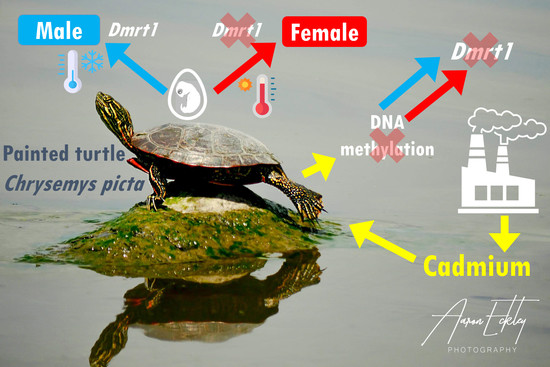
Cadmium Ecotoxic Effects on Embryonic Dmrt1 and Aromatase Expression in Chrysemys picta Turtles May Implicate Changes in DNA Methylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Egg Collection, Incubation, and Cadmium (Cd) Treatment
2.2. DNA and RNA Extractions, cDNA Conversion
2.3. qPCR Assay and Data Analysis
2.4. PCR Test of DNA Methylation
3. Results
3.1. Cadmium Induces High Embryonic Mortality
3.2. Cadmium Exposure Alters Gene Expression
3.3. Cadmium Exposure Induces Similar DNA Methylation Profiles of Dmrt1, a Testicular Development Regulator
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizoguchi, B.A.; Valenzuela, N. Ecotoxicological Perspectives of Sex Determination. Sex. Dev. 2016, 10, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S. Can Xenobiotics Alter the Sex Ratio of Crocodilians in the Wild? Sex. Dev. 2021, 15, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Adams, D.C.; Janzen, F.J. Pattern does not equal process: Exactly when is sex environmentally determined? Am. Nat. 2003, 161, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Stöck, M.; Kratochvíl, L.; Kuhl, H.; Rovatsos, M.; Evans, B.J.; Suh, A.; Valenzuela, N.; Veyrunes, F.; Zhou, Q.; Gamble, T.; et al. A brief review of vertebrate sex evolution with a pledge for integrative research: Towards ‘sexomics’. Philos. Trans. R. Soc. Lond. Ser. B 2021, 376. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, S.L.; Castelli, M.A.; Dissanayake, D.S.B.; Holleley, C.E.; Georges, A. Temperature-induced sex reversal in reptiles: Prevalence, discovery, and evolutionary implications. Sex. Dev. 2021, 15, 148–156. [Google Scholar] [CrossRef]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex Determination: Why So Many Ways of Doing It? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, N.; Lance, V.A. (Eds.) Temperature Dependent Sex Determination in Vertebrates; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Bista, B.; Valenzuela, N. Turtle insights into the evolution of the reptilian karyotype and the genomic architecture of sex determination. Genes 2020, 11, 416. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, N.; Badenhorst, D.; Montiel, E.E.; Literman, R. Molecular cytogenetic search for cryptic sex chromosomes in painted turtles Chrysemys picta. Cytogenet. Genome Res. 2014, 144, 39–46. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Literman, R.; Mizoguchi, B.; Valenzuela, N. MeDIP-seq and nCpG analyses illuminate sexually dimorphic methylation of gonadal development genes with high historic methylation in turtle hatchlings with temperature-dependent sex determination. Epigenet. Chromatin 2017, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Piferrer, F. Epigenetics of sex determination and gonadogenesis. Dev. Dyn. 2013, 242, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Head, J.A. Patterns of DNA Methylation in Animals: An Ecotoxicological Perspective. Integr. Comp. Biol. 2014, 54, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrott, B.B.; Kohno, S.; Cloy-McCoy, J.A.; Guillette, L.J. Differential Incubation Temperatures Result in Dimorphic DNA Methylation Patterning of the SOX9 and Aromatase Promoters in Gonads of Alligator (Alligator mississippiensis) Embryos1. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Hannigan, B.; Crews, D. Temperature shift alters DNA methylation and histone modification patterns in gonadal aromatase (cyp19a1) gene in species with temperature-dependent sex determination. PLoS ONE 2016, 11, e0167362. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.; Marmolejo-Valencia, A.; Valdes-Quezada, C.; Govenzensky, T.; Recillas-Targa, F.; Merchant-Larios, H. Dimorphic DNA methylation during temperature-dependent sex determination in the sea turtle Lepidochelys olivacea. Gen. Comp. Endocrinol. 2016, 236, 35–41. [Google Scholar] [CrossRef]
- Ge, C.; Ye, J.; Zhang, H.; Zhang, Y.; Sun, W.; Sang, Y.; Capel, B.; Qian, G. Dmrt1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development 2017, 144, 2222–2233. [Google Scholar] [CrossRef] [Green Version]
- Piferrer, F.; Anastasiadi, D.; Valdivieso, A.; Sánchez-baizán, N.; Moraleda-Prados, J.; Ribas, L. The Model of the Conserved Epigenetic Regulation of Sex. Front. Genet. 2019, 10, 857. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zheng, J.; Chi, M.; Liu, S.; Jiang, W.; Cheng, S.; Gu, Z.; Chen, L. Molecular identification of dmrt1 and its promoter CpG methylation in correlation with gene expression during gonad development in Culter alburnus. Fish Physiol. Biochem. 2019, 45, 245–252. [Google Scholar] [CrossRef]
- He, Y.; Wu, X.; Zhu, Y.; Yang, D. Expression Profiles of dmrt1 in Schizothorax kozlovi, and Their Relation to CpG Methylation of Its Promoter and Temperature Relation to CpG Methylation of Its Promoter and Temperature. Zool. Sci. 2020, 37, 140–147. [Google Scholar] [CrossRef]
- Ou, M.; Chen, K.; Gao, D.; Wu, Y.; Luo, Q.; Liu, H.; Zhao, J. Characterization, expression and CpG methylation analysis of Dmrt1 and its response to steroid hormone in blotched snakehead (Channa maculata). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110672. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Buemio, A.; Chu, R.; Vafaee, M.; Crews, D. Epigenetic Control of Gonadal Aromatase (cyp19a1) in Temperature-Dependent Sex Determination of Red-Eared Slider Turtles. PLoS ONE 2013, 8, e63599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, M. Sources of cadmium in the environment. Ecotoxicol. Environ. Saf. 1983, 7, 9–24. [Google Scholar] [CrossRef]
- Takiguchi, M.; Achanzar, W.E.; Qu, W.; Li, G.; Waalkes, M.P. Effects of cadmium on DNA- (Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp. Cell Res. 2003, 286, 355–365. [Google Scholar] [CrossRef]
- Kitana, N.; Callard, I.P. Effect of cadmium on gonadal development in freshwater turtle (Trachemys scripta, Chrysemys picta) embryos. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2008, 43, 262–271. [Google Scholar] [CrossRef]
- Valenzuela, N.; Neuwald, J.L.; Literman, R. Transcriptional evolution underlying vertebrate sexual development. Dev. Dyn. 2013, 242, 307–319. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Literman, R.; Neuwald, J.; Severin, A.; Valenzuela, N. Transcriptomic responses to environmental temperature by turtles with temperaturedependent and genotypic sex determination assessed by RNAseq inform the genetic architecture of embryonic gonadal development. PLoS ONE 2017, 12, e0172044. [Google Scholar] [CrossRef]
- Valenzuela, N. Egg incubation and collection of painted turtle embryos. Cold Spring Harb. Protoc. 2009, 4, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Rollinson, N.; Brooks, R.J. Optimal offspring provisioning when egg size is “constrained”: A case study with the painted turtle Chrysemys picta. Oikos 2008, 117, 144–151. [Google Scholar] [CrossRef]
- Rowe, J.W. Egg Size and Shape Variation within and among Nebraskan Painted Turtle (Chrysemys picta bellii) Populations: Relationships to Clutch and Maternal Body Size. Copeia 1994, 1994, 1034–1040. [Google Scholar] [CrossRef]
- Janzen, F.J.; Warner, D.A. Parent–offspring conflict and selection on egg size in turtles. J. Evol. Biol. 2009, 22, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- de Solla, S.R.; Martin, P.A. Absorption of current use pesticides by snapping turtle (Chelydra serpentina) eggs in treated soil. Chemosphere 2011, 85, 820–825. [Google Scholar] [CrossRef]
- Booth, D. Incubation of rigid-shelled turtle eggs: Do hydric conditions matter? J. Comp. Physiol. B 2022, 172, 627–633. [Google Scholar] [CrossRef]
- Bowden, R.M.; Ewert, M.A.; Nelson, C.E. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. Biol. Sci. 2000, 267. [Google Scholar] [CrossRef] [Green Version]
- Bull, J.J.; Vogt, R.C. Temperature-sensitive periods of sex determination in Emydid turtles. J. Exp. Zool. 1981, 218, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, B.; Valenzuela, N. Alternative splicing and thermosensitive expression of Dmrt1 during urogenital development in the painted turtle, Chrysemys picta. PeerJ 2020, 8, e8639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High Aromatase Expression in Uterine Leiomyoma Tissues of African-American Women. J. Clin. Endocrinol. Metab. 2009, 94, 1752–1756. [Google Scholar] [CrossRef]
- Lambard, S.; Galeraud-Denis, I.; Bouraïma, H.; Bourguiba, S.; Chocat, A.; Carreau, S. Expression of aromatase in human ejaculated spermatozoa: A putative marker of motility. Mol. Hum. Reprod. 2003, 9, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Carreau, S.; Wolczynski, S.; Galeraud-Denis, I. Aromatase, oestrogens and human male reproduction. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Caetano, L.C.; Gennaro, F.G.O.; Coelho, K.; Araújo, F.M.; Vila, R.A.; Araújo, A.; de Melo Bernardo, A.; Marcondes, C.R.; Chuva de Sousa Lopes, S.M.; Ramos, E.S. Differential expression of the MHM region and of sex-determining-related genes during gonadal development in chicken embryos. Genet. Mol. Res. 2014, 13, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, V.; Literman, R.; Neuwald, J.L.; Mizoguchi, B.; Iverson, J.B.; Riley, J.L.; Litzgus, J.D. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 2019, 9, 4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elf, P.K. Yolk steroid hormones and sex determination in reptiles with TSD. Gen. Comp. Endocrinol. 2003, 132, 349–355. [Google Scholar] [CrossRef]
- Eggers, S.; Ohnesorg, T.; Sinclair, A. Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 2014, 10, 673–683. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, L.; Song, S.; Zhu, C.; Wu, Q.; Zhang, L.; Wu, L. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology 2008, 244, 49–55. [Google Scholar] [CrossRef]
- Pierron, F.; Baillon, L.; Sow, M.; Gotreau, S.; Gonzalez, P. Effect of low-dose cadmium exposure on DNA methylation in the endangered European eel. Environ. Sci. Technol. 2014, 48, 797–803. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Li, J.; Wang, J.; He, B.; Xu, S. Effects of subchronic cadmium poisoning on DNA methylation in hens. Environ. Toxicol. Pharmacol. 2009, 27, 345–349. [Google Scholar] [CrossRef]
- Rie, M.T.; Lendas, K.A.; Callard, I.P. Cadmium: Tissue distribution and binding protein induction in the painted turtle, Chrysemys picta. Comp. Biochem. Physiology. Toxicol. Pharmacol. 2001, 130, 41–51. [Google Scholar] [CrossRef]
- Willingham, E. Endocrine-disrupting compounds and mixtures: Unexpected dose-response. Arch. Environ. Contam. Toxicol. 2004, 46, 265–269. [Google Scholar] [CrossRef]



| Primers and probes used in qPCR for gene expression assay | |||
| Forward | Reverse | Probe | |
| Dmrt1 | 5′ CCAACACATTCAACAAACA 3′ | 5′ ACTGCTGTAGTAGGTGGAGTC 3′ | 5′/FAM/ATCAGAGGGACGGATGCTCATTCAG 3′ |
| ß-actin | 5′ TGTGCTGCTTACAGAGG 3′ | 5′ GTACGACCAGAGGCCTA 3′ | 5′/CY5/GCCAACAGAGAAAAGATGACACAGATC 3′ |
| Aromatase | 5′ CTGTATGGGAATTGGTCC 3′ | 5′ TAATAATGAGTGTTTCCTCTCCACT 3′ | N/A |
| GAPDH | 5′ GGAGTGAGTATGACTCTTCCT 3′ | 5′ CAGCATCTCCCCACTTGA 3′ | N/A |
| Primers used in methylation-sensitive PCR assay | |||
| Forward 1 | Reverse 1 | Reverse 2 | |
| Dmrt1 | 5′ TGTCTTATTTGGGTCTCATC 3′ | 5′ GGAAGGAGCTAATGTTTCA 3′ | 5′ CTTTTTACACTGACAGTCCC 3′ |
| Aromatase | 5′ GCTAAGCAGAAAATAACTGG 3′ | 5′ ATGCCTTCTTTTTCACCTC 3′ | 5′ ACAACTGTATGTATCAGAAGTGG 3′ |
| Dmrt1 | Aromatase | |||||
|---|---|---|---|---|---|---|
| Developmental Stage | t | df | p-Value | t | df | p-Value |
| Stage 09 | 1.17728 | 14 | 0.098 | 1.6955 | 14 | 0.1121 |
| Stage 15 | 1.8221 | 11 | 0.0957 | 0.693 | 11 | 0.5027 |
| Stage 22 | 1.3729 | 9 | 0.2032 | 1.0124 | 8 | 0.341 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, B.; Topping, N.E.; Lavin, A.M.; Valenzuela, N. Cadmium Ecotoxic Effects on Embryonic Dmrt1 and Aromatase Expression in Chrysemys picta Turtles May Implicate Changes in DNA Methylation. Genes 2022, 13, 1318. https://doi.org/10.3390/genes13081318
Mizoguchi B, Topping NE, Lavin AM, Valenzuela N. Cadmium Ecotoxic Effects on Embryonic Dmrt1 and Aromatase Expression in Chrysemys picta Turtles May Implicate Changes in DNA Methylation. Genes. 2022; 13(8):1318. https://doi.org/10.3390/genes13081318
Chicago/Turabian StyleMizoguchi, Beatriz, Nicholas E. Topping, Andrew M. Lavin, and Nicole Valenzuela. 2022. "Cadmium Ecotoxic Effects on Embryonic Dmrt1 and Aromatase Expression in Chrysemys picta Turtles May Implicate Changes in DNA Methylation" Genes 13, no. 8: 1318. https://doi.org/10.3390/genes13081318
APA StyleMizoguchi, B., Topping, N. E., Lavin, A. M., & Valenzuela, N. (2022). Cadmium Ecotoxic Effects on Embryonic Dmrt1 and Aromatase Expression in Chrysemys picta Turtles May Implicate Changes in DNA Methylation. Genes, 13(8), 1318. https://doi.org/10.3390/genes13081318