
- Delgado-Viscogliosi, P.; Brugerolle, G.; Viscogliosi, E. Tubulin post-translational modifications in the primitive protest Trichomonas vaginalis. Cell Motil. Cytoskeleton 1996, 33, 288–297. [Google Scholar] [CrossRef]
- Schneider, A.; Plessmann, U.; Felleisen, R.; Weber, K. Posttranslational modifications of trichomonad tubulins; identification of multiple glutamylation sites. FEBS 1998, 429, 399–402. [Google Scholar] [CrossRef] [Green Version]
- Noël, C.; Gerbod, D.; Fast, N.M.; Wintjens, R.; Delgado-Viscogliosi, P.; Doolittle, W.F.; Viscogliosi, E. Tubulins in Trichomonas vaginalis: Molecular characterization of alpha-tubulin genes, posttranslational modifications, and homology modeling of the tubulin dimer. J. Eukaryot. Microbiol. 2001, 48, 647–654. [Google Scholar] [CrossRef]
- Coutinho Lopes, L.; Ribeiro, K.C.; Benchimol, M. Immunolocalization of tubulin isoforms and post-translational modifications in the protists Tritrichomonas foetus and Trichomonas vaginalis. Histochem. Cell Biol. 2001, 116, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lecke, S.B.; Tasca, T.; Souto, A.A.; De Carli, G.A. Trichomonas vaginalis: Microtubule cytoskeleton distribution using fluorescent taxoid. Exp. Parasitol. 2002, 102, 113–116. [Google Scholar] [CrossRef]
- Ribeiro, K.C.; Pereira-Neves, A.; Benchimol, M. The mitotic spindle and associated membranes in the closed mitosis of trichomonads. Biol. Cell 2002, 94, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Vieira, P.B.; Borges, F.P.; Gottardi, B.; Stuepp, C.; Larré, A.B.; Tasca, T.; De Carli, G.A. Analysis of microtubule cytoskeleton distribution using a fluorescent taxoid in two trichomonadid protozoa: Trichomonas gallinae and Tritrichomonas foetus. Exp. Parasitol. 2008, 119, 186–191. [Google Scholar] [CrossRef]
- Furtado, M.B.; Benchimol, M. Observation of membrane fusion on the interaction of Trichomonas vaginalis with human vaginal epithelial cells. Parasitol. Res. 1998, 84, 213–220. [Google Scholar] [CrossRef]
- Rupp, I.; Sologub, L.; Williamson, K.C.; Scheuermayer, M.; Reininger, L.; Doerig, C.; Eksi, S.; Kombila, D.U.; Frank, M.; Pradel, G. Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 2011, 21, 683–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, R.; Karhu, E.; Wang, J.S.; Delgado, S.; Zukerman, R.; Mittal, J.; Jhaveri, V.M. Cell communication by tunneling nanotubes: Implications in disease and therapeutic applications. J. Cell. Physiol. 2019, 234, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Walters, H.E.; Cox, L.S. Intercellular transfer of mitochondria between senescent cells through cytoskeleton-supported intercellular bridges requires mTOR and CDC42 signalling. Oxid. Med. Cell. Longev. 2021, 2021, 6697861. [Google Scholar] [CrossRef] [PubMed]
- Cordero Cervantes, D.; Zurzolo, C. Peering into tunneling nanotubes—The path forward. EMBO J. 2021, 40, e105789. [Google Scholar] [CrossRef]
- Goulas, T.; Cuppari, A.; Garcia-Castellanos, R.; Snipas, S.; Glockshuber, R.; Arolas, J.L.; Gomis-Ruth, F.X. The pCri System: A vector collection for recombinant protein expression and purification. PLoS ONE 2014, 9, e112643. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.B.; Ortega-López, J.; Cárdenas-Guerra, R.E.; Reséndiz-Cardiel, G.; Chávez-Munguía, B.; Lagunes-Guillen, A.; Arroyo, R. Characterization of a novel endoegenous cysteine proteinase inhibitor, trichocystatin-3 (TC-3), localized on the surface of Trichomonas vaginalis. Int. J. Biochem. Cell. Biol. 2018, 102, 87–100. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Liston, R.D.; Johnson, J.P. Analysis of a ubiquitous promoter element in a primitive eukaryote: Early evolution of the initiator element. Mol. Cell. Biol. 1999, 19, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Liston, D.R.; Lau, A.O.; Ortiz, D.; Smale, S.T.; Johnson, P.J. Initiator recognition in a primitive eukaryote: IBP39, an initiator-binding protein from Trichomonas vaginalis. Mol. Cell. Biol. 2001, 21, 7872–7882. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Johnson, P. Gene expression in the unicellular eukaryote Trichomonas vaginalis. Res. Microbiol. 2011, 162, 646–654. [Google Scholar] [CrossRef]
- Espinosa, N.; Hernández, R.; López-Griego, L.; López-Villaseñor, I. Separable putative polyadenylation and cleavage motifs in Trichomonas vaginalis. Gene 2002, 289, 81–86. [Google Scholar] [CrossRef]
- Fuentes, V.; Barrera, G.; Sánchez, J.; Hernández, R.; López-Villaseñor, I. Functional analysis of sequence motifs involved in the polyadenylation of Trichomonas vaginalis mRNAs. Eukaryot. Cell. 2012, 11, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadders, A.M.; Agromayor, M.; Obita, T.; Perisic, O.; Caballe, A.; Kloc, M.; Lamers, H.M.; Williams, L.R.; Martin-Serrano, J. ESCRT-III binding protein MITD1 is involved in cytokinesis and has an unanticipated PLD fold that binds membranes. Proc. Natl. Acad. Sci. USA 2012, 109, 17424–17429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, K.C.; Monteiro-Leal, L.H.; Benchimol, M. Contributions of the axostyle and flagella to closed mitosis in the protists Tritrichomonas foetus and Trichomonas vaginalis. J. Eukaryot. Microbiol. 2000, 47, 481–492. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perri, B.J.; Ervasti, J.M. The actin gene family: Function follows isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell. Sci. 2001, 114, 2723–2733. [Google Scholar] [CrossRef]
- Joseph, J.M.; Fey, P.; Ramalingam, N.; Liu, X.I.; Rohlfs, M.; Noegel, A.A.; Müller-Taubenberger, A.; Glöckner, G.; Schleicher, M. The Actinome of Dictyostelium discoideum in Comparison to actins and actin-related proteins from other organisms. PLoS ONE 2008, 3, e2654. [Google Scholar] [CrossRef] [Green Version]
- McKinney, E.C.; Meagher, R.B. Members of the Arabidopsis actin gene family are widely dispersed in the genome. Genetics 1998, 149, 663–675. [Google Scholar] [CrossRef]
- Oakley, B.R. An abundance of tubulins. Trends Cell. Biol. 2000, 12, 537–542. [Google Scholar] [CrossRef]
- Raff, E.C.; Fackenthal, J.D.; Hutchens, J.A.; Hoyle, H.D.; Turner, F.R. Microtubule architecture specified by a beta-tubulin isoform. Science 1997, 275, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Terman, J.R.; Kashina, A. Post-translational modification and regulation of actin. Curr. Opin. Cell Biol. 2013, 25, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varland, S.; Vandekerckhove, J.; Drazic, A. Actin post-translational modifications: The cinderella of cytoskeletal control. Trends Biochem. Sci. 2019, 44, 502–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wloga, D.; Joachimiak, E.; Louka, P.; Gaerting, J. Posttranslational modifications of tubulin and cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shu, S.; Hong, M.S.; Levine, R.L.; Korn, E.D. Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments. Proc. Natl. Acad. Sci. USA 2006, 103, 13694–13699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budniak, A.; O’Day, D.H. Tyrosine phosphorylation of actin during microcyst formation and germination in Polysphondylium pallidum. Protist 2011, 62, 490–502. [Google Scholar] [CrossRef]
- Krtková, J.; Xu, J.; Lalle, M.; Steele-Ogus, M.; Alas, G.C.M.; Sept, D.; Paredez, A.R. 14-3-3 Regulates actin filament formation in the deep-branching eukaryote Giardia lamblia. mSphere 2017, 2, e00248.17. [Google Scholar] [CrossRef] [Green Version]
- Cappuccinelli, P.; Sellito, C.; Zicconi, D.; Juliano, C. Structural and molecular organization of Trichomonas vaginalis cytoskeleton. Acta Univ. Carolinae Biol. 1986, 30, 211–217. [Google Scholar]
- Talamás-Rohana, P.; Ríos, A. Actin fibers in Entamoeba histolytica induced by fibronectin. Arch. Med. Res. 2000, 31, S131–S133. [Google Scholar] [CrossRef]
- Brugerolle, G. Etude de la cryptopleuromitose et de la morphogénèse de division chez Trichomonas vaginalis et chez plusieurs genres de Trichomonadines primitives. Protistologica 1975, 11, 457–468. [Google Scholar]
- Austefjord, M.W.; Gerdes, H.H.; Wang, X. Tunneling Nanotubes: Diversity in Morphology and Structure. Commun. Integr. Biol. 2014, 7, e27934. [Google Scholar] [CrossRef] [PubMed]

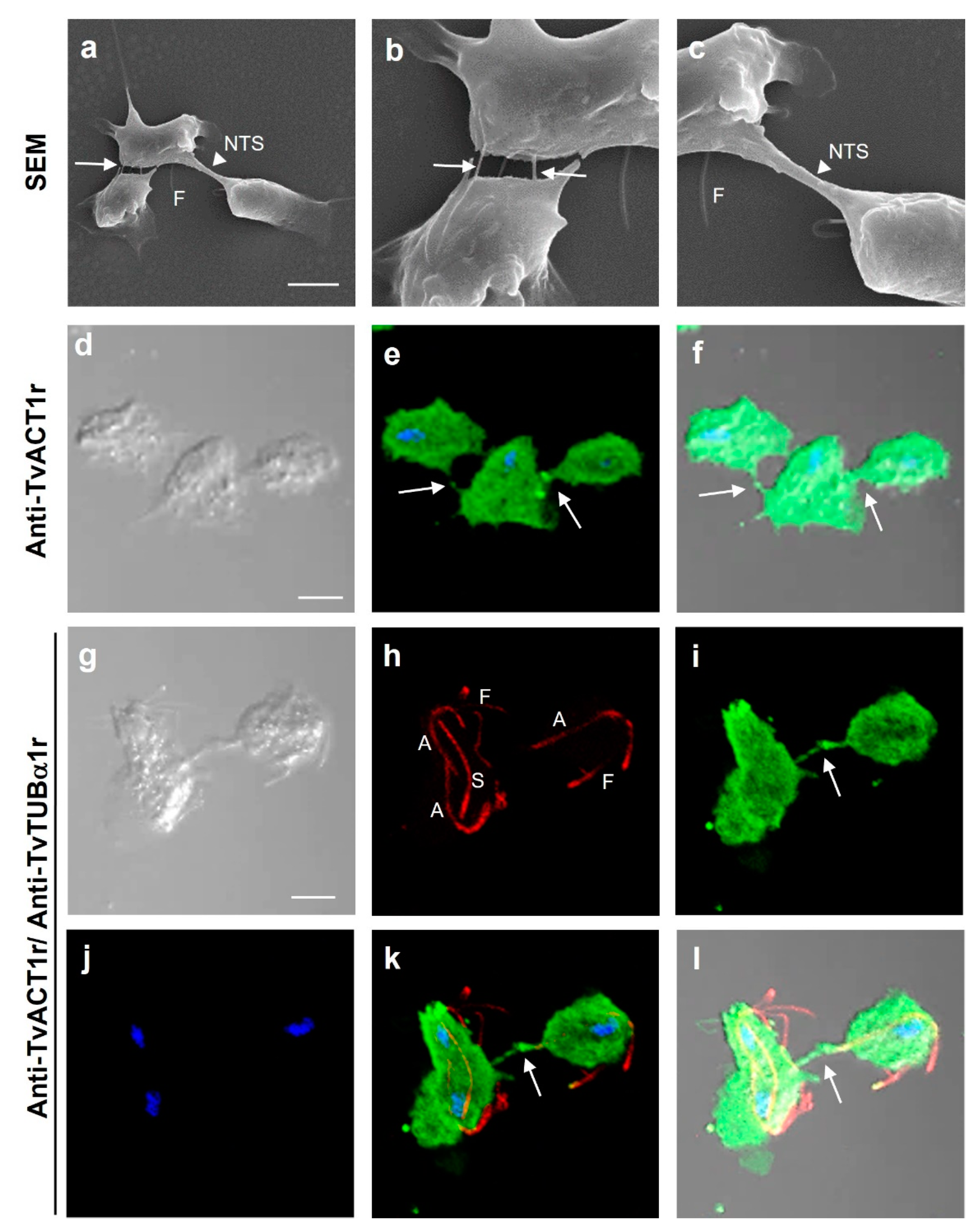
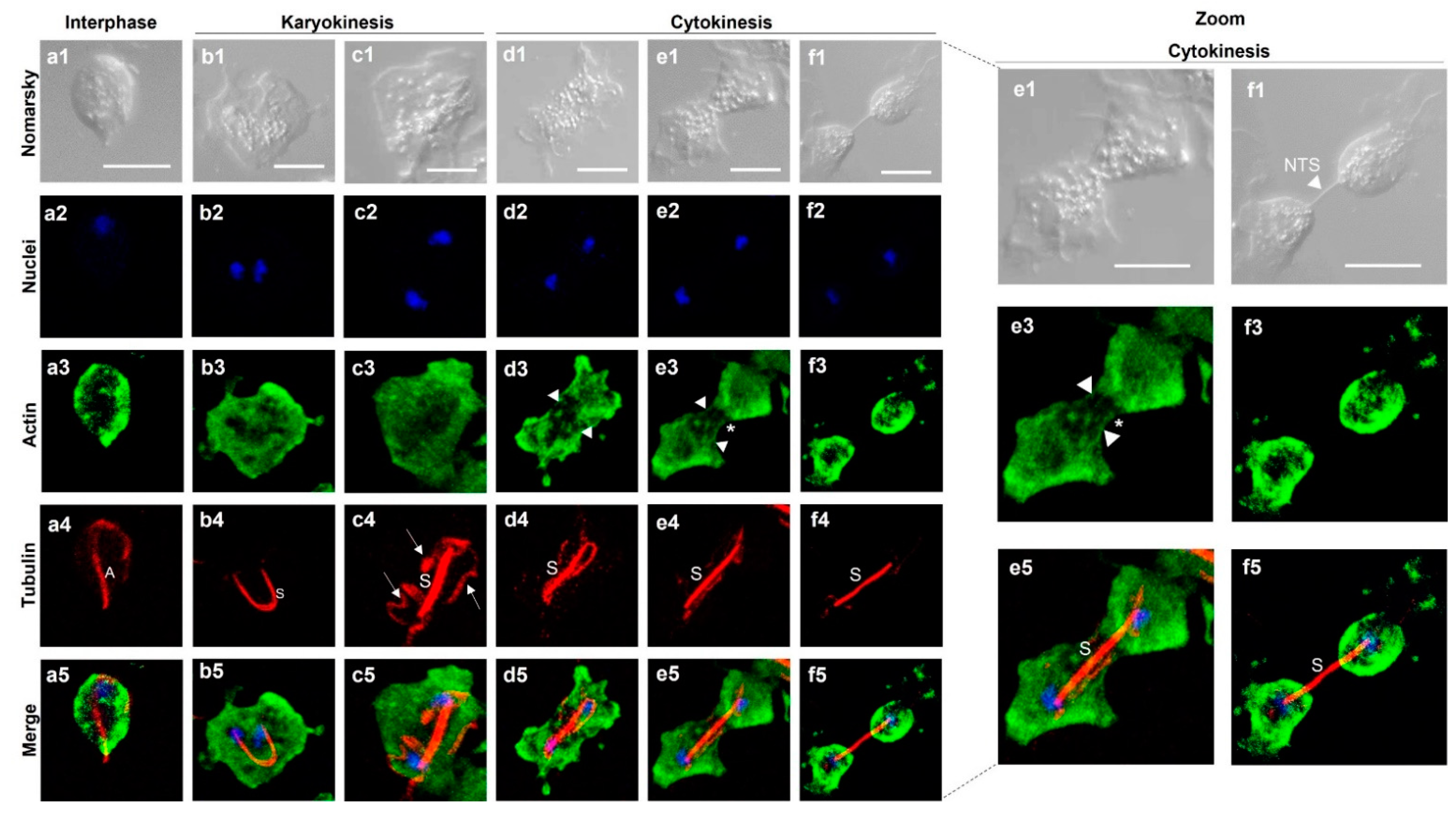
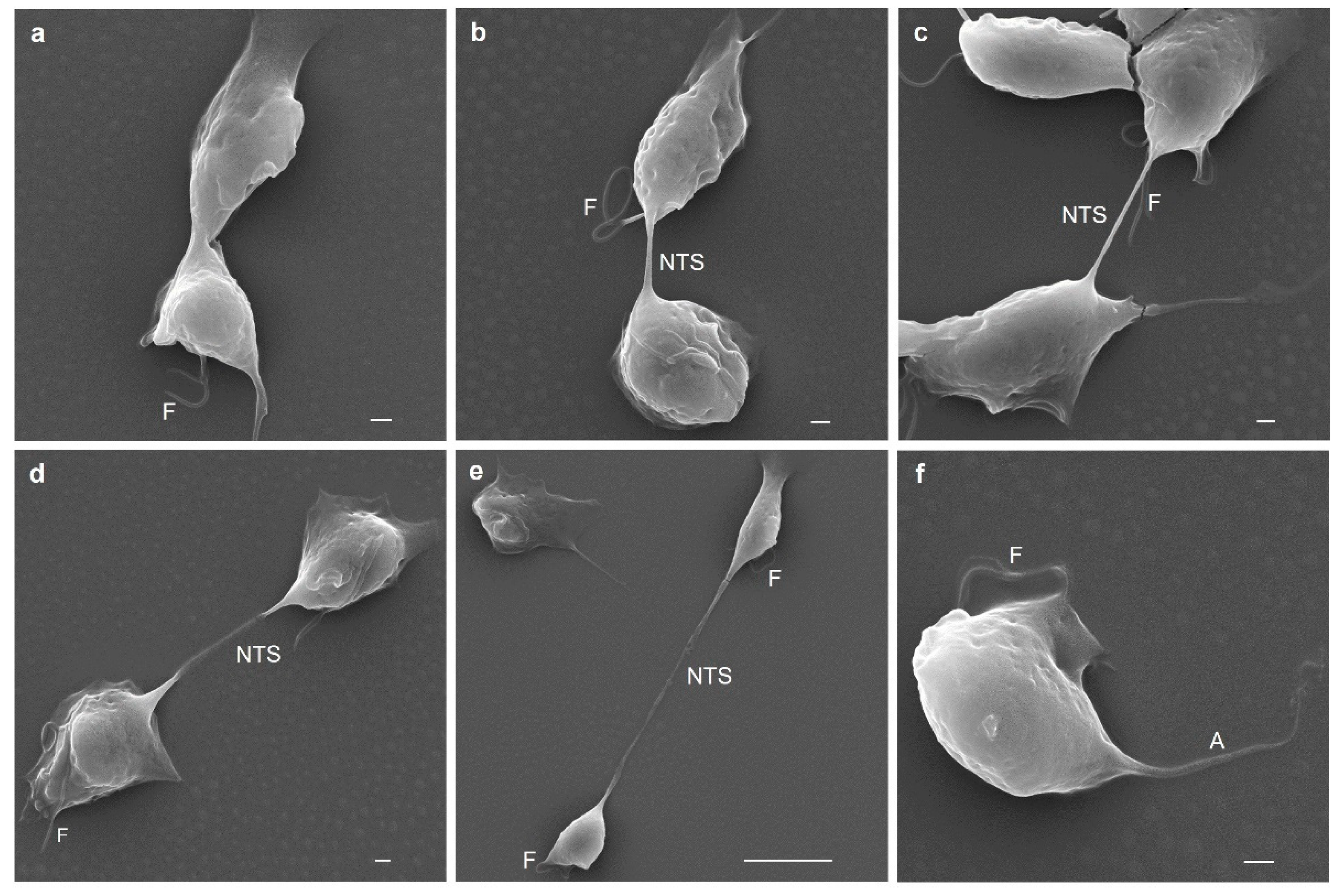
| Antibody | Spot Code 1 | Protein Type 2 | Locus Tag 3 TVAG_ | Protein Number 4 | Coverage (%) 5 | Theoretical Molecular Mass (kDa) |
|---|---|---|---|---|---|---|
| anti-TvACT1r | 1 | Actin | 249200 | TvACT4 | 54.52 | 42.2 |
| 2 | Actin | 249200 | TvACT4 | 54.52 | 42.2 | |
| 3 | Actin | 249200 | TvACT4 | 42.55 | 42.2 | |
| anti-TvTUBα1r | 1 | α-tubulin | 456920 | TvTUBβ2 | 15.21 | 50.5 |
| 2 | β-tubulin | 359090 | TvTUBα1 | 24.56 | 50.7 | |
| γ-tubulin | 525430/456920 | TvTUBβ2 | 25.88/23.00 | 45.2/50.5 | ||
| 3 | α-tubulin | 456920 | TvTUBβ2 | 34.68 | 50.5 | |
| β-tubulin | 359090 | TvTUBα1 | 20.13 | 50.7 | ||
| 4 | γ-tubulin | 525430/456920 | TvTUBβ2 | 25.88/23.00 | 45.2/50.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Benito, S.; Rivera-Rivas, L.A.; Sánchez-Ayala, L.; Ortega-López, J.; Montes-Flores, O.; Talamás-Lara, D.; Arroyo, R. Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis. Genes 2022, 13, 1067. https://doi.org/10.3390/genes13061067
Lorenzo-Benito S, Rivera-Rivas LA, Sánchez-Ayala L, Ortega-López J, Montes-Flores O, Talamás-Lara D, Arroyo R. Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis. Genes. 2022; 13(6):1067. https://doi.org/10.3390/genes13061067
Chicago/Turabian StyleLorenzo-Benito, Sebastián, Luis Alberto Rivera-Rivas, Lizbeth Sánchez-Ayala, Jaime Ortega-López, Octavio Montes-Flores, Daniel Talamás-Lara, and Rossana Arroyo. 2022. "Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis" Genes 13, no. 6: 1067. https://doi.org/10.3390/genes13061067
APA StyleLorenzo-Benito, S., Rivera-Rivas, L. A., Sánchez-Ayala, L., Ortega-López, J., Montes-Flores, O., Talamás-Lara, D., & Arroyo, R. (2022). Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis. Genes, 13(6), 1067. https://doi.org/10.3390/genes13061067







