Differences and Similarities in Adaptive Functioning between Children with Autism Spectrum Disorder and Williams–Beuren Syndrome: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Molecular Analysis
2.3. Assessment Tools
2.3.1. Adaptive Level
2.3.2. Cognitive/Developmental Level
- Griffiths Mental Development Scales—Extended Revised (GMDS-ER) [42], a tool used to assess the level of development in children from birth to 8 years. GMDS-ER provides an overall score of developmental level (DQ), which has been used to compare the youngest children to the other patients included in our sample.
- The Leiter-R [43], a non-verbal cognitive tool administered to people from 2 years to 20 years and 11 months of age. This test does not require language abilities. In this study, the nonverbal brief intelligence quotient (hereinafter referred to as IQ) has been used for comparisons.
- Raven’s Colored Progressive Matrices (R-CPM) [44], a standardized test for children between 5 and 11 years of age. The assessment comprises 36 items and participants are asked to complete a missing piece out of six/eight options. This test provides a performance percentile and a corresponding intelligent quotient (hereinafter referred to as IQ).
2.4. Procedure
- A clinical diagnosis of WBS confirmed positive fluorescent in situ hybridization test or Array-CGH;
- A clinical diagnosis of “Autistic Disorder”, “Asperger’s Disorder”, and “Pervasive Developmental Disorders Not Otherwise Specified” based on Diagnostic and Statistical Manual of Mental Disorders, 4th, text revision criteria (DSM IV-TR) [47], or ASD based on Diagnostic and Statistical Manual of Mental Disorders-5 criteria (DSM 5) [3] and confirmed by gold standard assessment tools: Autism Diagnostic Observation Schedule-Generic (ADOS-G) [48], Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) [49] and Autism Diagnostic Interview—Revised (ADI-R) [50].
- Age between 2 and 17.11 years.
- At least two assessments of adaptive profile by means of VABS [51].
- Assessment of cognitive/developmental level by means of appropriate developmental tools during the first evaluation (Time 0).
3. Statistical Analysis
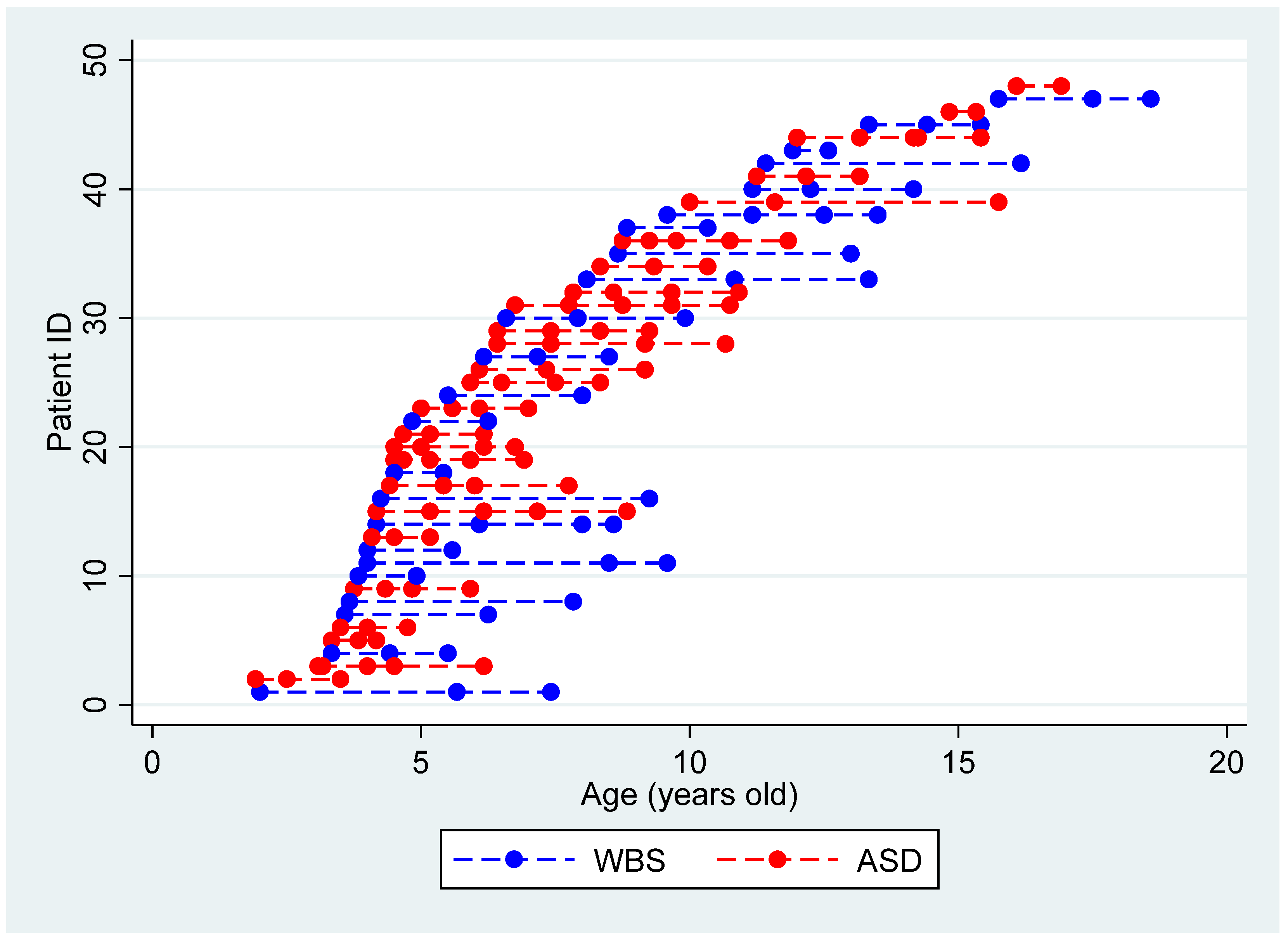
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tassé, M.J.; Luckasson, R.; Nygren, M. AAIDD proposed recommendations for ICD-11 and the condition previously known as mental retardation. Intellect. Dev. Disabil. 2013, 51, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales Vineland-II: Survey Forms Manual; Pearson: Minneapolis, MN, USA, 2005. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12, Erratum in MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 503. [Google Scholar]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 29, e5. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors with Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef]
- Osborne, L.R.; Li, M.; Pober, B.; Chitayat, D.; Bodurtha, J.; Mandel, A.; Costa, T.; Grebe, T.; Cox, S.; Tsui, L.-C.; et al. A 1.5 million–base pair inversion polymorphism in families with Williams-Beuren syndrome. Nat. Genet. 2001, 29, 321–325. [Google Scholar] [CrossRef]
- Bayés, M.; Magano, L.F.; Rivera, N.; Flores, R.; Jurado, L.A.P. Mutational Mechanisms of Williams-Beuren Syndrome Deletions. Am. J. Hum. Genet. 2003, 73, 131–151. [Google Scholar] [CrossRef] [Green Version]
- Strømme, P.; Bjømstad, P.G.; Ramstad, K. Prevalence Estimation of Williams Syndrome. J. Child Neurol. 2002, 17, 269–271. [Google Scholar] [CrossRef]
- Vicari, S.; Caselli, M.C.; Gagliardi, C.; Tonucci, F.; Volterra, V. Language acquisition in special populations: A comparison between Down and Williams syndromes. Neuropsychologia 2002, 40, 2461–2470. [Google Scholar] [CrossRef]
- Järvinen-Pasley, A.; Bellugi, U.; Reilly, J.; Debra, L.; Galaburda, A.; Reiss, A.L.; Korenberg, J.R. Defining the social phenotype in Williams syndrome: A model for linking gene, the brain, and behavior. Dev. Psychopathol. 2008, 20, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, P.; Menghini, D.; Marotta, L.; De Peppo, L.; Ravà, L.; Salvaguardia, F.; Varuzza, C.; Vicari, S. A comparison between linguistic skills and socio-communicative abilities in Williams syndrome. J. Intellect. Disabil. Res. 2017, 61, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, G.; Hamner, T.; Lee, N.R. Neurodevelopmental disorders affecting sociability: Recent Research Advances and Future Directions in Autism Spectrum Disorder and Williams Syndrome. Curr. Neurol. Neurosci. Rep. 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.S.; Volkmar, F.R.; Sparrow, S.S.; Wang, J.-J.; Lord, C.; Dawson, G.; Fombonne, E.; Loveland, K.; Mesibov, G.; Schopler, E. The Vineland Adaptive Behavior Scales: Supplementary norms for individuals with autism. J. Autism Dev. Disord. 1998, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Greer, M.K.; Brown, F.R.; Pai, G.S.; Choudry, S.H.; Klein, A.J. Cognitive, adaptive, and behavioral characteristics of Williams syndrome. Am. J. Med Genet. 1997, 74, 521–525. [Google Scholar] [CrossRef]
- Mervis, C.B.; Klein-Tasman, B.P. Williams syndrome: Cognition, personality, and adaptive behavior. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 148–158. [Google Scholar] [CrossRef]
- Mervis, C.B.; Klein-Tasman, B.P.; Mastin, M.E. Adaptive behavior of 4-through 8-year-old children with Williams syndrome. Am. J. Ment. Retard. 2001, 106, 82–93. [Google Scholar] [CrossRef]
- Kanne, S.M.; Gerber, A.J.; Quirmbach, L.M.; Sparrow, S.S.; Cicchetti, D.V.; Saulnier, C.A. The Role of Adaptive Behavior in Autism Spectrum Disorders: Implications for Functional Outcome. J. Autism Dev. Disord. 2010, 41, 1007–1018. [Google Scholar] [CrossRef]
- Estes, A.; Network, I.; Zwaigenbaum, L.; Gu, H.; John, T.S.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Hahn, L.; Fidler, D.J.; Hepburn, S.L. Adaptive Behavior and Problem Behavior in Young Children with Williams Syndrome. Am. J. Intellect. Dev. Disabil. 2014, 119, 49–63. [Google Scholar] [CrossRef]
- Kirchner, R.M.; Martens, M.A.; Andridge, R.R. Adaptive behavior and development of infants and toddlers with Williams syndrome. Front. Psychol. 2016, 7, 598. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Paynter, J.M.; Gilmore, L. Vineland Adaptive Behavior Scales: II Profile of Young Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 46, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatham, C.H.; Taylor, K.I.; Charman, T.; D'Ardhuy, X.L.; Eule, E.; Fedele, A.; Hardan, A.Y.; Loth, E.; Murtagh, L.; Rubido, M.D.V.; et al. Adaptive behavior in autism: Minimal clinically important differences on the Vineland-II. Autism Res. 2017, 11, 270–283. [Google Scholar] [CrossRef]
- Szatmari, P.; Georgiades, S.; Duku, E.; Bennett, T.A.; Bryson, S.; Fombonne, E.; Mirenda, P.; Roberts, W.; Smith, I.M.; Vaillancourt, T.; et al. Developmental Trajectories of Symptom Severity and Adaptive Functioning in an Inception Cohort of Preschool Children with Autism Spectrum Disorder. JAMA Psychiatry 2015, 72, 276–283. [Google Scholar] [CrossRef]
- Szatmari, P.; Bryson, S.; Duku, E.; Vaccarella, L.; Zwaigenbaum, L.; Bennett, T.; Boyle, M.H. Similar developmental trajectories in autism and Asperger syndrome: From early childhood to adolescence. J. Child Psychol. Psychiatry 2009, 50, 1459–1467. [Google Scholar] [CrossRef]
- Baghdadli, A.; Assouline, B.; Sonié, S.; Pernon, E.; Darrou, C.; Michelon, C.; Picot, M.-C.; Aussilloux, C.; Pry, R. Developmental Trajectories of Adaptive Behaviors from Early Childhood to Adolescence in a Cohort of 152 Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 42, 1314–1325. [Google Scholar] [CrossRef]
- Pugliese, C.E.; Anthony, L.G.; Strang, J.F.; Dudley, K.; Wallace, G.; Naiman, D.Q.; Kenworthy, L. Longitudinal Examination of Adaptive Behavior in Autism Spectrum Disorders: Influence of Executive Function. J. Autism Dev. Disord. 2015, 46, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Farmer, C.A.; Chilakamarri, P.; Thurm, A.E.; Swedo, S.E.; Holmes, G.L.; Buckley, A.W. Spindle activity in young children with autism, developmental delay, or typical development. Neurology 2018, 91, e112–e122. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Zöller, D.; Gentaz, E.; Glaser, B.; Wood de Wilde, H.; Kojovic, N.; Eliez, S.; Schaer, M. Early adaptive functioning trajectories in preschoolers with autism spectrum disorders. J. Pediatric Psychol. 2018, 43, 800–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewski, B.; Hepburn, S.; Blakeley-Smith, A.; Rogers, S.J. Developmental Trajectories of Adaptive Behavior from Toddlerhood to Middle Childhood in Autism Spectrum Disorder. Am. J. Intellect. Dev. Disabil. 2020, 125, 155–169. [Google Scholar] [CrossRef]
- Mervis, C.B.; Pitts, C.H. Children with Williams syndrome: Developmental trajectories for intellectual abilities, vocabulary abilities, and adaptive behavior. Am. J. Med. Genet. Part C Semin. Med Genet. 2015, 169, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.H.; Lense, M.; Dykens, E.M. Longitudinal trajectories of intellectual and adaptive functioning in adolescents and adults with Williams syndrome. J. Intellect. Disabil. Res. 2016, 60, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, C.E.; Anthony, L.; Strang, J.F.; Dudley, K.; Wallace, G.; Kenworthy, L. Increasing Adaptive Behavior Skill Deficits from Childhood to Adolescence in Autism Spectrum Disorder: Role of Executive Function. J. Autism Dev. Disord. 2014, 45, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Dressler, A.; Perelli, V.; Bozza, M.; Bargagna, S. The autistic phenotype in Down syndrome: Differences in adaptive behaviour versus Down syndrome alone and autistic disorder alone. Funct. Neurol. 2011, 26, 151. [Google Scholar]
- Will, M.N.; Currans, K.; Smith, J.; Weber, S.; Duncan, A.; Burton, J.M.; Kroeger-Geoppinger, K.; Miller, V.; Stone, M.; Mays, L.; et al. Evidenced-Based Interventions for Children with Autism Spectrum Disorder. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Hamner, T.; Lee, N.R.; Hocking, D.R.; Vivanti, G. Shared and syndrome-specific adaptive difficulties in preschoolers with Williams syndrome and autism spectrum disorder: A cross-syndrome study. J. Intellect. Disabil. Res. 2019, 63, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, P.; Scibelli, F.; Digilio, M.C.; Novello, R.L.; Caciolo, C.; Valeri, G.; Vicari, S. Comparison of Adaptive Functioning in Children with Williams Beuren Syndrome and Autism Spectrum Disorder: A Cross-Syndrome Study. Autism Res. 2020, 14, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.; Moon, M.J.; Brian, J.; Georgiades, S.; Levy, T.; Anagnostou, E.; Nicolson, R.; Schachar, R.; Crosbie, J. Concurrent Validity of the ABAS-II Questionnaire with the Vineland II Interview for Adaptive Behavior in a Pediatric ASD Sample: High Correspondence Despite Systematically Lower Scores. J. Autism Dev. Disord. 2020, 51, 1417–1427. [Google Scholar] [CrossRef]
- Fenton, G.; D’ardia, C.; Valente, D.; Del Vecchio, I.; Fabrizi, A.; Bernabei, P. Vineland adaptive behavior profiles in children with autism and moderate to severe developmental delay. Autism 2003, 7, 269–287. [Google Scholar] [CrossRef]
- Perry, A.; Flanagan, H.E.; Geier, J.D.; Freeman, N.L. Brief Report: The Vineland Adaptive Behavior Scales in Young Children with Autism Spectrum Disorders at Different Cognitive Levels. J. Autism Dev. Disord. 2009, 39, 1066–1078. [Google Scholar] [CrossRef]
- Luiz, D.M.; Foxcroft, C.D.; Povey, J.-L. The Griffiths scales of mental development: A factorial validity study. S. Afr. J. Psychol. 2006, 36, 192–214. [Google Scholar] [CrossRef]
- Roid, G.L.; Miller, L.J. Leiter International Performance Scale—Revised (1997); Italian edition (2002); Stoelting: Wood Dale, IL, USA; Giunti Psychometrics: Florence, Italy, 1997. [Google Scholar]
- Raven, J.C.; Court, J.H.; Raven, J. Coloured Progressive Matrices; Psychology Press: Oxford, UK, 1990. [Google Scholar]
- Weschler, D. Wechsler Intelligence Scale for Children—Third Edition (WISC-III); The Psychological Corporation: San Antonio, TX, USA, 1991. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Orsini, A., Pezzuti, L., Picone, L., Eds.; Italian edition; The Psychological Corporation: San Antonio, TX, USA; Giunti Psychometrics: Florence, Italy, 2014. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Text Revision, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland Adaptive Behavior Scales VABS: Expanded Form Manual; American Guidance Service: Washington, DC, USA, 1984. [Google Scholar]
- Plesa Skwerer, D.; Verbalis, A.; Schofield, C.; Faja, S.; Tager-Flusberg, H. Social-perceptual abilities in adolescents and adults with Williams syndrome. Cogn. Neuropsychol. 2006, 23, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Dykens, E.M.; Rosner, B.A. Refining behavioral phenotypes: Personality—Motivation in Williams and Prader-Willi syndromes. Am. J. Ment. Retard. 1999, 104, 158–169. [Google Scholar] [CrossRef]



| ASD | WBS | |
|---|---|---|
| M: F | 3:21 | 13:11 |
| Age in months (SD) [Min-Max] | 80.75 (45.05) (23–193) | 82.63 (44.14) (24–189) |
| IQ/DQ | 66.96 (17.12) (39–87) | 65.96 (19.24) (39–102) |
| REC | ASD | WBS | p-Value |
|---|---|---|---|
| Communication | 0.53 | 0.70 | 0.021 * |
| Daily Living Skills | 0.51 | 0.55 | 0.373 |
| Socialization | 0.44 | 0.55 | 0.029 * |
| Scale | Coefficient (β) | p-Value | 95% CI |
|---|---|---|---|
| Communication Age Diagnosis ASD (ref) WBS | |||
| −0.01 | 0.033 | (−0.025; 0.001) | |
| 0.12 | 0.055 | (−0.002; 0.243) | |
| Daily Living Skills Age Diagnosis ASD (ref) WBS | |||
| −0.02 | <0.001 | (−0.025; 0.009) | |
| −0.01 | 0.781 | (−0.084; 0.063) | |
| Socialization Age Diagnosis ASD (ref) WBS | |||
| −0.01 | 0.005 | (−0.024; −0.004) | |
| 0.12 | 0.010 | (0.029; 0.216) | |
| Total Age Diagnosis ASD (ref) WBS | |||
| −0.01 | 0.014 | (−0.020; −0.002) | |
| 0.67 | 0.134 | (−0.021; 0.157) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, P.; Scibelli, F.; Montanaro, F.A.M.; Digilio, M.C.; Ravà, L.; Valeri, G.; Vicari, S. Differences and Similarities in Adaptive Functioning between Children with Autism Spectrum Disorder and Williams–Beuren Syndrome: A Longitudinal Study. Genes 2022, 13, 1266. https://doi.org/10.3390/genes13071266
Alfieri P, Scibelli F, Montanaro FAM, Digilio MC, Ravà L, Valeri G, Vicari S. Differences and Similarities in Adaptive Functioning between Children with Autism Spectrum Disorder and Williams–Beuren Syndrome: A Longitudinal Study. Genes. 2022; 13(7):1266. https://doi.org/10.3390/genes13071266
Chicago/Turabian StyleAlfieri, Paolo, Francesco Scibelli, Federica Alice Maria Montanaro, Maria Cristina Digilio, Lucilla Ravà, Giovanni Valeri, and Stefano Vicari. 2022. "Differences and Similarities in Adaptive Functioning between Children with Autism Spectrum Disorder and Williams–Beuren Syndrome: A Longitudinal Study" Genes 13, no. 7: 1266. https://doi.org/10.3390/genes13071266
APA StyleAlfieri, P., Scibelli, F., Montanaro, F. A. M., Digilio, M. C., Ravà, L., Valeri, G., & Vicari, S. (2022). Differences and Similarities in Adaptive Functioning between Children with Autism Spectrum Disorder and Williams–Beuren Syndrome: A Longitudinal Study. Genes, 13(7), 1266. https://doi.org/10.3390/genes13071266








