Genetic Contributors of Efficacy and Adverse Metabolic Effects of Chlorthalidone in African Americans from the Genetics of Hypertension Associated Treatments (GenHAT) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. ALLHAT Treatment
2.3. Response Phenotypes
2.4. Genotyping and Imputation
2.5. Statistical Analysis
2.6. Replication Populations
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprint Research Group; Lewis, C.E.; Fine, L.J.; Beddhu, S.; Cheung, A.K.; Cushman, W.C.; Cutler, J.A.; Evans, G.W.; Johnson, K.C.; Kitzman, D.W.; et al. Final Report of a Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2021, 384, 1921–1930. [Google Scholar] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics–2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) Public Use Data Files, 2015–2018. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 1 April 2020).
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief. 2017, 289, 1–8. [Google Scholar]
- Lackland, D.T. Racial differences in hypertension: Implications for high blood pressure management. Am. J. Med. Sci. 2014, 348, 135–138. [Google Scholar] [CrossRef] [Green Version]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A. Ethnic differences in cardiovascular drug response: Potential contribution of pharmacogenetics. Circulation 2008, 118, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar]
- Roush, G.C.; Buddharaju, V.; Ernst, M.E.; Holford, T.R. Chlorthalidone: Mechanisms of action and effect on cardiovascular events. Curr. Hypertens. Rep. 2013, 15, 514–521. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q. Association of Thiazide-Type Diuretics With Glycemic Changes in Hypertensive Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J. Clin. Hypertens. 2016, 18, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Barzilay, J.I.; Davis, B.R.; Cutler, J.A.; Pressel, S.L.; Whelton, P.K.; Basile, J.; Margolis, K.L.; Ong, S.T.; Sadler, L.S.; Summerson, J.; et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch. Intern. Med. 2006, 166, 2191–2201. [Google Scholar]
- Johnson, J.A. Advancing management of hypertension through pharmacogenomics. Ann. Med. 2012, 44 (Suppl. 1), S17–S22. [Google Scholar] [CrossRef]
- Adeyemo, A.; Gerry, N.; Chen, G.; Herbert, A.; Doumatey, A.; Huang, H.; Zhou, J.; Lashley, K.; Chen, Y.; Christman, M.; et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009, 5, e1000564. [Google Scholar] [CrossRef] [Green Version]
- Basson, J.; Simino, J.; Rao, D.C. Between candidate genes and whole genomes: Time for alternative approaches in blood pressure genetics. Curr. Hypertens. Rep. 2012, 14, 46–61. [Google Scholar] [CrossRef]
- Doris, P.A. The genetics of blood pressure and hypertension: The role of rare variation. Cardiovasc. Ther. 2011, 29, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Kato, N.; Loh, M.; Takeuchi, F.; Verweij, N.; Wang, X.; Zhang, W.; Kelly, T.N.; Saleheen, D.; Lehne, B.; Leach, I.M.; et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet. 2015, 47, 1282–1293. [Google Scholar] [CrossRef]
- Kraja, A.T.; Hunt, S.C.; Rao, D.C.; Davila-Roman, V.G.; Arnett, D.K.; Province, M.A. Genetics of hypertension and cardiovascular disease and their interconnected pathways: Lessons from large studies. Curr. Hypertens. Rep. 2011, 13, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.C.; Province, M.A.; Leppert, M.F.; Oberman, A.; Heiss, G.; Ellison, R.C.; Arnett, D.K.; Eckfeldt, J.H.; Schwander, K.; Mockrin, S.C.; et al. A genome-wide affected sibpair linkage analysis of hypertension: The HyperGEN network. Am. J. Hypertens. 2003, 16, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Simino, J.; Shi, G.; Kume, R.; Schwander, K.; Province, M.A.; Gu, C.C.; Kardia, S.; Chakravarti, A.; Ehret, G.; Olshen, R.A.; et al. Five blood pressure loci identified by an updated genome-wide linkage scan: Meta-analysis of the Family Blood Pressure Program. Am. J. Hypertens. 2011, 24, 347–354. [Google Scholar] [CrossRef]
- Sung, Y.J.; Basson, J.; Cheng, N.; Nguyen, K.D.; Nandakumar, P.; Hunt, S.C.; Arnett, D.K.; Dávila-Román, V.G.; Rao, D.C.; Chakravarti, A. The role of rare variants in systolic blood pressure: Analysis of ExomeChip data in HyperGEN African Americans. Hum. Hered. 2015, 79, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Young, J.H.; Fox, E.; Keating, B.J.; Franceschini, N.; Kang, S.; Tayo, B.; Adeyemo, A.; Sun, Y.V.; Li, Y.; et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: Contributions from the CARe consortium. Hum. Mol. Genet. 2011, 20, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000, 283, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Boerwinkle, E.; Davis, B.R.; Eckfeldt, J.; Ford, C.E.; Black, H. Pharmacogenetic approaches to hypertension therapy: Design and rationale for the Genetics of Hypertension Associated Treatment (GenHAT) study. Pharmacogenom. J. 2002, 2, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Cushman, W.C.; Ford, C.E.; Einhorn, P.T.; Wright, J.T., Jr.; Preston, R.A.; Davis, B.R.; Basile, J.N.; Whelton, P.K.; Weiss, R.J.; Bastien, A.; et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J. Clin. Hypertens. 2008, 10, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Do, A.N.; Lynch, A.I.; Claas, S.A.; Boerwinkle, E.; Davis, B.R.; Ford, C.E.; Eckfeldt, J.H.; Tiwari, H.K.; Arnett, D.K.; Irvin, M.R. The effects of genes implicated in cardiovascular disease on blood pressure response to treatment among treatment-naive hypertensive African Americans in the GenHAT study. J. Hum. Hypertens. 2016, 30, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.R.; Cutler, J.A.; Furberg, C.D.; Wright, J.T.; Farber, M.A.; Felicetta, J.V.; Stokes, J.D.; ALLHAT Collaborative Research Group. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: Further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial. Ann. Intern. Med. 2002, 137, 313–320. [Google Scholar]
- Irvin, M.R.; Lynch, A.I.; Kabagambe, E.K.; Tiwari, H.K.; Barzilay, J.I.; Eckfeldt, J.H.; Boerwinkle, E.; Davis, B.R.; Ford, C.E.; Arnett, D.K. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT Study. J. Hypertens. 2010, 28, 2076–2083. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Forer, L.; Schonherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [Green Version]
- Fuchsberger, C.; Abecasis, G.R.; Hinds, D.A. minimac2: Faster genotype imputation. Bioinformatics 2015, 31, 782–784. [Google Scholar] [CrossRef] [Green Version]
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- McCaw, Z.R.; Lane, J.M.; Saxena, R.; Redline, S.; Lin, X. Operating characteristics of the rank-based inverse normal transformation for quantitative trait analysis in genome-wide association studies. Biometrics 2020, 76, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boughton, A.P.; Welch, R.P.; Flickinger, M.; VandeHaar, P.; Taliun, D.; Abecasis, G.R.; Boehnke, M. LocusZoom.js: Interactive and embeddable visualization of genetic association study results. Bioinformatics 2021, 37, 3017–3018. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Schwartz, G.L.; Boerwinkle, E.; Turner, S.T. Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int. 2002, 61, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A.; Boerwinkle, E.; Zineh, I.; Chapman, A.B.; Bailey, K.; Cooper-DeHoff, R.M.; Gums, J.; Curry, R.W.; Gong, Y.; Beitelshees, A.L.; et al. Pharmacogenomics of antihypertensive drugs: Rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am. Heart J. 2009, 157, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Hamadeh, I.S.; Langaee, T.Y.; Dwivedi, R.; Garcia, S.; Burkley, B.M.; Skaar, T.C.; Chapman, A.B.; Gums, J.G.; Turner, S.T.; Gong, Y.; et al. Impact of CYP2D6 polymorphisms on clinical efficacy and tolerability of metoprolol tartrate. Clin. Pharmacol. Ther. 2014, 96, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Sa, A.C.C.; Webb, A.; Gong, Y.; McDonough, C.W.; Shahin, M.H.; Datta, S.; Langaee, T.Y.; Turner, S.T.; Beitelshees, A.L.; Chapman, A.B.; et al. Blood pressure signature genes and blood pressure response to thiazide diuretics: Results from the PEAR and PEAR-2 studies. BMC Med. Genom. 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- McDonough, C.W.; Warren, H.R.; Jack, J.R.; Motsinger-Reif, A.A.; Armstrong, N.D.; Bis, J.C.; House, J.S.; Singh, S.; Rouby, N.M.E.; Gong, Y.; et al. Adverse Cardiovascular Outcomes and Antihypertensive Treatment: A Genome-Wide Interaction Meta-Analysis in the International Consortium for Antihypertensive Pharmacogenomics Studies. Clin. Pharmacol. Ther. 2021, 110, 723–732. [Google Scholar] [CrossRef]
- National Library of Medicine, National Center for Biotechnology Information. Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 27 January 2022).
- Wojczynski, M.K.; Li, M.; Bielak, L.F.; Kerr, K.F.; Reiner, A.P.; Wong, N.D.; Yanek, L.R.; Qu, L.; White, C.C.; Lange, L.A.; et al. Genetics of coronary artery calcification among African Americans, a meta-analysis. BMC Med. Genet. 2013, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Motro, M.; Shemesh, J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension 2001, 37, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Strutz, F.; Zeisberg, M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2992–2998. [Google Scholar] [CrossRef]
- Thedieck, C.; Kalbacher, H.; Kuczyk, M.; Muller, G.A.; Muller, C.A.; Klein, G. Cadherin-9 is a novel cell surface marker for the heterogeneous pool of renal fibroblasts. PLoS ONE 2007, 2, e657. [Google Scholar] [CrossRef] [Green Version]
- Krapf, R.; Hulter, H.N. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin. J. Am. Soc. Nephrol. 2009, 4, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Liu, J.; Asuncion-Chin, M.; Blaskova, E.; Bannister, J.P.; Dopico, A.M.; Jaggar, J.H. A novel CaV1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J. Biol. Chem. 2007, 282, 29211–29221. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.H.; Rusch, N.J. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation 2002, 9, 243–257. [Google Scholar] [CrossRef]
- Bremer, T.; Man, A.; Kask, K.; Diamond, C. CACNA1C polymorphisms are associated with the efficacy of calcium channel blockers in the treatment of hypertension. Pharmacogenomics 2006, 7, 271–279. [Google Scholar] [CrossRef]
- Kamide, K.; Yang, J.; Matayoshi, T.; Takiuchi, S.; Horio, T.; Yoshii, M.; Miwa, Y.; Yasuda, H.; Yoshihara, F.; Nakamura, S.; et al. Genetic polymorphisms of L-type calcium channel α1C and α1D subunit genes are associated with sensitivity to the antihypertensive effects of L-type dihydropyridine calcium-channel blockers. Circ. J. 2009, 73, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Beitelshees, A.L.; Navare, H.; Wang, D.; Gong, Y.; Wessel, J.; Moss, J.I.; Langaee, T.Y.; Cooper-DeHoff, R.M.; Sadee, W.; Pepine, C.J.; et al. CACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment response. Circ. Cardiovasc. Genet. 2009, 2, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Liu, F.; Li, M.; He, J.; Huang, J.; Rao, D.C.; Hixson, J.E.; Gu, C.; Kelly, T.N.; Chen, S.; et al. Associations of Variants in the CACNA1A and CACNA1C Genes with Longitudinal Blood Pressure Changes and Hypertension Incidence: The GenSalt Study. Am. J. Hypertens. 2016, 29, 1301–1306. [Google Scholar] [CrossRef] [Green Version]
- Wain, L.V.; Verwoert, G.C.; O’Reilly, P.F.; Shi, G.; Johnson, T.; Johnson, A.D.; Bochud, M.; Rice, K.M.; Henneman, P.; Smith, A.V.; et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 2011, 43, 1005–1011. [Google Scholar] [CrossRef]
- Sumida, T.; Naito, A.T.; Nomura, S.; Nakagawa, A.; Higo, T.; Hashimoto, A.; Okada, K.; Sakai, T.; Ito, M.; Yamaguchi, T.; et al. Complement C1q-induced activation of beta-catenin signalling causes hypertensive arterial remodelling. Nat. Commun. 2015, 6, 6241. [Google Scholar] [CrossRef] [Green Version]
- Abou Ziki, M.D.; Mani, A. Wnt signaling, a novel pathway regulating blood pressure? State of the art review. Atherosclerosis 2017, 262, 171–178. [Google Scholar] [CrossRef]
- Van de Schans, V.A.; van den Borne, S.W.; Strzelecka, A.E.; Janssen, B.J.; van der Velden, J.L.; Langen, R.C.; Wynshaw-Boris, A.; Smits, J.F.M.; Blankesteijn, W.M. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension 2007, 49, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.A.; Zavadzkas, J.A.; Chang, E.I.; Sheats, N.; Koval, C.; Stroud, R.E.; Spinale, F.G.; Ikonomidis, J.S. Cellular phenotype transformation occurs during thoracic aortic aneurysm development. J. Thorac. Cardiovasc. Surg. 2010, 140, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Oklu, R.; Hesketh, R. The latent transforming growth factor beta binding protein (LTBP) family. Biochem. J. 2000, 352, 601–610. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Bao, M.; Li, J.; Liu, X.; Tan, X.; Zhou, Y.; Chen, Y.; Wu, S.; Chen, S.; et al. Genome Wide Association Study Identifies L3MBTL4 as a Novel Susceptibility Gene for Hypertension. Sci. Rep. 2016, 6, 30811. [Google Scholar] [CrossRef]
- Hui, Y.; Cheng, Y.; Smalera, I.; Jian, W.; Goldhahn, L.; Fitzgerald, G.A.; Funk, C.D. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation 2004, 110, 3360–3366. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.C.; Hammarstrom, S.; Samuelsson, B. Leukotriene C: A slow-reacting substance from murine mastocytoma cells. Proc. Natl. Acad. Sci. USA 1979, 76, 4275–4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.T.; Laine, A.P.; Soderhall, C.; Gruzieva, O.; Rautio, S.; Melen, E.; Pershagen, G.; Lähdesmäki, H.J.; Knip, M.; Ilonen, J.; et al. GIMAP GTPase family genes: Potential modifiers in autoimmune diabetes, asthma, and allergy. J. Immunol. 2015, 194, 5885–5894. [Google Scholar] [CrossRef] [PubMed]
- Divers, J.; Palmer, N.D.; Langefeld, C.D.; Brown, W.M.; Lu, L.; Hicks, P.J.; Smith, S.C.; Xu, J.; Terry, J.G.; Register, T.C.; et al. Genome-wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 2017, 18, 105. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Qi, Q.; Lu, L.; Gan, W.; Loos, R.J.; Lin, X. Common variants in or near FGF5, CYP17A1 and MTHFR genes are associated with blood pressure and hypertension in Chinese Hans. J. Hypertens. 2011, 29, 70–75. [Google Scholar] [CrossRef]
- Ren, Y.; Jiao, X.; Zhang, L. Expression level of fibroblast growth factor 5 (FGF5) in the peripheral blood of primary hypertension and its clinical significance. Saudi J. Biol. Sci. 2018, 25, 469–473. [Google Scholar] [CrossRef]
- Chittani, M.; Zaninello, R.; Lanzani, C.; Frau, F.; Ortu, M.F.; Salvi, E.; Fresu, G.; Citterio, L.; Braga, D.; Piras, D.A.; et al. TET2 and CSMD1 genes affect SBP response to hydrochlorothiazide in never-treated essential hypertensives. J. Hypertens. 2015, 33, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, T.P.; Donner, K.M.; Sarin, A.P.; Saarela, J.; Ripatti, S.; Chapman, A.B.; Gums, J.G.; Gong, Y.; Cooper-DeHoff, R.M.; Frau, F.; et al. Pharmacogenomics of hypertension: A genome-wide, placebo-controlled cross-over study, using four classes of antihypertensive drugs. J. Am. Heart Assoc. 2015, 4, e001521. [Google Scholar] [CrossRef] [Green Version]
- Wegner, B.; Al-Momany, A.; Kulak, S.C.; Kozlowski, K.; Obeidat, M.; Jahroudi, N.; Paes, J.; Berryman, M.; Ballerman, B.J. CLIC5A, a component of the ezrin-podocalyxin complex in glomeruli, is a determinant of podocyte integrity. Am. J. Physiol. Renal Physiol. 2010, 298, F1492–F1503. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Luo, Y.; Peng, H. EBF1 gene polymorphism and its interaction with smoking and drinking on the risk of coronary artery disease for Chinese patients. Biosci. Rep. 2018, 38, BSR20180324. [Google Scholar] [CrossRef] [Green Version]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Nitritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; McDonough, C.W.; Gong, Y.; Alghamdi, W.A.; Arwood, M.J.; Bargal, S.A.; Dumeny, L.; Li, W.-Y.; Mehanna, M.; Stockard, B.; et al. Genome Wide Association Study Identifies the HMGCS2 Locus to be Associated With Chlorthalidone Induced Glucose Increase in Hypertensive Patients. J. Am. Heart Assoc. 2018, 7, e007339. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; McDonough, C.W.; Gong, Y.; Bailey, K.R.; Boerwinkle, E.; Chapman, A.B.; Gums, J.G.; Turner, S.T.; Cooper-DeHoff, R.M.; Johnson, J.A. Genome Wide Analysis Approach Suggests Chromosome 2 Locus to be Associated with Thiazide and Thiazide Like-Diuretics Blood Pressure Response. Sci. Rep. 2019, 9, 17323. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Wang, Z.; Shahin, M.H.; Langaee, T.Y.; Gong, Y.; Turner, S.T.; Chapman, A.B.; Gums, J.G.; McDonough, C.W.; Bailey, K.R.; et al. Targeted sequencing identifies a missense variant in the BEST3 gene associated with antihypertensive response to hydrochlorothiazide. Pharmacogenet. Genom. 2018, 28, 251–255. [Google Scholar] [CrossRef]

| N | 4297 |
|---|---|
| Sex, % Female | 55.71% (2394) |
| Age, years | 66.14 ± 7.73 |
| BMI, kg/m2 | 30.46 ± 6.54 |
| T2D status | 40.66% (1747) |
| Cigarette smoking status | 27.44% (1000) |
| eGFR, mL/min/1.73 m2 | 82.66 ± 21.49 |
| SBP, mmHg | 146.14 ± 15.67 |
| DBP, mmHg | 84.86 ± 21.49 |
| FG, mg/dL | 127.40 ± 65.30 |
| K, mmol/L | 4.22 ± 0.54 |
| N | Mean Difference ± SD | |
|---|---|---|
| ΔSBP, mmHg | 3982 | −6.69 ± 19.30 |
| ΔDBP, mmHg | 3982 | −3.06 ± 10.82 |
| ΔFG, mg/dL | 1127 | 6.71 ± 58.65 |
| rsID | CHR:BP | A1/A2 | EAF | β 2 | 95% CI | p3 | Location | Gene |
|---|---|---|---|---|---|---|---|---|
| ΔSBP | ||||||||
| rs191702725 | 5:176580424 | A/G | 0.003 | 1.08 | 0.73, 1.43 | 2.40 × 10−9 | intronic | CDHR2 |
| rs6009272 | 22:49475763 | G/C | 0.444 | 0.11 | 0.07, 0.15 | 2.14 × 10−8 | intergenic | LINC01310; MIR3667 |
| rs186198403 | 5:176649236 | A/G | 0.003 | 1.02 | 0.66, 1.39 | 3.15 × 10−8 | intronic | TSPAN17 |
| ΔDBP | ||||||||
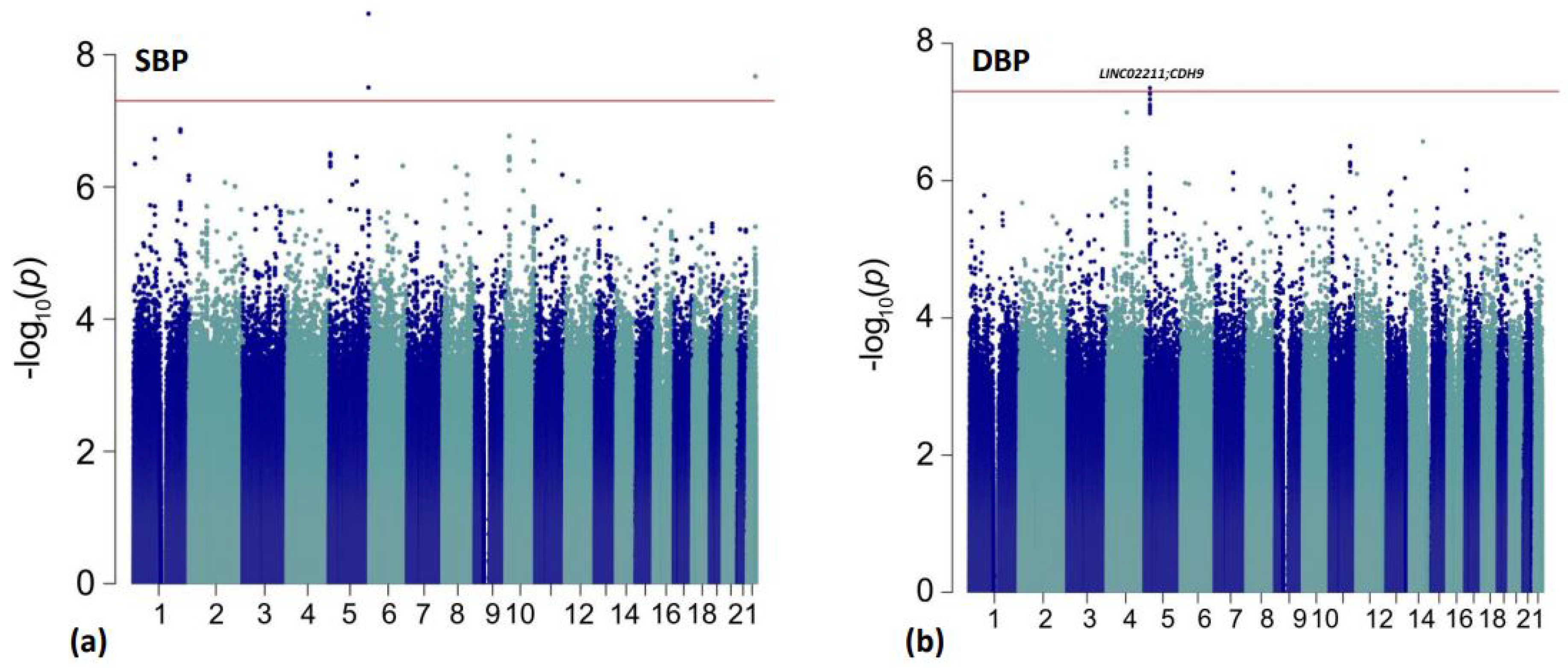
| rs10440665 | 5:25918567 | G/A | 0.267 | −0.11 | −0.15, −0.07 | 4.48 × 10−8 | intergenic | LINC02211; CDH9 |
| rs1593983 | 5:25919837 | A/G | 0.267 | −0.11 | −0.15, −0.07 | 5.18 × 10−8 | intergenic | LINC02211; CDH9 |
| rs28413118 | 5:25929421 | A/C | 0.270 | −0.11 | −0.15, −0.07 | 5.37 × 10−8 | intergenic | LINC02211; CDH9 |
| rs10050387 | 5:25928779 | C/T | 0.268 | −0.11 | −0.15, −0.07 | 5.44 × 10−8 | intergenic | LINC02211; CDH9 |
| rs4701513 | 5:25925888 | T/A | 0.268 | −0.11 | −0.15, −0.07 | 5.44 × 10−8 | intergenic | LINC02211; CDH9 |
| rs10440666 | 5:25918597 | G/A | 0.267 | −0.11 | −0.15, −0.07 | 6.47 × 10−8 | intergenic | LINC02211; CDH9 |
| rs12697671 | 5:25929723 | A/C | 0.268 | −0.11 | −0.15, −0.07 | 6.61 × 10−8 | intergenic | LINC02211; CDH9 |
| rs72748996 | 5:25924670 | G/A | 0.268 | −0.11 | −0.15, −0.07 | 7.83 × 10−8 | intergenic | LINC02211; CDH9 |
| rs13164498 | 5:25931554 | T/A | 0.275 | −0.11 | −0.15, −0.07 | 7.98 × 10−8 | intergenic | LINC02211; CDH9 |
| rs6884731 | 5:25921084 | G/A | 0.268 | −0.11 | −0.15, −0.07 | 8.72 × 10−8 | intergenic | LINC02211; CDH9 |
| rs10440667 | 5:25919034 | G/A | 0.267 | −0.11 | −0.15, −0.07 | 9.58 × 10−8 | intergenic | LINC02211; CDH9 |
| rsID | CHR:BP | A1/A2 | EAF | β 2 | 95% CI | p 3 | Location | Gene |
|---|---|---|---|---|---|---|---|---|
| rs114758661 | 12:2472136 | G/A | 0.017 | 1.26 | 0.84, 1.68 | 1.12 × 10−8 | intronic | CACNA1C |
| rs540857940 | 12:2475156 | A/G | 0.017 | 1.26 | 0.84, 1.68 | 1.12 × 10−8 | intronic | CACNA1C |
| rs141391468 | 12:2470148 | G/A | 0.019 | 1.17 | 0.77, 1.57 | 3.59 × 10−8 | intronic | CACNA1C |
| rs7905470 | 10:7247513 | A/C | 0.199 | −0.44 | −0.59, −0.29 | 4.72 × 10−8 | intronic | SFMBT2 |
| rs80214621 | 5:157232090 | T/G | 0.017 | 1.40 | 0.91, 1.89 | 6.14 × 10−8 | intronic | ITK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armstrong, N.D.; Srinivasasainagendra, V.; Chekka, L.M.S.; Nguyen, N.H.K.; Nahid, N.A.; Jones, A.C.; Tanner, R.M.; Hidalgo, B.A.; Limdi, N.A.; Claas, S.A.; et al. Genetic Contributors of Efficacy and Adverse Metabolic Effects of Chlorthalidone in African Americans from the Genetics of Hypertension Associated Treatments (GenHAT) Study. Genes 2022, 13, 1260. https://doi.org/10.3390/genes13071260
Armstrong ND, Srinivasasainagendra V, Chekka LMS, Nguyen NHK, Nahid NA, Jones AC, Tanner RM, Hidalgo BA, Limdi NA, Claas SA, et al. Genetic Contributors of Efficacy and Adverse Metabolic Effects of Chlorthalidone in African Americans from the Genetics of Hypertension Associated Treatments (GenHAT) Study. Genes. 2022; 13(7):1260. https://doi.org/10.3390/genes13071260
Chicago/Turabian StyleArmstrong, Nicole D., Vinodh Srinivasasainagendra, Lakshmi Manasa S. Chekka, Nam H. K. Nguyen, Noor A. Nahid, Alana C. Jones, Rikki M. Tanner, Bertha A. Hidalgo, Nita A. Limdi, Steven A. Claas, and et al. 2022. "Genetic Contributors of Efficacy and Adverse Metabolic Effects of Chlorthalidone in African Americans from the Genetics of Hypertension Associated Treatments (GenHAT) Study" Genes 13, no. 7: 1260. https://doi.org/10.3390/genes13071260
APA StyleArmstrong, N. D., Srinivasasainagendra, V., Chekka, L. M. S., Nguyen, N. H. K., Nahid, N. A., Jones, A. C., Tanner, R. M., Hidalgo, B. A., Limdi, N. A., Claas, S. A., Gong, Y., McDonough, C. W., Cooper-DeHoff, R. M., Johnson, J. A., Tiwari, H. K., Arnett, D. K., & Irvin, M. R. (2022). Genetic Contributors of Efficacy and Adverse Metabolic Effects of Chlorthalidone in African Americans from the Genetics of Hypertension Associated Treatments (GenHAT) Study. Genes, 13(7), 1260. https://doi.org/10.3390/genes13071260







