The Relationship between ACE, ACTN3 and MCT1 Genetic Polymorphisms and Athletic Performance in Elite Rugby Union Players: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Design
2.3. Anthropometry and Body Composition
2.4. Physical Performance
2.5. Molecular Biology Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehlert, T.; Simon, P.; Moser, D.A. Epigenetics in Sports. Sports Med. 2013, 43, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, I.I.; Egorova, E.S.; Gabdrakhmanova, L.J.; Fedotovskaya, O.N. Genes and Athletic Performance: An Update. Med. Sport Sci. 2016, 61, 41–54. [Google Scholar] [PubMed]
- Ghosh, A.; Mahajan, P.B. Can Genotype Determine the Sports Phenotype? A Paradigm Shift in Sports Medicine. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Margiotti, K.; Longo, U.G.; Loppini, M.; Fazio, V.M.; Denaro, V. The Genetics of Sports Injuries and Athletic Performance. Muscles Ligaments Tendon J. 2013, 3, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.-E.; Silva, M.-R.G.; Cerqueira, F.; Tavares, V.; Medeiros, R. Genomic Profile in Association with Sport-Type, Sex, Ethnicity, Psychological Traits and Sport Injuries of Elite Athletes. J. Sports Med. Phys. Fit. 2022, 62, 418–434. [Google Scholar] [CrossRef]
- Bonen, A. The Expression of Lactate Transporters (MCT1 and MCT4) in Heart and Muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef]
- Sawczuk, M.; Banting, L.K.; Cieszczyk, P.; Maciejewska-Karłowska, A.; Zarebska, A.; Leońska-Duniec, A.; Jastrzebski, Z.; Bishop, D.J.; Eynon, N. MCT1 A1470T: A Novel Polymorphism for Sprint Performance? J. Sci. Med. Sport 2015, 18, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Woods, D. Angiotensin-Converting Enzyme, Renin-Angiotensin System and Human Performance. Med. Sport Sci. 2009, 54, 72–87. [Google Scholar]
- MacArthur, D.G.; North, K.N. A Gene for Speed? The Evolution and Function of α-Actinin-3. BioEssays 2004, 26, 786–795. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. ACTN3: More than Just a Gene for Speed. Front. Physiol. 2017, 8, 1080. [Google Scholar] [CrossRef] [Green Version]
- Fedotovskaya, O.N.; Mustafina, L.J.; Popov, D.V.; Vinogradova, O.L.; Ahmetov, I.I. A Common Polymorphism of the MCT1 Gene and Athletic Performance. Int. J. Sports Physiol. Perform. 2014, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cupeiro, R.; Benito, P.J.; Maffulli, N.; Calderón, F.J.; González-Lamuño, D. MCT1 Genetic Polymorphism Influence in High Intensity Circuit Training: A Pilot Study. J. Sci. Med. Sport 2010, 13, 526–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilherme, J.P.L.F.; Bertuzzi, R.; Lima-Silva, A.E.; da Pereira, A.C.; Lancha Junior, A.H. Analysis of Sports-Relevant Polymorphisms in a Large Brazilian Cohort of Top-Level Athletes. Ann. Hum. Genet. 2018, 82, 254–264. [Google Scholar] [CrossRef]
- Duthie, G.; Pyne, D.; Hooper, S. Applied Physiology and Game Analysis of Rugby Union. Sport. Med. 2003, 33, 973–991. [Google Scholar] [CrossRef] [PubMed]
- Ziv, G.; Lidor, R. On-Field Performance of Rugby Union Players—A Review. J. Strength Cond. Res. 2016, 30, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.; Antrobus, M.; Stebbings, G.K.; Day, S.H.; Callus, P.; Erskine, R.M.; Bennett, M.A.; Kilduff, L.P.; Williams, A.G. Anthropometric and Physiological Characteristics of Elite Male Rugby Athletes. J. Strength Cond. Res. 2020, 34, 1790–1801. [Google Scholar] [CrossRef]
- Austin, D.; Gabbett, T.; Jenkins, D. The Physical Demands of Super 14 Rugby Union. J. Sci. Med. Sport 2011, 14, 259–263. [Google Scholar] [CrossRef]
- Heffernan, S.M.; Kilduff, L.P.; Day, S.H.; Pitsiladis, Y.P.; Williams, A.G. Genomics in Rugby Union: A Review and Future Prospects. Eur. J. Sport Sci. 2015, 15, 460–468. [Google Scholar] [CrossRef]
- Quarrie, K.L.; Handcock, P.; Waller, A.E.; Chalmers, D.J.; Toomey, M.J.; Wilson, B.D. The New Zealand Rugby Injury and Performance Project. III. Anthropometric and Physical Performance Characteristics of Players. Br. J. Sports Med. 1995, 29, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, S.M.; Kilduff, L.P.; Erskine, R.M.; Day, S.H.; McPhee, J.S.; McMahon, G.E.; Stebbings, G.K.; Neale, J.P.H.; Ribbans, W.J.; Cook, C.J.; et al. Association of ACTN3 R577X but Not ACE I/D Gene Variants with Elite Rugby Union Player Status and Playing Position. Physiol. Genom. 2016, 48, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.P.; Chew, K.; Koh, A.; Guan, M.; Wong, Y.S.; Sum, C.F. The Relationship between ACE Gene ID Polymorphism and Aerobic Capacity in Asian Rugby Players. Singap. Med. J. 2009, 50, 997–1003. [Google Scholar]
- Bell, W.; Colley, J.P.; Gwynne, J.R.; Glazier, P.; Evans, W.D.; Darlington, S.E. ACE ID Genotype and Leg Power in Rugby Union Players. J. Sports Med. Phys. Fit. 2010, 50, 350–355. [Google Scholar]
- Bell, W.; Colley, J.P.; Evans, W.D.; Darlington, S.E.; Cooper, S. ACTN3 Genotypes of Rugby Union Players: Distribution, Power Output and Body Composition. Ann. Hum. Biol. 2012, 39, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, D. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, NZ, USA, 2011. [Google Scholar]
- Zemski, A.J.; Slater, G.J.; Broad, E.M. Body Composition Characteristics of Elite Australian Rugby Union Athletes According to Playing Position and Ethnicity. J. Sports Sci. 2015, 33, 970–978. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaughton, L.R.; Polman, R.; Marchant, D. Criteria for Determination of Maximal Oxygen Uptake. Sport. Med. 2007, 37, 1019–1028. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo Intermittent Recovery Test: A Useful Tool for Evaluation of Physical Performance in Intermittent Sports. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef]
- Preatoni, E.; Colombo, A.; Verga, M.; Galvani, C.; Faina, M.; Rodano, R.; Ennio, P.; Cardinale, M. The Effects of Whole-Body Vibration in Isolation or Combined with Strength Training in Female Athletes. J. Strenght Cond. Res. 2012, 26, 2496–2506. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Smart, D.J.; Hopkins, W.G.; Gill, N.D. Differences and Changes in the Physical Charactersitics of Professional and Amateur Rugby Union Players. J. Strength Cond. Res. 2013, 27, 3033–3044. [Google Scholar] [CrossRef]
- Vachon, A.; Berryman, N.; Mujika, I.; Paquet, J.-B.; Bosquet, L. Fitness Determinants of Repeated High-Intensity Effort Ability in Elite Rugby Union Players. Int. J. Sports Physiol. Perform. 2021, 16, 1103–1110. [Google Scholar] [CrossRef]
- Eynon, N.; Duarte, J.A.; Oliveira, J.; Sagiv, M.; Yamin, C.; Meckel, Y.; Sagiv, M.; Goldhammer, E. ACTN3 R577X Polymorphism and Israeli Top-Level Athletes. Int. J. Sports Med. 2009, 30, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.; Zentgraf, K.; Krüger, K.; Alack, K. Effects of Genetic Variation on Endurance Performance, Muscle Strength, and Injury Susceptibility in Sports: A Systematic Review. Front. Physiol. 2021, 12, 694411. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Kiely, J.; Suraci, B.; Collins, D.; De Lorenzo, D.; Pickering, C.; Grimaldi, K. A Genetic-Based Algorithm for Personalized Resistance Training. Biol. Sport 2016, 33, 117–126. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. Can Genetic Testing Predict Talent? A Case Study of Five Elite Athletes. Int. J. Sports Physiol. Perform. 2020, 16, 429–434. [Google Scholar] [CrossRef] [PubMed]

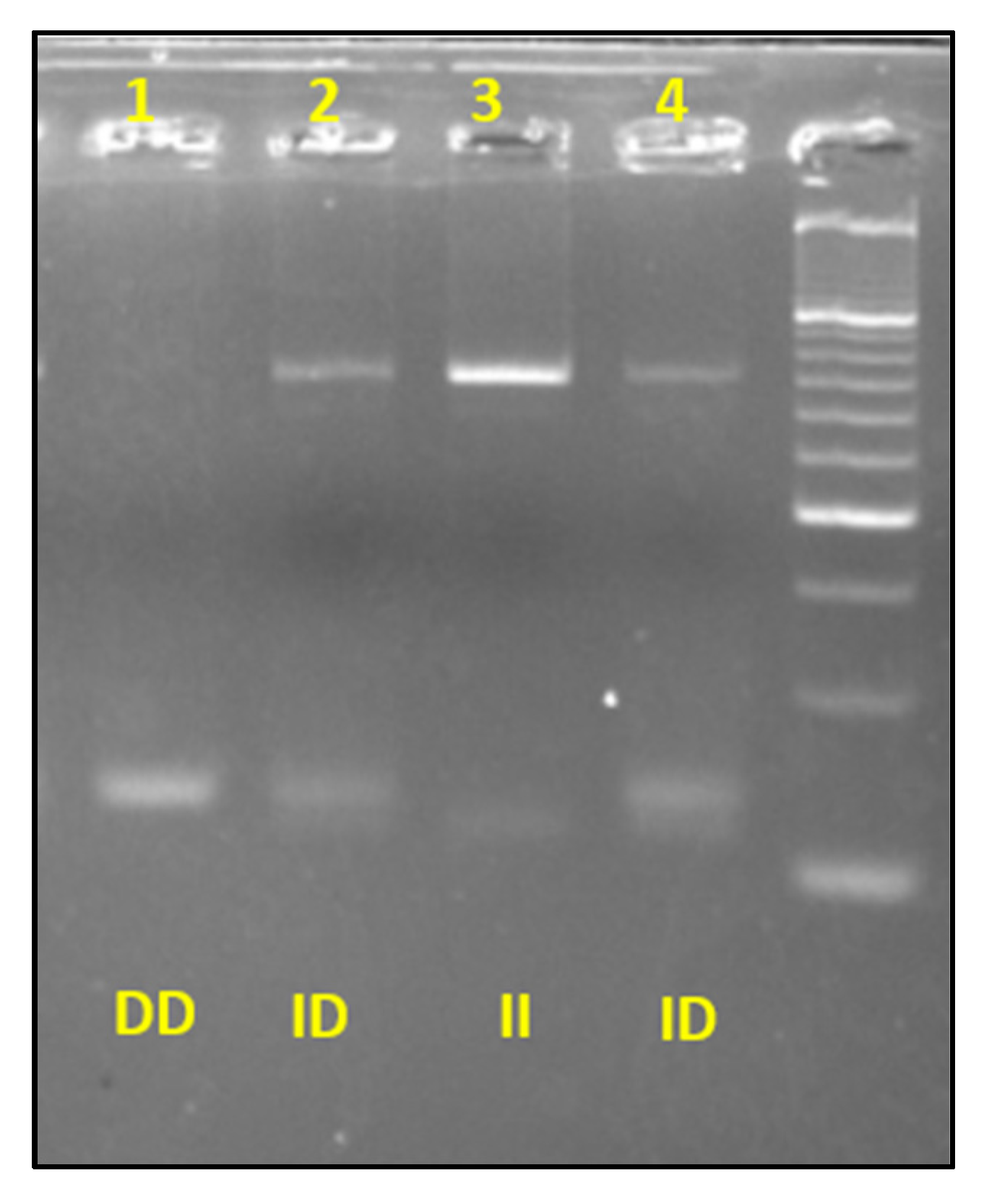
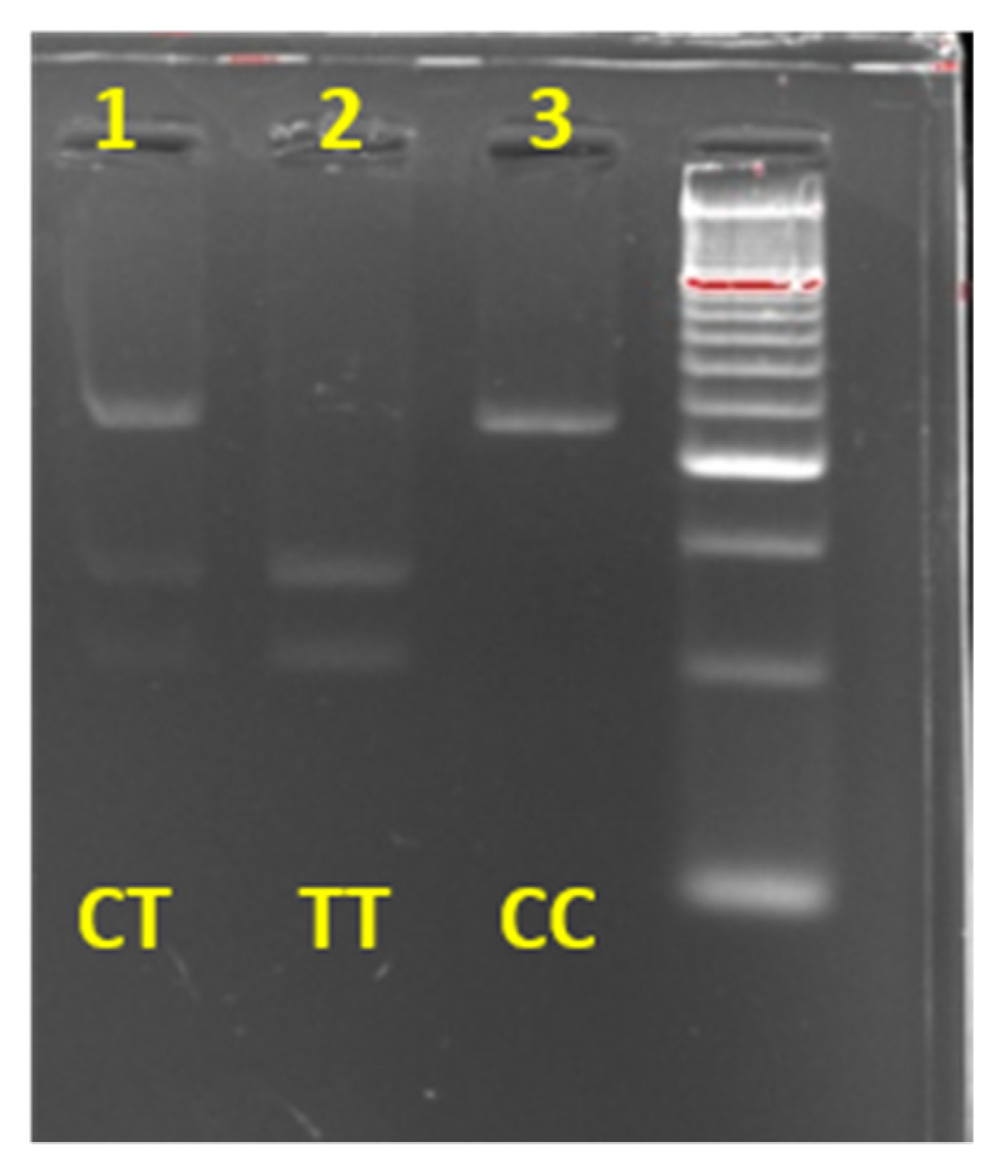

| Gene | Primers Sequence | Annealing Temperature for PCR |
|---|---|---|
| ACE | Forward F1, 5′-CCCATTTCTCTAGACCTGCT-3′ Forward F2, 5′-TGGGATTACAGGCGTGAT-3′ Reverse R1, 5′-AGAGCTGGAATAAAATTGGC-3′ | 55° |
| ACTN3 | Forward, 5′-GGGCACACTGCTGCCCTTTC-3′ Reverse, 5′-GATGTCCTGCGGGCTGAG-3′ | 61° |
| MCT1 | Forward, 5′-AGACCAGTATAGATGTTGCTGGG-3′ Reverse, 5′-CCACTGGTAGATTACAGGCCA-3′ | 58° |
| Forwards (n = 13) | Backs (n = 14) | p-Value | ES | |
|---|---|---|---|---|
| Weight (kg) | 106.7 ± 10.1 | 81.5 ± 9.6 | <0.0001 | 2.48 |
| Height (cm) | 184.4 ± 5.5 | 177.4 ± 6.9 | 0.0055 | 1.14 |
| LMI (kg/mm0.14) | 55.1 ± 4.6 | 45.8 ± 4.8 | <0.0001 | 1.93 |
| Peak vertical power (W/kg) | 69.5 ± 17.7 | 84.3 ± 23.6 | 0.978 | 0.01 |
| 505 agility test (s) | 2.35 ± 0.16 | 2.18 ± 0.10 | 0.0032 | 1.22 |
| 20 m sprint test (s) | 3.20 ± 0.16 | 2.95 ± 0.14 | 0.0002 | 1.62 |
| RSA total sprint time (s) | 41.94 ± 2.43 | 38.17 ± 0.93 | <0.0001 | 2.01 |
| V’O2max (mlO2/kg/min) | 46.1 ± 3.2 | 51.1 ± 3.2 | 0.0004 | 1.52 |
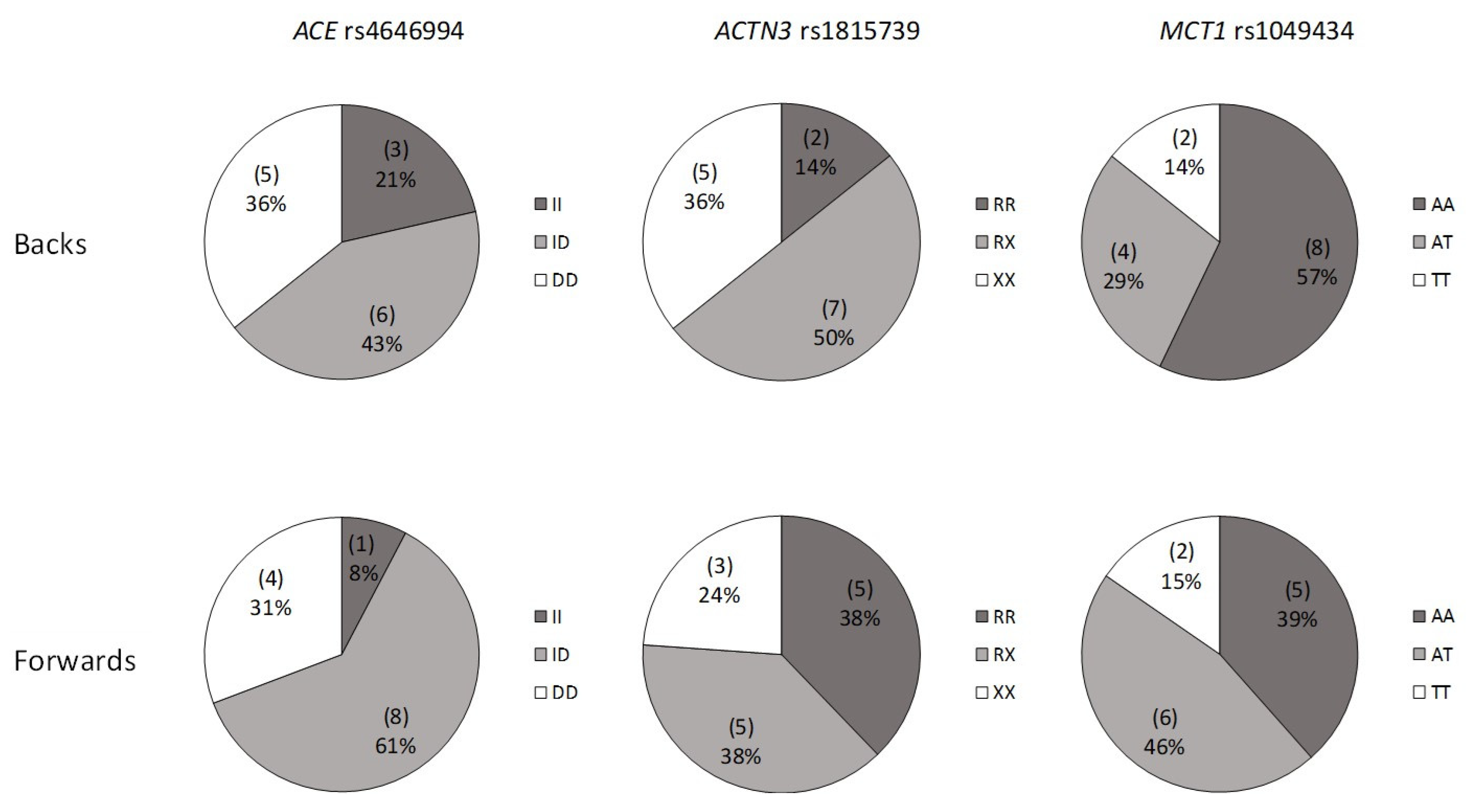
| Genotype | |||
| ACE | II (n = 4) | ID (n = 14) | DD (n = 9) |
| Physical characteristics | |||
| LMI (kg/mm0.14) | 54.4 ± 6.6 | 49.7 ± 6.7 | 49.5 ± 6.6 |
| Peak vertical power (W/kg) | 81.9 ± 26.7 | 76.9 ± 26.1 | 73.6 ± 19.1 |
| 505 agility test (s) | 2.34 ± 0.30 | 2.25 ± 0.10 | 2.25 ± 0.16 |
| 20 m sprint (s) | 3.12 ± 0.31 | 3.09 ± 0.15 | 3.03 ± 0.22 |
| RSA total sprint time (s) | 40.48 ± 4.28 | 40.13 ± 2.39 | 39.54 ± 2.39 |
| V’O2max (mlO2/kg/min) | 47.7 ± 4.6 | 48.9 ± 4.6 | 48.9 ± 3.0 |
| Genotype | |||
| ACTN3 | RR | RX | XX |
| Physical characteristics | (n = 7) | (n = 12) | (n = 8) |
| LMI (kg/mm0.14) | 50.7 ± 5.7 | 50.5 ± 6.2 | 49.7 ± 8.6 |
| Peak vertical power (W/kg) | 78.2 ± 27.8 | 74.5 ± 17.6 | 78.2 ± 29.1 |
| 505 agility test (s) | 2.27 ± 0.13 | 2.25 ± 0.13 | 2.28 ± 0.22 |
| 20 m sprint (s) | 3.06 ± 0.20 | 3.07 ± 0.20 | 3.08 ± 0.21 |
| RSA total sprint time (s) | 40.10 ± 1.85 | 39.98 ± 2.88 | 39.88 ± 3.07 |
| V’O2max (mlO2/kg/min) | 48.3 ± 4.5 | 48.0 ± 3.6 | 50.1 ± 4.3 |
| Genotype | |||
| MCT1 | AA | AT | TT |
| Physical characteristics | (n = 13) | (n = 10) | (n = 4) |
| LMI (kg/mm0.14) | 49.1 ± 7.1 | 52.0 ± 7.0 | 50.2 ± 3.4 |
| Peak vertical power (W/kg) | 78.6 ± 26.2 | 67.5 ± 19.1 | 92.6 ± 14.9 * |
| 505 agility test (s) | 2.19 ± 0.09 * | 2.36 ± 0.19 | 2.26 ± 0.12 |
| 20 m sprint (s) | 2.98 ± 0.17 * | 3.19 ± 0.19 | 3.09 ± 0.19 |
| RSA total sprint time (s) | 39.23 ± 2.16 | 41.03 ± 3.28 | 39.82 ± 1.52 |
| V’O2max (mlO2/kg/min) | 41.3 ± 3.5 | 48.2 ± 5.1 | 48.9 ± 3.5 |
| Dominant Model | ||||
| ACE Physical characteristics | II + ID (n = 18) | DD (n = 9) | p-Value | ES |
| LMI (kg/mm0.14) | 50.6 ± 6.6 | 49.5 ± 6.6 | 0.640 | 0.19 |
| Peak vertical power (W/kg) | 78.0 ± 25.5 | 73.6 ± 19.1 | 0.653 | 0.18 |
| 505 agility test (s) | 2.27 ± 0.16 | 2.25 ± 0.16 | 0.714 | 0.15 |
| 20 m sprint (s) | 3.09 ± 0.19 | 3.03 ± 0.22 | 0.446 | 0.31 |
| RSA total sprint time (s) | 40.21 ± 2.76 | 39.54 ± 2.39 | 0.542 | 0.25 |
| V’O2max (mlO2/kg/min) | 48.6 ± 4.5 | 48.9 ± 3.0 | 0.841 | 0.08 |
| Dominant model | ||||
| ACTN3 | RR + RX | XX | p-Value | ES |
| Physical characteristics | (n = 19) | (n = 8) | ||
| LMI (kg/mm0.14) | 50.6 ± 5.8 | 49.7 ± 8.6 | 0.767 | 0.12 |
| Peak vertical power (W/kg) | 75.9 ± 21.3 | 78.2 ± 29.1 | 0.820 | 0.09 |
| 505 agility test (s) | 2.26 ± 0.13 | 2.28 ± 0.22 | 0.771 | 0.12 |
| 20 m sprint (s) | 3.07 ± 0.19 | 3.08 ± 0.21 | 0.934 | 0.03 |
| RSA total sprint time (s) | 40.02 ± 2.49 | 39.88 ± 3.07 | 0.901 | 0.05 |
| V’O2max (mlO2/kg/min) | 48.1 ± 3.8 | 50.1 ± 4.3 | 0.242 | 0.49 |
| Dominant model | ||||
| MCT1 | TT + AT | AA | p-Value | ES |
| Physical characteristics | (n = 14) | (n = 13) | ||
| LMI (kg/mm0.14) | 51.4 ± 6.1 | 49.1 ± 7.1 | 0.359 | 0.35 |
| Peak vertical power (W/kg) | 74.6 ± 21.0 | 78.6 ± 26.2 | 0.668 | 0.16 |
| 505 agility test (s) | 2.33 ± 0.17 | 2.19 ± 0.09 | 0.013 | 1.00 |
| 20 m sprint (s) | 3.16 ± 0.19 | 2.98 ± 0.17 | 0.017 | 0.95 |
| RSA total sprint time (s) | 40.69 ± 2.88 | 39.23 ± 2.16 | 0.152 | 0.55 |
| V’O2max (mlO2/kg/min) | 48.4 ± 4.6 | 49.1 ± 3.5 | 0.641 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualetti, M.; Onori, M.E.; Canu, G.; Moretti, G.; Minucci, A.; Baroni, S.; Mordente, A.; Urbani, A.; Galvani, C. The Relationship between ACE, ACTN3 and MCT1 Genetic Polymorphisms and Athletic Performance in Elite Rugby Union Players: A Preliminary Study. Genes 2022, 13, 969. https://doi.org/10.3390/genes13060969
Pasqualetti M, Onori ME, Canu G, Moretti G, Minucci A, Baroni S, Mordente A, Urbani A, Galvani C. The Relationship between ACE, ACTN3 and MCT1 Genetic Polymorphisms and Athletic Performance in Elite Rugby Union Players: A Preliminary Study. Genes. 2022; 13(6):969. https://doi.org/10.3390/genes13060969
Chicago/Turabian StylePasqualetti, Massimo, Maria Elisabetta Onori, Giulia Canu, Giacomo Moretti, Angelo Minucci, Silvia Baroni, Alvaro Mordente, Andrea Urbani, and Christel Galvani. 2022. "The Relationship between ACE, ACTN3 and MCT1 Genetic Polymorphisms and Athletic Performance in Elite Rugby Union Players: A Preliminary Study" Genes 13, no. 6: 969. https://doi.org/10.3390/genes13060969







