The Effect of Angiotensin Converting Enzyme (ACE) I/D Polymorphism on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk in Non-Hemodialyzed Chronic Kidney Disease: The Mediating Role of Plasma ACE Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
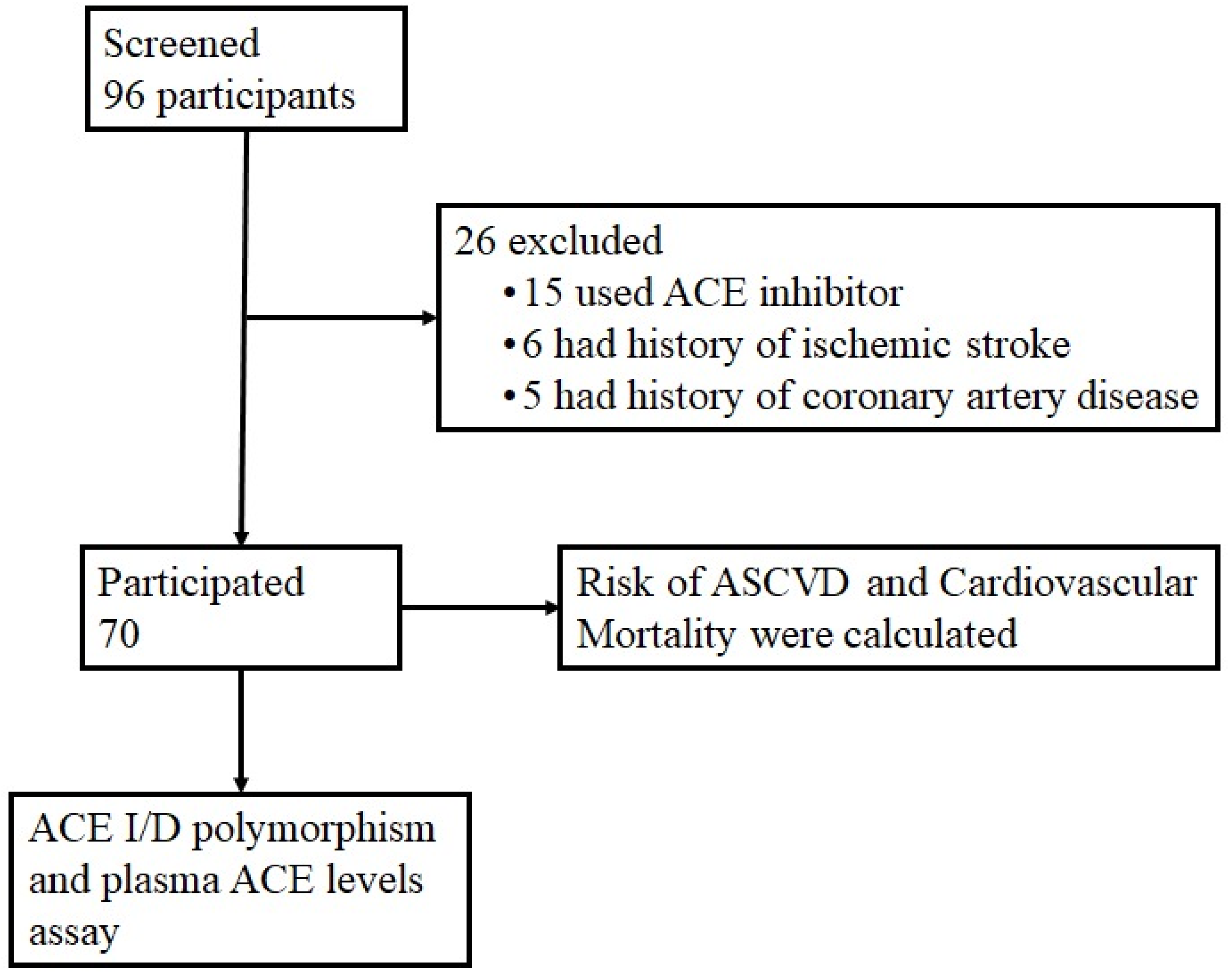
2.2. Sample Criteria and Data Collection
2.3. DNA Isolation
2.4. Genotyping of ACE I/D Polymorphism
2.5. Plasma ACE Levels
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.; Mone, P.; Jankauskas, S.S.; Gambardella, J.; Santulli, G.; Ba, S.W. Chronic kidney disease: Definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J. Clin. Hypertens. 2021, 23, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, N.K.; Galougahi, K.K.; Paz, Y.; Nazif, T.; Moses, J.W.; Leon, M.B.; Stone, G.W.; Kirtane, A.J.; Karmpaliotis, D.; Bokhari, S.; et al. Diagnosis and Management of Cardiovascular Disease in Advanced and End-Stage Renal Disease. J. Am. Heart Assoc. 2016, 5, e003648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Maharani, A.; Sujarwoto Praveen, D.; Oceandy, D.; Tampubolon, G.; Patel, A. Cardiovascular disease risk factor prevalence and estimated 10-year cardiovascular risk scores in Indonesia: The SMARThealth Extend study. PLoS ONE 2019, 14, e0215219. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Alsagaff, M.Y.; Pikir, B.S.; Thaha, M.T.; Susilo, H. Correlations between Total Antioxidant Capacity and 8-Hydroxydeoxyguanosine with Carotid-Femoral Pulse Wave Velocity in Chronic Kidney Disease. Indones. Biomed. J. 2020, 12, 267–274. [Google Scholar] [CrossRef]
- Rossaint, J.; Oehmichen, J.; Van Aken, H.; Reuter, S.; Pavenstädt, H.J.; Meersch, M.; Unruh, M.; Zarbock, A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Investig. 2016, 126, 962–974. [Google Scholar] [CrossRef] [Green Version]
- Alsagaff, M.Y.; Thaha, M.; Pikir, B.S.; Susilo, H.; Wungu, C.D.K.; Suryantoro, S.D.; Haryati, M.R.; Ramadhani, R.; Agustin, E.D.; Putra, M.R.A.; et al. The role of oxidative stress markers in Indonesian chronic kidney disease patients: A cross sectional study. F1000Research 2022, 11, 132. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470410/ (accessed on 4 March 2022).
- Lv, Y.; Zhao, W.; Yu, L.; Yu, J.-G.; Zhao, L. Angiotensin-Converting Enzyme Gene D/I Polymorphism in Relation to Endothelial Function and Endothelial-Released Factors in Chinese Women. Front. Physiol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, L.; Zhou, Y.; Lu, N. Genetic polymorphism of angiotensin-converting enzyme and hypertrophic cardiomyopathy risk. Medicine 2017, 96, e8639. [Google Scholar] [CrossRef]
- Nouryazdan, N.; Adibhesami, G.; Birjandi, M.; Heydari, R.; Yalameha, B.; Shahsavari, G. Study of angiotensin-converting enzyme insertion/deletion polymorphism, enzyme activity and oxidized low density lipoprotein in Western Iranians with atherosclerosis: A case-control study. BMC Cardiovasc. Disord. 2019, 19, 184. [Google Scholar] [CrossRef]
- Dai, S.; Ding, M.; Liang, N.; Li, Z.; Li, D.; Guan, L.; Liu, H. Associations of ACE I/D polymorphism with the levels of ACE, kallikrein, angiotensin II and interleukin-6 in STEMI patients. Sci. Rep. 2019, 9, 19719. [Google Scholar] [CrossRef] [Green Version]
- Sabir, J.S.M.; Omri, A.E.; Ali Khan, I.; Banaganapalli, B.; Hajrah, N.H.; Zrelli, H.; Omar, A.M.S.; Alharbi, M.G.; Alhebshi, A.M.; Jansen, R.K.; et al. ACE insertion/deletion genetic polymorphism, serum ACE levels and high dietary salt intake influence the risk of obesity development among the Saudi adult population. J. Renin Angiotensin Aldosterone Syst. 2019, 20, 1470320319870945. [Google Scholar] [CrossRef] [Green Version]
- Seckin, D.; Ilhan, N.; Ilhan, N.; Ozbay, Y. The relationship between ACE insertion/deletion polymorphism and coronary artery disease with or without myocardial infarction. Clin. Biochem. 2006, 39, 50–54. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0009912005002870 (accessed on 15 January 2022). [CrossRef]
- Benenemissi, I.H.; Sifi, K.; Sahli, L.K.; Semmam, O.; Abadi, N.; Satta, D. Angiotensin-converting enzyme insertion/deletion gene polymorphisms and the risk of glioma in an Algerian population. Pan Afr. Med. J. 2019, 32, 197. [Google Scholar] [CrossRef]
- Rahimi, Z. ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J. Nephropathol. 2012, 1, 143–151. [Google Scholar] [CrossRef]
- Andrus, B.; Lacaille, D. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. J. Am. Coll. Cardiol. 2014, 63, 2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representat). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Matsushita, K.; Jassal, S.K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Arnlov, J.; Bansal, N.; Bozic, M.; Brenner, H.; et al. Incorporating kidney disease measures into cardiovascular risk prediction: Development and validation in 9 million adults from 72 datasets. eClinicalMedicine 2020, 27, 100552. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Srivastava, N.; Amit, S.; Prasad, S.; Misra, M.; Ateeq, B. Association of AGTR1 (A1166C) and ACE (I/D) Polymorphisms with Breast Cancer Risk in North Indian Population. Transl. Oncol. 2018, 11, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Pablos, I.; Machado, Y.; de Jesus, H.C.R.; Mohamud, Y.; Kappelhoff, R.; Lindskog, C.; Vlok, M.; Bell, P.A.; Butler, G.S.; Grin, P.M.; et al. Mechanistic insights into COVID-19 by global analysis of the SARS-CoV-2 3CLpro substrate degradome. Cell Rep. 2021, 37, 109892. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Banerjee, M. Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update. High Blood Press. Cardiovasc. Prev. 2021, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ribichini, F.; Steffenino, G.; Dellavalle, A.; Matullo, G.; Colajanni, E.; Camilla, T.; Vado, A.; Benetton, G.; Uslenghi, E.; Piazza, A. Plasma Activity and Insertion/Deletion Polymorphism of Angiotensin I–Converting Enzyme. Circulation 1998, 97, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, K.; Lone, N.M.; Sadique, S.; Sultan, S.; Eupash, A.Z.; Riaz, S. Association of Angiotensin-Converting Enzyme gene polymorphism in Pakistani women with the atypical steroidogenesis in Polycystic ovarian syndrome: A case-control study. Saudi J. Biol. Sci. 2021, 28, 3483–3489. [Google Scholar] [CrossRef]
- Cambien, F.; Costerousse, O.; Tiret, L.; Poirier, O.; Lecerf, L.; Gonzales, M.F.; Evans, A.; Arveiler, D.; Cambou, J.P.; Luc, G. Plasma level and gene polymorphism of angiotensin-converting enzyme in relation to myocardial infarction. Circulation 1994, 90, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Borzyszkowska, J.; Stanislawska-Sachadyn, A.; Wirtwein, M.; Sobiczewski, W.; Ciecwierz, D.; Targonski, R.; Gruchala, M.; Rynkiewicz, A.; Limon, J. Angiotensin converting enzyme gene polymorphism is associated with severity of coronary artery disease in men with high total cholesterol levels. J. Appl. Genet. 2012, 53, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Vaisi-Raygani, A.; Ghaneialvar, H.; Rahimi, Z.; Nomani, H.; Saidi, M.; Bahrehmand, F.; Vaisi-Raygani, A.; Tavilani, H.; Pourmotabbed, T. The angiotensin converting enzyme D allele is an independent risk factor for early onset coronary artery disease. Clin. Biochem. 2010, 43, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Ismail, P.; Stanslas, J.; Shamsudin, N.; Moin, S.; Jas, R.M. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J. Renin Angiotensin Aldosterone Syst. 2008, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, B.A.; Robitaille, N.M.; Bogaty, P.; Labbé, L.; Turgeon, J.; Zakrzewski-Jakubiak, M. Increased Risk of Myocardial Infarction Associated With Angiotensin-Converting Enzyme Gene Polymorphism Is Age Dependent. J. Clin. Pharmacol. 2011, 51, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Fedor, R.; Asztalos, L.; Locsey, L.; Szabó, L.; Mányiné, I.S.; Fagyas, M.; Lizanecz, E.; Tóth, A. Insertion/Deletion Polymorphism of the Angiotensin-Converting Enzyme Predicts Left Ventricular Hypertrophy After Renal Transplantation. Transpl. Proc. 2011, 43, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, F. From an ACE polymorphism to genome-wide searches for eQTL. J. Clin. Investig. 2013, 123, 111–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturrock, E.D.; Anthony, C.S.; Danilov, S.M. Peptidyl-Dipeptidase A/Angiotensin I-Converting Enzyme. Handb. Proteolytic Enzym. 2013, 1, 480–494. [Google Scholar] [CrossRef]
- Sayed-Tabatabaei, F.A.; Oostra, B.A.; Isaacs, A.; Van Duijn, C.M.; Witteman, J.C.M. ACE polymorphisms. Circ. Res. 2006, 98, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Investig. 1990, 86, 1343–1346. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tikellis, C.; Thomas, M.; Golledge, J. Angiotensin converting enzyme 2 and atherosclerosis. Atherosclerosis 2012, 226, 3–8. [Google Scholar] [CrossRef]
- Silva, G.M.; França-Falcão, M.S.; Calzerra, N.T.M.; Luz, M.S.; Gadelha, D.D.A.; Balarini, C.M.; Queiroz, T.M. Role of Renin-Angiotensin System Components in Atherosclerosis: Focus on Ang-II, ACE2, and Ang-1–7. Front. Physiol. 2020, 11, 1067. [Google Scholar] [CrossRef]
- Kamilic, J.; Lely, A.T.; van Goor, H.; Buikema, H.; Tent, H.; Navis, G.J.; Korstanje, R. Differential ACE expression among tissues in allele-specific Wistar rat lines. Mamm. Genome 2009, 20, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjadj, S.; Tarnow, L.; Forsblom, C.; Kazeem, G.; Marre, M.; Groop, P.H.; Parving, H.H.; Cambien, F.; Tregouet, D.A.; Gut, I.G.; et al. Association between Angiotensin-Converting Enzyme Gene Polymorphisms and Diabetic Nephropathy: Case-Control, Haplotype, and Family-Based Study in Three European Populations. J. Am. Soc. Nephrol. 2007, 18, 1284–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, D.P.K.; Tai, B.C.; Koh, D.; Tan, K.W.; Chia, K.S. Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: A meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia 2005, 48, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Nikzamir, A.; Nakhjavani, M.; Esteghamati, A.; Rashidi, A. Correlates of ACE activity in macroalbuminuric type 2 diabetic patients treated with chronic ACE inhibition. Nephrol. Dial. Transplant. 2007, 23, 1274–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed-Tabatabaei, F.A.; Schut, A.F.C.; Vásquez, A.A.; Bertoli-Avella, A.M.; Hofman, A.; Witteman, J.C.M.; van Duijn, C.M. Angiotensin converting enzyme gene polymorphism and cardiovascular morbidity and mortality: The Rotterdam Study. J. Med. Genet. 2005, 42, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Sman-De Beer, F.; Verhagen, C.; Rombach, S.M.; Boorsma, P.; Van Manen, J.G.; Korevaar, J.C.; Van Den Bogaard, R.; Boeschoten, E.W.; Krediet, R.T.; Navis, G.J.; et al. ACE I/D polymorphism is associated with mortality in a cohort study of patients starting with dialysis. Kidney Int. 2005, 68, 2237–2243. [Google Scholar] [CrossRef] [Green Version]
- Bloem, L.J.; Manatunga, A.K.; Pratt, J.H. Racial Difference in the Relationship of an Angiotensin I–Converting Enzyme Gene Polymorphism to Serum Angiotensin I–Converting Enzyme Activity. Hypertension 1996, 27, 62–66. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Angiotensin-converting enzyme gene polymorphism: I and D alleles from some different countries. Clin. Appl. Thromb. 2004, 10, 179–182. [Google Scholar] [CrossRef]
- Cambien, F.; Poirier, O.; Lecerf, L.; Evans, A.; Cambou, J.-P.; Arveiler, D.; Luc, G.; Bard, J.-M.; Bara, L.; Ricard, S.; et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 1992, 359, 641–644. [Google Scholar] [CrossRef]
- Schunkert, H.; Hence, H.W.; Holmer, S.R.; Stender, M.; Perz, S.; Keil, U.; Lorell, B.H.; Riegger, G. Association between a Deletion Polymorphism of the Angiotensin-Converting-Enzyme Gene and Left Ventricular Hypertrophy. N. Engl. J. Med. 2010, 330, 1634–1638. [Google Scholar] [CrossRef]
- Abdel Azeem, N.E.; Attallah, D.A.; Hussein, A.A.; Alzzubidi, N.A.S. The angiotensin-converting enzyme insertion/deletion polymorphism of vitiligo in a population in upper Egypt: A hospital-based study. J. Egypt Women’s Dermatol. Soc. 2016, 13, 129–132. [Google Scholar] [CrossRef]
- Gómez, J.; Albaiceta, G.M.; García-Clemente, M.; López-Larrea, C.; Amado-Rodríguez, L.; Lopez-Alonso, I.; Hermida, T.; Enriquez, A.I.; Herrero, P.; Melón, S.; et al. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene 2020, 762, 145102. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Annunziata, A.; Coppola, A.; Pafundi, P.C.; Guarino, S.; Di Spirito, V.; Maddaloni, V.; Pepe, N.; Fiorentino, G. ACE Gene I/D Polymorphism and Acute Pulmonary Embolism in COVID19 Pneumonia: A Potential Predisposing Role. Front. Med. 2021, 7, 631148. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Abbas, M.; Verma, S.; Khan, F.H.; Raza, S.T.; Siddiqi, Z.; Ahmad, I.; Mahdi, F. Impact of I/D polymorphism of angiotensin-converting enzyme 1 (ACE1) gene on the severity of COVID-19 patients. Infect. Genet. Evol. 2021, 91, 104801. [Google Scholar] [CrossRef] [PubMed]
- Nitiyanant, W.; Sriussadaporn, S.; Ploybutr, S.; Watanakejorn, P.; Tunlakit, M.; Bejrachandra, S. Angiotensin converting enzyme gene polymorphism in healthy Thais and patients with non-insulin dependent diabetes mellitus. J. Med. Assoc. Thail. 1997, 80, 747–752. [Google Scholar]
- Um, J.Y.; Kim, H.J.; Choi, T.J.; Jin, C.S.; Park, S.T.; Lee, K.C.; Rhee, H.S.; Lee, K.-M.; Lee, Y.-M.; Kim, H.-M.; et al. Polymorphism of the angiotensin-converting enzyme gene in patients with cerebral infarction in Koreans. J. Mol. Neurosci. 2001, 17, 279–283. [Google Scholar] [CrossRef]
- Sinorita, H.; Madiyan, M.; Pramono, R.B.; Purnama, L.B.; Ikhsan, M.R.; Asdie, A.H. ACE gene insertion/deletion polymorphism among patients with type 2 diabetes, and its relationship with metabolic syndrome at Sardjito Hospital Yogyakarta, Indonesia. Acta Med. Indones 2010, 42, 12–16. [Google Scholar]
- Handayani, M.D.N.; Sadewa, A.H.; Farmawati, A.; Rochmah, W. Deletion Polymorphism of Angiotensin-Converting Enzyme Gene Is Associated with Low Muscle Mass in Elderly People in Jakarta, Indonesia. Kobe J. Med. Sci. 2018, 64, E119–E125. [Google Scholar]
- Vegter, S.; Perna, A.; Hiddema, W.; Ruggenenti, P.; Remuzzi, G.; Navis, G.; Postma, M.J. Cost-effectiveness of ACE inhibitor therapy to prevent dialysis in nondiabetic nephropathy: Influence of the ACE insertion/deletion polymorphism. Pharm. Genom. 2009, 19, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Felehgari, V.; Rahimi, Z.; Mozafari, H.; Vaisi-Raygani, A. ACE gene polymorphism and serum ACE activity in Iranians type II diabetic patients with macroalbuminuria. Mol. Cell. Biochem. 2010, 346, 23–30. [Google Scholar] [CrossRef]
- Heidari, F.; Vasudevan, R.; Mohd Ali, S.Z.; Ismail, P.; Etemad, A.; Pishva, S.R.; Othman, F.; Bakar, S.A. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene among Malay male hypertensive infjects in response to ACE inhibitors. JRAAS J. Renin Angiotensin Aldosterone Syst. 2015, 16, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, Y.; Nonoguchi, H.; Kohda, Y.; Inoue, H.; Memetimin, H.; Izumi, Y.; Tomita, K. Different Mechanisms for the Progression of CKD with ACE Gene Polymorphisms. Nephron Clin. Pract. 2009, 111, c240–c246. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Chaturvedi, N.; Talmud, P.J.; Cotroneo, P.; Manto, A.; Nannipieri, M.; Luong, L.A.; Fuller, J.H. Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: Findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 1998, 47, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Schelleman, H.; Klungel, O.H.; van Duijn, C.M.; Witteman, J.C.M.; Hofman, A.; de Boer, A.; Stricker, B.H.C. Insertion/deletion polymorphism of the ACE gene and adherence to ACE inhibitors. Br. J. Clin. Pharmacol. 2005, 59, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Harrap, S.B.; Tzourio, C.; Cambien, F.; Poirier, O.; Raoux, S.; Chalmers, J.; Chapman, N.; Colman, S.; Leguennec, S.; MacMahon, S.; et al. The ACE Gene I/D Polymorphism Is Not Associated With the Blood Pressure and Cardiovascular Benefits of ACE Inhibition. Hypertension 2003, 42, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3–5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. Available online: https://academic.oup.com/eurheartj/article/42/34/3227/6358713 (accessed on 22 February 2022). [CrossRef]




| Variable | CKD Stage | All Patients (n= 70) | p | |||
|---|---|---|---|---|---|---|
| Stage 2 (n = 3) | Stage 3 (n = 36) | Stage 4 (n = 20) | Stage 5 (n = 11) | |||
| Gender, male% | 3 (100) | 19 (52.8) | 11 (20) | 4 (36.4) | 37 (52.9) | 0.271 A |
| Age (years) | 58.0 ± 12.17 | 58.47 ± 5.92 | 58.65 ± 6.39 | 54.64 ± 10.62 | 57.90 ± 7.19 | 0.716 B |
| Diabetes n (%) | 3 (100) | 28 (77.8) | 15 (75) | 8 (72.7) | 40 (57.1) | 0.784 A |
| Hypertension n (%) | 3 (100) | 32 (88.9) | 16 (80) | 10 (90.9) | 53 (75.7) | 0.866 A |
| Smoking history | 0.521 A | |||||
| Non-smoker n (%) | 2 (66.7) | 26 (72.2) | 12 (60) | 9 (81.8) | 49 (70.0) | |
| Current smoker n (%) | 0 (0) | 1 (2.8) | 3 (15) | 0 (0) | 4 (5.7) | |
| Former smoker n (%) | 1 (33.3) | 9 (25) | 5 (25) | 2 (18.2) | 17 (24.3) | |
| Body mass index (kg/m2) | 28.32 ± 5.45 | 26.04 ± 5.42 | 27.09 ± 5.28 | 24.39 ± 4.48 | 26.18 ± 5.23 | 0.387 B |
| Systolic blood pressure (mmHg) | 134.33 ± 3.06 | 145.44 ± 23.33 | 141.95 ± 21.95 | 147.27 ± 28.33 | 144.26 ± 23.09 | 0.800 C |
| Diastolic blood pressure (mmHg) | 79.33 ± 4.73 | 81.42 ± 12.33 | 81.30 ± 10.51 | 81.27 ± 15.58 | 81.27 ± 11.98 | 0.887 B |
| Total cholesterol (mg/dL) | 192.0 ± 52.72 | 181.22 ± 52.44 | 184.25 ± 52.23 | 184.09 ± 47.75 | 183.00 ± 50.63 | 0.902 B |
| High-density lipoprotein (mg/dL) | 37.0 ± 1.73 | 43.75 ± 15.43 | 36.65 ± 5.79 | 34.18 ± 7.36 | 39.93 ± 12.42 | 0.022 B |
| Serum creatinine (mg/dL) | 1.3 ± 0.04 | 1.71 ± 0.27 | 2.92 ± 0.61 | 5.46 ± 2.21 | 2.63 ± 1.63 | 0.000 *,B |
| eGFR (mL/min/1.73 m2) | 63.67 ± 3.79 | 40.94 ± 7.27 | 21.70 ± 4.13 | 11.09 ± 3.33 | 31.73 ± 14.81 | 0.000 *,B |
| Urine ACR (mg/g) | 28.19 ± 31.28 | 313.7 ± 475.92 | 590.53 ± 777.54 | 1805.69 ± 1352.2 | 615.01 ± 913.74 | 0.000 *,B |
| Plasma ACE (pg/mL) | 3417.83 ± 546.8 | 3908.79 ± 1158.18 | 4151.9 ± 1134.18 | 4145.85 ± 752.48 | 3994.46 ± 1074.47 | 0.637 B |
| Variable | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Ten-year risk of atherosclerotic cardiovascular disease (%) | 0.8 | 80.8 | 23.54 | 18.79 |
| Ten-year risk of cardiovascular mortality (%) | 0.3 | 88.4 | 16.3 | 17.02 |
| Variables | Risk of Atherosclerotic Cardiovascular Disease | Risk of Cardiovascular Mortality | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.596 | 0.000 * | 0.508 | 0.000 * |
| Body mass index | 0.111 | 0.361 | 0.094 | 0.437 |
| Systolic blood pressure | 0.280 | 0.019 * | 0.421 | 0.000 * |
| Diastolic blood pressure | 0.115 | 0.342 | 0.185 | 0.126 |
| Smoking history | 0.431 | 0.000 * | 0.450 | 0.000 * |
| Total cholesterol | 0.101 | 0.405 | 0.193 | 0.110 |
| High-density lipoprotein | −0.337 | 0.004 * | −0.202 | 0.093 |
| Serum creatinine | 0.237 | 0.048 * | 0.365 | 0.002 * |
| CKD stage | 0.160 | 0.186 | 0.308 | 0.009 * |
| eGFR | −0.143 | 0.238 | −0.284 | 0.017 * |
| Urine ACR | 0.340 | 0.004 * | 0.457 | 0.000 * |
| Plasma ACE | 0.391 | 0.001 * | 0.318 | 0.007 * |
| CKD Groups | Plasma ACE Levels and ASCVD Risk | Plasma ACE Levels and Cardiovascular Mortality Risk | ||
|---|---|---|---|---|
| r | p | r | p | |
| Mild-moderate CKD | 0.319 | 0.048 * | 0.16 | 0.332 |
| Severe CKD | 0.426 | 0.017 * | 0.362 | 0.045 * |
| Genotype | n | Frequency (%) |
|---|---|---|
| II | 43 | 61.4 |
| ID | 24 | 34.3 |
| DD | 3 | 4.3 |
| Total | 70 | 100 |
| Recessive model | n | Frequency (%) |
| II | 43 | 61.4 |
| ID + DD | 27 | 38.6 |
| Total | 70 | 100 |
| Allele | n | Frequency (%) |
| I | 110 | 78.6 |
| D | 30 | 21.4 |
| Total | 140 | 100 |
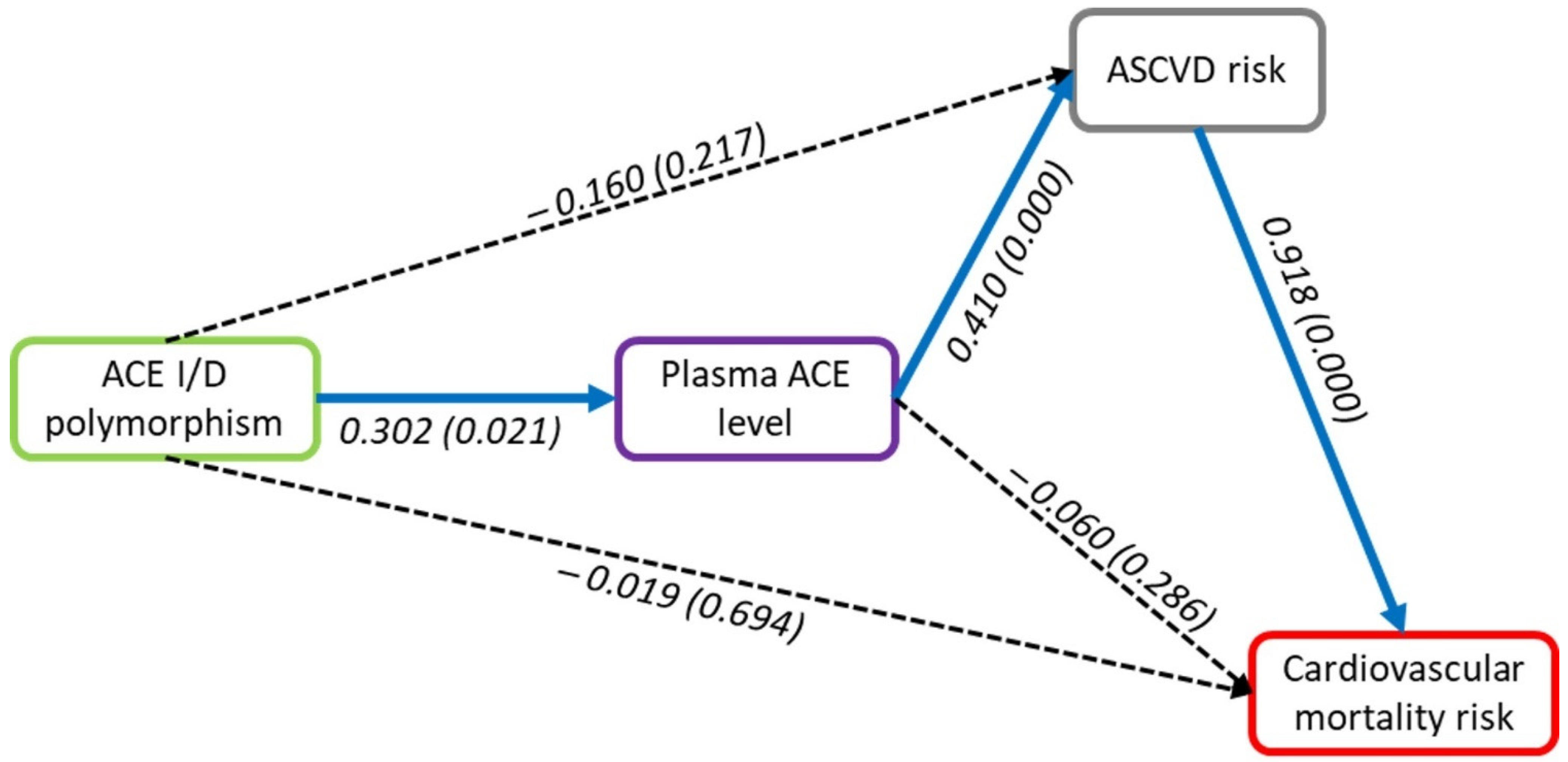
| Outcome | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|
| Cardiovascular mortality risk | |||
| ASCVD risk > Cardiovascular mortality risk | 0.918 * | 0.918 * | |
| Plasma ACE level > Cardiovascular mortality risk | −0.06 | 0.376 * | 0.316 * |
| ACE I/D polymorphism > Cardiovascular mortality risk | −0.019 | −0.051 | −0.071 |
| ASCVD risk | |||
| Plasma ACE level > ASCVD risk | 0.41 * | 0.41 * | |
| ACE I/D polymorphism > ASCVD risk | −0.16 | 0.124 * | −0.036 |
| Plasma ACE level | |||
| ACE I/D polymorphism > Plasma ACE level | 0.302 * | 0.302 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susilo, H.; Pikir, B.S.; Thaha, M.; Alsagaff, M.Y.; Suryantoro, S.D.; Wungu, C.D.K.; Wafa, I.A.; Pakpahan, C.; Oceandy, D. The Effect of Angiotensin Converting Enzyme (ACE) I/D Polymorphism on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk in Non-Hemodialyzed Chronic Kidney Disease: The Mediating Role of Plasma ACE Level. Genes 2022, 13, 1121. https://doi.org/10.3390/genes13071121
Susilo H, Pikir BS, Thaha M, Alsagaff MY, Suryantoro SD, Wungu CDK, Wafa IA, Pakpahan C, Oceandy D. The Effect of Angiotensin Converting Enzyme (ACE) I/D Polymorphism on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk in Non-Hemodialyzed Chronic Kidney Disease: The Mediating Role of Plasma ACE Level. Genes. 2022; 13(7):1121. https://doi.org/10.3390/genes13071121
Chicago/Turabian StyleSusilo, Hendri, Budi Susetyo Pikir, Mochammad Thaha, Mochamad Yusuf Alsagaff, Satriyo Dwi Suryantoro, Citrawati Dyah Kencono Wungu, Ifan Ali Wafa, Cennikon Pakpahan, and Delvac Oceandy. 2022. "The Effect of Angiotensin Converting Enzyme (ACE) I/D Polymorphism on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk in Non-Hemodialyzed Chronic Kidney Disease: The Mediating Role of Plasma ACE Level" Genes 13, no. 7: 1121. https://doi.org/10.3390/genes13071121
APA StyleSusilo, H., Pikir, B. S., Thaha, M., Alsagaff, M. Y., Suryantoro, S. D., Wungu, C. D. K., Wafa, I. A., Pakpahan, C., & Oceandy, D. (2022). The Effect of Angiotensin Converting Enzyme (ACE) I/D Polymorphism on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk in Non-Hemodialyzed Chronic Kidney Disease: The Mediating Role of Plasma ACE Level. Genes, 13(7), 1121. https://doi.org/10.3390/genes13071121







