PHEXL222P Mutation Increases Phex Expression in a New ENU Mouse Model for XLH Disease
Abstract
:1. Introduction
2. Materials and Methods
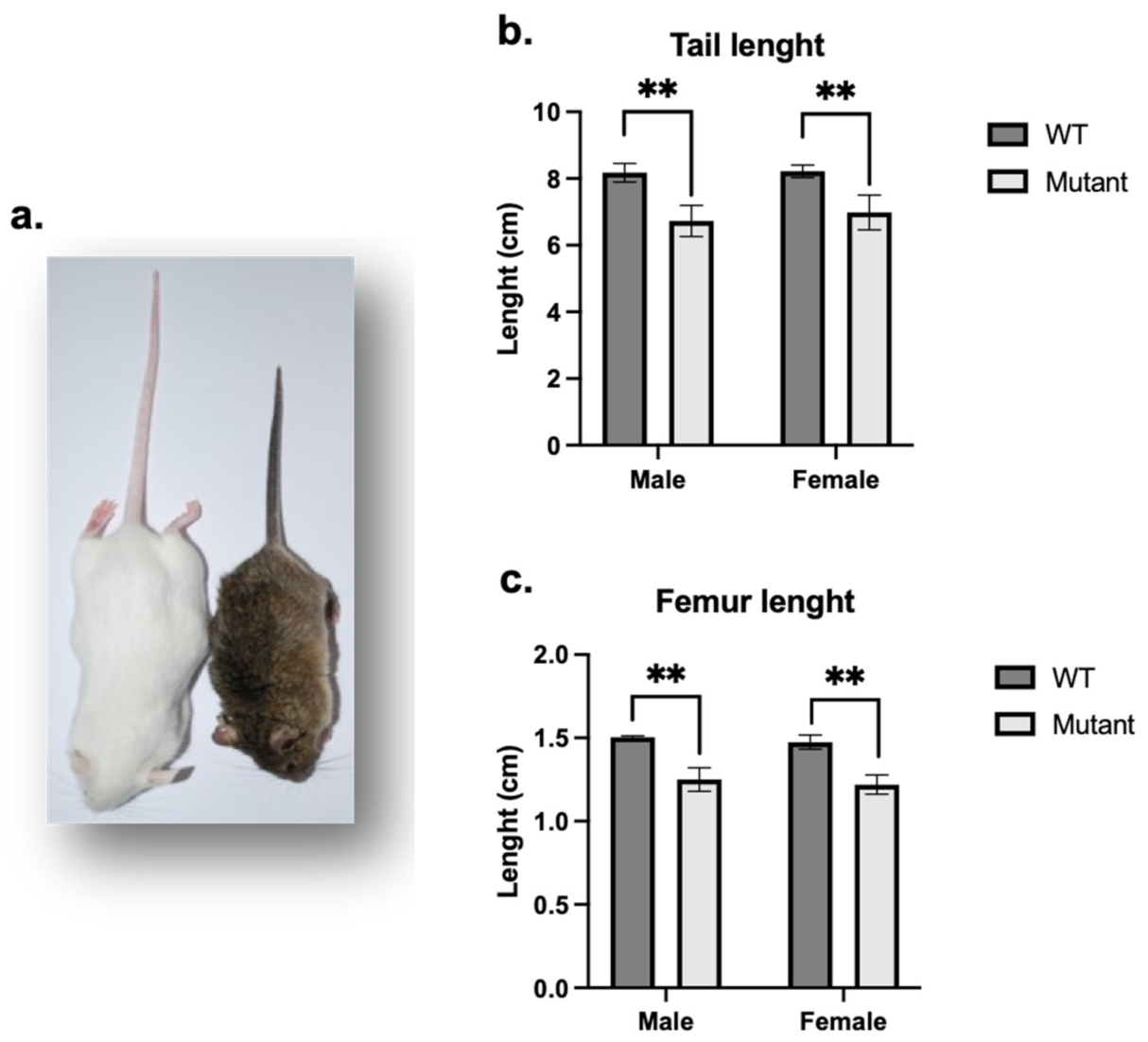
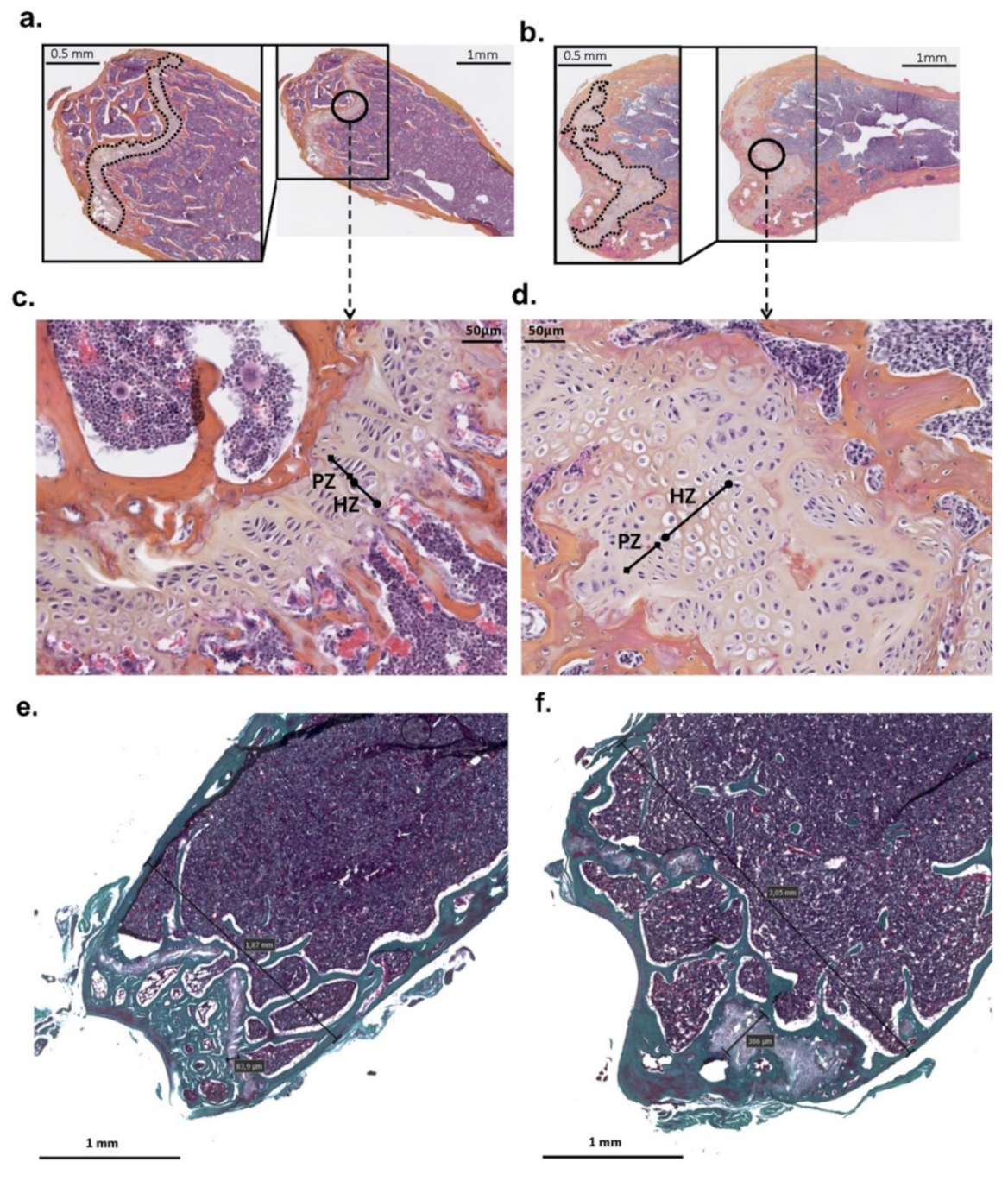
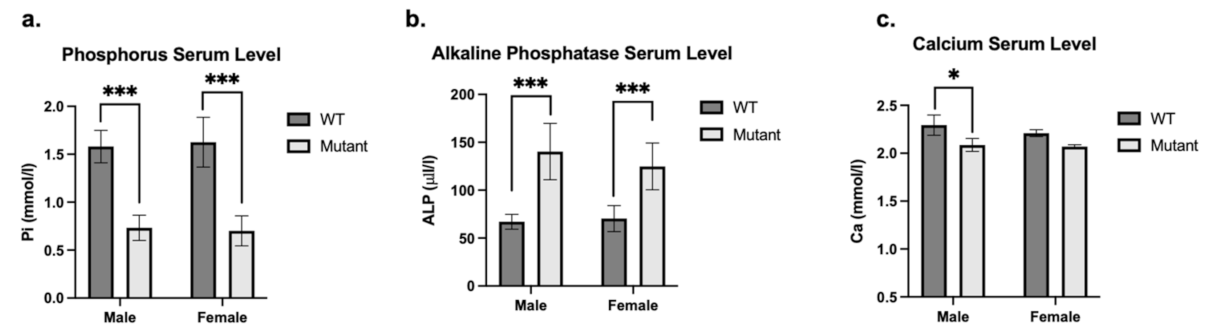
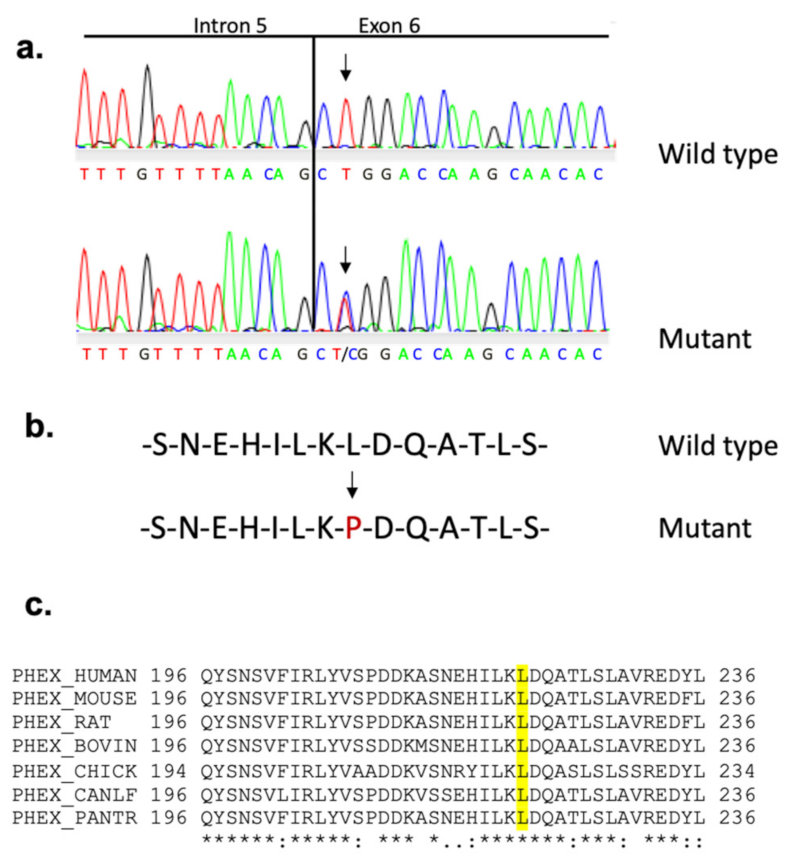
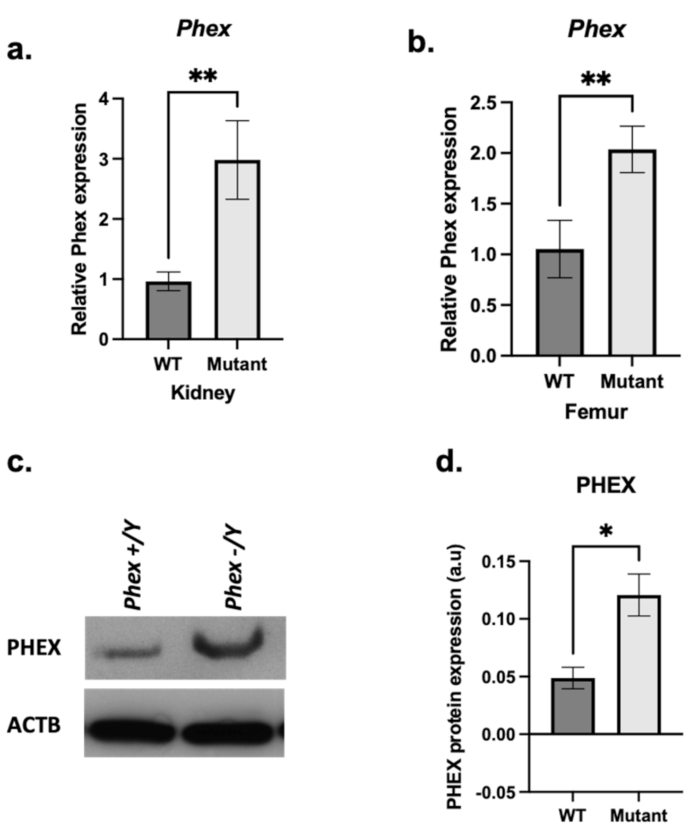
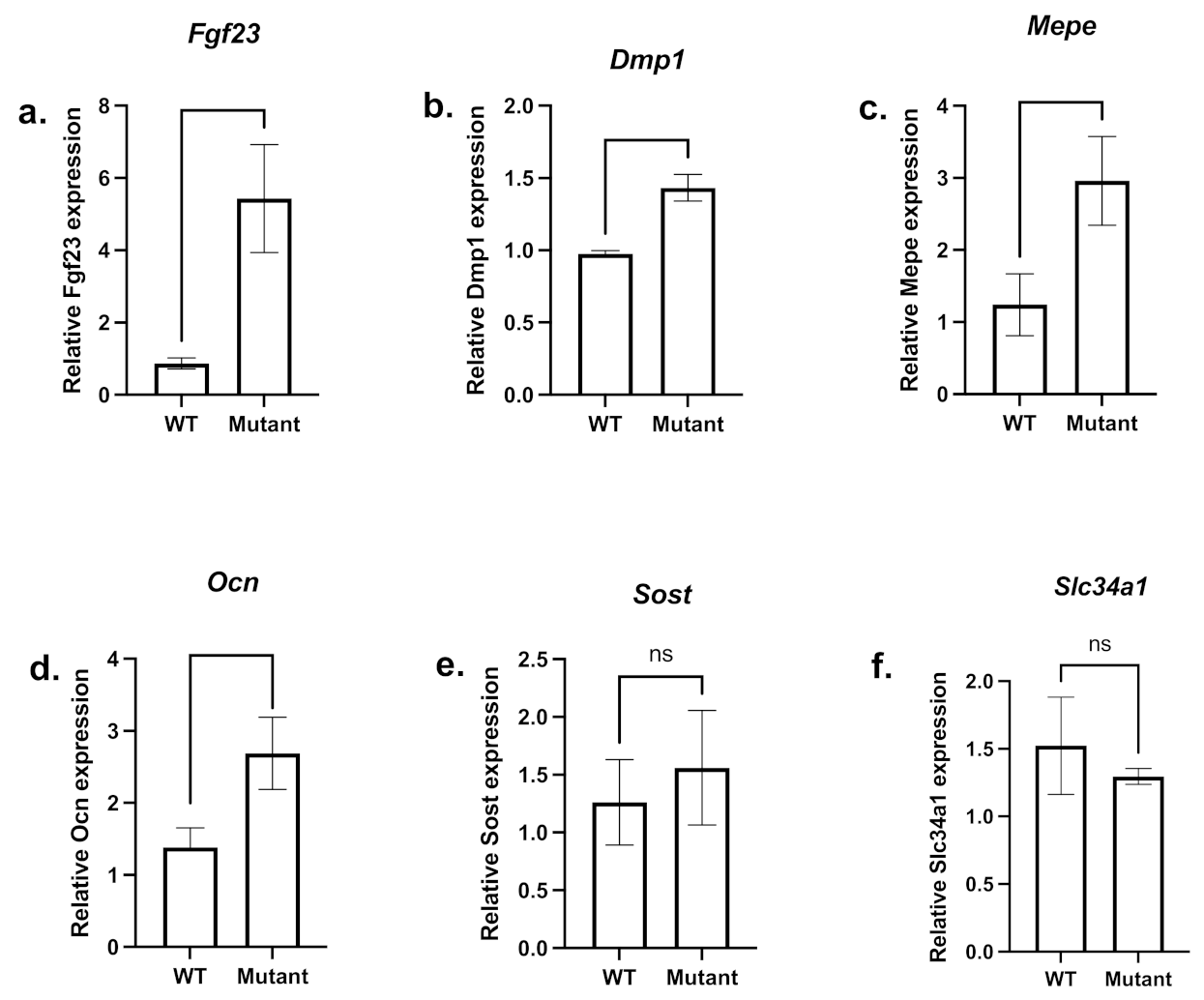
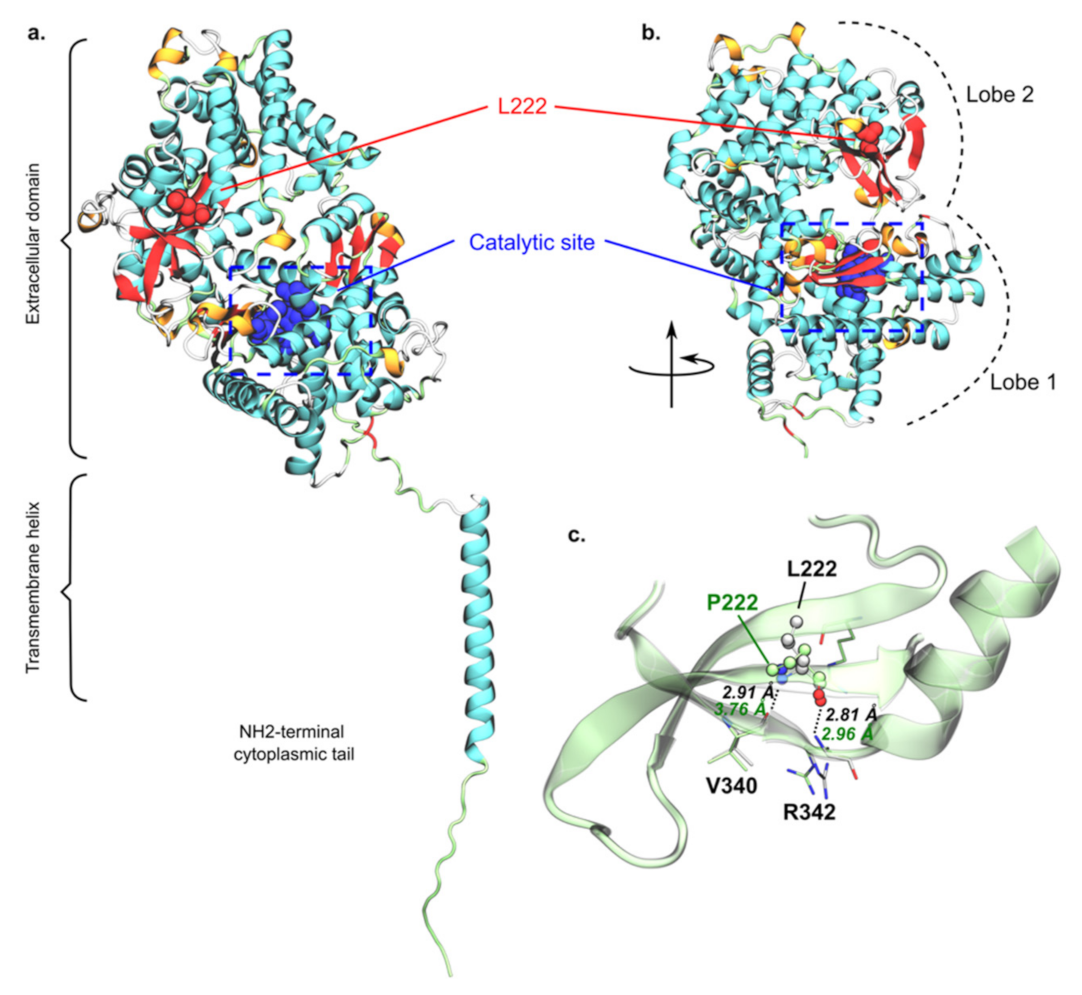
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obara-Moszynska, M.; Rojek, A.; Kolesinska, Z.; Jurkiewicz, D.; Chrzanowska, K.H.; Niedziela, M. X-linked hypophosphataemic rickets in children: Clinical phenotype, therapeutic strategies, and molecular background. Endokrynol. Pol. 2021, 72, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.L.; Vega-Warner, V.; Gillies, C.; Sampson, M.G.; Kher, V.; Sethi, S.K.; Otto, E.A. Whole Exome Sequencing Reveals Novel PHEX Splice Site Mutations in Patients with Hypophosphatemic Rickets. PLoS ONE 2015, 10, e0130729. [Google Scholar] [CrossRef] [Green Version]
- Marik, B.; Bagga, A.; Sinha, A.; Hari, P.; Sharma, A. Genetics of Refractory Rickets: Identification of Novel PHEX Mutations in Indian Patients and a Literature Update. J. Pediatr. Genet. 2018, 7, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.L.; Buckalew, V.M.; Frederickson, E.D.; Rhodes, D.J.; Conner, D.A.; Seidman, J.G.; Seidman, C.E. CLCN5 mutation Ser244Leu is associated with X-linked renal failure without X-linked recessive hypophosphatemic rickets. Kidney Int. 1998, 53, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Econs, M.J.; McEnery, P.T. Autosomal dominant hypophosphatemic rickets/osteomalacia: Clinical characterization of a novel renal phosphate-wasting disorder. J. Clin. Endocrinol. Metab. 1997, 82, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Depiereux, B.; Bastepe, M.; Benet-Pagès, A.; Amyere, M.; Wagenstaller, J.; Müller-Barth, U.; Badenhoop, K.; Kaiser, S.M.; Rittmaster, R.S.; Shlossberg, A.H.; et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006, 38, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.-F.; et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagtap, V.S.; Sarathi, V.; Lila, A.R.; Bandgar, T.; Menon, P.; Shah, N.S. Hypophosphatemic rickets. Indian J. Endocrinol. Metab. 2012, 16, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tenenhouse, H.S.; Werner, A.; Biber, J.; Ma, S.; Martel, J.; Roy, S.; Murer, H. Renal Na(+)-phosphate cotransport in murine X-linked hypophosphatemic rickets. Molecular characterization. J. Clin. Investig. 1994, 93, 671–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenhouse, H.S. X-linked hypophosphataemia: A homologous disorder in humans and mice. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1999, 14, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Murayama, T.; Iwatsubo, R.; Akiyama, S.; Amano, A.; Morisaki, I. Familial hypophosphatemic vitamin D-resistant rickets: Dental findings and histologic study of teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Hamdy, N.A.T.; Kant, S.G.; Papapoulos, S.E. Normal growth and muscle dysfunction in X-linked hypophosphatemic rickets associated with a novel mutation in the PHEX gene. J. Clin. Endocrinol. Metab. 2008, 93, 1386–1389. [Google Scholar] [CrossRef]
- Morey, M.; Castro-Feijóo, L.; Barreiro, J.; Cabanas, P.; Pombo, M.; Gil, M.; Bernabeu, I.; Díaz-Grande, J.M.; Rey-Cordo, L.; Ariceta, G.; et al. Genetic diagnosis of X-linked dominant hypophosphatemic rickets in a cohort study: Tubular reabsorption of phosphate and 1,25(OH)2D serum levels are associated with PHEX mutation type. BMC Med. Genet. 2011, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosse Duplan, M.; Coyac, B.R.; Bardet, C.; Zadikian, C.; Rothenbuhler, A.; Kamenicky, P.; Briot, K.; Linglart, A.; Chaussain, C. Phosphate and Vitamin D Prevent Periodontitis in X-Linked Hypophosphatemia. J. Dent. Res. 2017, 96, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Boukpessi, T.; Hoac, B.; Coyac, B.R.; Leger, T.; Garcia, C.; Wicart, P.; Whyte, M.P.; Glorieux, F.H.; Linglart, A.; Chaussain, C.; et al. Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia. Bone 2017, 95, 151–161. [Google Scholar] [CrossRef]
- Francis, F.; Strom, T.M.; Hennig, S.; Böddrich, A.; Lorenz, B.; Brandau, O.; Mohnike, K.L.; Cagnoli, M.; Steffens, C.; Klages, S.; et al. Genomic organization of the human PEX gene mutated in X-linked dominant hypophosphatemic rickets. Genome Res. 1997, 7, 573–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, Y.; Boileau, G.; DesGroseillers, L.; Tenenhouse, H.S. Disease-causing missense mutations in the PHEX gene interfere with membrane targeting of the recombinant protein. Hum. Mol. Genet. 2001, 10, 1539–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, Y.; Boileau, G.; Campos, M.; Carmona, A.K.; Tenenhouse, H.S. Structure and function of disease-causing missense mutations in the PHEX gene. J. Clin. Endocrinol. Metab. 2003, 88, 2213–2222. [Google Scholar] [CrossRef]
- Veilleux, L.-N.; Cheung, M.; Ben Amor, M.; Rauch, F. Abnormalities in muscle density and muscle function in hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 2012, 97, E1492–E1498. [Google Scholar] [CrossRef] [Green Version]
- Beck-Nielsen, S.S.; Mughal, Z.; Haffner, D.; Nilsson, O.; Levtchenko, E.; Ariceta, G.; de Lucas Collantes, C.; Schnabel, D.; Jandhyala, R.; Mäkitie, O. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J. Rare Dis. 2019, 14, 58. [Google Scholar] [CrossRef]
- The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 1995, 11, 130–136. [CrossRef] [PubMed]
- Grieff, M.; Mumm, S.; Waeltz, P.; Mazzarella, R.; Whyte, M.P.; Thakker, R.V.; Schlessinger, D. Expression and Cloning of the Human X-Linked Hypophosphatemia Gene cDNA. Biochem. Biophys. Res. Commun. 1997, 231, 635–639. [Google Scholar] [CrossRef]
- Strom, T.M.; Francis, F.; Lorenz, B.; Böddrich, A.; Econs, M.J.; Lehrach, H.; Meitinger, T. Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum. Mol. Genet. 1997, 6, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Quarles, L.D. Cloning and sequencing of human PEX from a bone cDNA library: Evidence for its developmental stage-specific regulation in osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1997, 12, 1009–1017. [Google Scholar] [CrossRef]
- Ruchon, A.F.; Marcinkiewicz, M.; Siegfried, G.; Tenenhouse, H.S.; DesGroseillers, L.; Crine, P.; Boileau, G. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1998, 46, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.A., Jr.; Young, C.G.; Meyer, M.H.; Garges, P.L.; Price, D.K. Effect of age on the expression of Pex (Phex) in the mouse. Calcif. Tissue Int. 2000, 66, 282–287. [Google Scholar] [CrossRef]
- Bowe, A.E.; Finnegan, R.; Jan de Beur, S.M.; Cho, J.; Levine, M.A.; Kumar, R.; Schiavi, S.C. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem. Biophys. Res. Commun. 2001, 284, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Okazaki, R.; Shibata, M.; Hasegawa, Y.; Satoh, K.; Tajima, T.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Yamashita, T.; et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 2002, 87, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, R.; Simpson, L.G.; Xiao, Z.-S.; Burnham, C.E.; Quarles, L.D. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 2003, 278, 37419–37426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benet-Pagès, A.; Lorenz-Depiereux, B.; Zischka, H.; White, K.E.; Econs, M.J.; Strom, T.M. FGF23 is processed by proprotein convertases but not by PHEX. Bone 2004, 35, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Andrukhova, O.; Clinkenbeard, E.L.; White, K.E.; Erben, R.G. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol. 2016, 14, e1002427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhou, J.; Tang, W.; Jiang, X.; Rowe, D.W.; Quarles, L.D. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E38–E49. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.; Chen, F.; Flenniken, A.M.; Osborne, L.R.; Ichikawa, S.; Adamson, S.L.; Rossant, J.; Aubin, J.E. A novel Phex mutation in a new mouse model of hypophosphatemic rickets. J. Cell. Biochem. 2012, 113, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.S.N.; Garrett, I.R.; Schwarz, P.M.; Carnes, D.L.; Lafer, E.M.; Mundy, G.R.; Gutierrez, G.E. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: A model for impaired mineralization in X-linked rickets (HYP). Bone 2005, 36, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Rowe, P.S.N.; Matsumoto, N.; Jo, O.D.; Shih, R.N.J.; Oconnor, J.; Roudier, M.P.; Bain, S.; Liu, S.; Harrison, J.; Yanagawa, N. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone 2006, 39, 773–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addison, W.N.; Nakano, Y.; Loisel, T.; Crine, P.; McKee, M.D. MEPE-ASARM Peptides Control Extracellular Matrix Mineralization by Binding to Hydroxyapatite: An Inhibition Regulated by PHEX Cleavage of ASARM. J. Bone Miner. Res. 2008, 23, 1638–1649. [Google Scholar] [CrossRef]
- Addison, W.N.; Masica, D.L.; Gray, J.J.; McKee, M.D. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J. Bone Miner. Res. 2010, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Boukpessi, T.; Gaucher, C.; Léger, T.; Salmon, B.; Faouder, J.L.; Willig, C.; Rowe, P.S.; Garabédian, M.; Meilhac, O.; Chaussain, C. Abnormal Presence of the Matrix Extracellular Phosphoglycoprotein-Derived Acidic Serine- and Aspartate-Rich Motif Peptide in Human Hypophosphatemic Dentin. Am. J. Pathol. 2010, 177, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.M.; Hoac, B.; Neves, R.L.; Addison, W.N.; Assis, D.M.; Murshed, M.; Carmona, A.K.; McKee, M.D. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J. Bone Miner. Res. 2013, 28, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Minamizaki, T.; Yoshiko, Y. The bioactive acidic serine- and aspartate-rich motif peptide. Curr. Protein Pept. Sci. 2015, 16, 196–202. [Google Scholar] [CrossRef]
- Moriyama, K.; Hanai, A.; Mekada, K.; Yoshiki, A.; Ogiwara, K.; Kimura, A.; Takahashi, T. Kbus/Idr, a mutant mouse strain with skeletal abnormalities and hypophosphatemia: Identification as an allele of “Hyp”. J. Biomed. Sci. 2011, 18, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.D.; Meyer, R.A.; Iorio, R.J. Craniometric measurements of craniofacial malformations in the X-linked hypophosphatemic (Hyp) mouse on two different genetic backgrounds: C57BL/6J and B6C3H. Teratology 1992, 46, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, R.; Tu, Q.; Quarles, L.D. Overexpression of Phex in osteoblasts fails to rescue the Hyp mouse phenotype. J. Biol. Chem. 2002, 277, 3686–3697. [Google Scholar] [CrossRef] [Green Version]
- Carpinelli, M.R.; Wicks, I.P.; Sims, N.A.; O’Donnell, K.; Hanzinikolas, K.; Burt, R.; Foote, S.J.; Bahlo, M.; Alexander, W.S.; Hilton, D.J. An ethyl-nitrosourea-induced point mutation in phex causes exon skipping, x-linked hypophosphatemia, and rickets. Am. J. Pathol. 2002, 161, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Lorenz-Depiereux, B.; Guido, V.E.; Johnson, K.R.; Zheng, Q.Y.; Gagnon, L.H.; Bauschatz, J.D.; Davisson, M.T.; Washburn, L.L.; Donahue, L.R.; Strom, T.M.; et al. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2004, 15, 151–161. [Google Scholar]
- Karunaratne, A.; Esapa, C.R.; Hiller, J.; Boyde, A.; Head, R.; Bassett, J.H.D.; Terrill, N.J.; Williams, G.R.; Brown, M.A.; Croucher, P.I.; et al. Significant deterioration in nanomechanical quality occurs through incomplete extrafibrillar mineralization in rachitic bone: Evidence from in-situ synchrotron X-ray scattering and backscattered electron imaging. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Megerian, C.A.; Semaan, M.T.; Aftab, S.; Kisley, L.B.; Zheng, Q.Y.; Pawlowski, K.S.; Wright, C.G.; Alagramam, K.N. A mouse model with postnatal endolymphatic hydrops and hearing loss. Hear. Res. 2008, 237, 90–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, S.; Traxler, E.A.; Estwick, S.A.; Curry, L.R.; Johnson, M.L.; Sorenson, A.H.; Imel, E.A.; Econs, M.J. Mutational Survey of the PHEX Gene in Patients with X-linked Hypophosphatemic Rickets. Bone 2008, 43, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Magnol, L.; Monestier, O.; Vuillier-Devillers, K.; Wagner, S.; Cocquempot, O.; Chevallier, M.C.; Blanquet, V. A sensitised mutagenesis screen in the mouse to explore the bovine genome: Study of muscle characteristics. Animal 2011, 5, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Parenté, A.; Boukredine, A.; Baraige, F.; Duprat, N.; Gondran-Tellier, V.; Magnol, L.; Blanquet, V. GASP-2 overexpressing mice exhibit a hypermuscular phenotype with contrasting molecular effects compared to GASP-1 transgenics. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 4026–4040. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Kalajzic, Z.; Maye, P.; Braut, A.; Bellizzi, J.; Mina, M.; Rowe, D.W. Histological Analysis of GFP Expression in Murine Bone. J. Histochem. Cytochem. 2005, 53, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Buel, G.R.; Walters, K.J. Can AlphaFold2 predict the impact of missense mutations on structure? Nat. Struct. Mol. Biol. 2022, 29, 1–2. [Google Scholar] [CrossRef]
- Pak, M.A.; Markhieva, K.A.; Novikova, M.S.; Petrov, D.S.; Vorobyev, I.S.; Maksimova, E.S.; Kondrashov, F.A.; Ivankov, D.N. Using AlphaFold to predict the impact of single mutations on protein stability and function. BioRxiv 2021. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 27–28, 33–38. [Google Scholar] [CrossRef]
- Tang, H.; Thomas, P.D. PANTHER-PSEP: Predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinforma. Oxf. Engl. 2016, 32, 2230–2232. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, P.Y.; Fasman, G.D. Prediction of protein conformation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef]
- Khan, S.; Vihinen, M. Spectrum of disease-causing mutations in protein secondary structures. BMC Struct. Biol. 2007, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Graw, J.; Neuhäuser-Klaus, A.; Klopp, N.; Selby, P.B.; Löster, J.; Favor, J. Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1202–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quwailid, M.M.; Hugill, A.; Dear, N.; Vizor, L.; Wells, S.; Horner, E.; Fuller, S.; Weedon, J.; McMath, H.; Woodman, P.; et al. A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2004, 15, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Sedlmeier, R.; Peters, T.; Huffstadt, U.; Kochmann, E.; Simon, D.; Schöniger, M.; Garke-Mayerthaler, S.; Laufs, J.; Mayhaus, M.; et al. Efficient and fast targeted production of murine models based on ENU mutagenesis. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2005, 16, 405–413. [Google Scholar] [CrossRef]
- Sachs, A.J.; Schwendinger, J.K.; Yang, A.W.; Haider, N.B.; Nystuen, A.M. The mouse mutants recoil wobbler and nmf373 represent a series of Grm1 mutations. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2007, 18, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gondo, Y. Next-generation gene targeting in the mouse for functional genomics. BMB Rep. 2009, 42, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gondo, Y. Now and future of mouse mutagenesis for human disease models. J. Genet. Genom. 2010, 37, 559–572. [Google Scholar] [CrossRef]
- Kim, B.J.; Zaveri, H.P.; Shchelochkov, O.A.; Yu, Z.; Hernández-García, A.; Seymour, M.L.; Oghalai, J.S.; Pereira, F.A.; Stockton, D.W.; Justice, M.J.; et al. An allelic series of mice reveals a role for RERE in the development of multiple organs affected in chromosome 1p36 deletions. PLoS ONE 2013, 8, e57460. [Google Scholar] [CrossRef]
- El Hakam Kamareddin, C.; Magnol, L.; Blanquet, V. A new Otogelin ENU mouse model for autosomal-recessive nonsyndromic moderate hearing impairment. SpringerPlus 2015, 4, 730. [Google Scholar] [CrossRef] [Green Version]
- Brommage, R.; Ohlsson, C. High Fidelity of Mouse Models Mimicking Human Genetic Skeletal Disorders. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wang, H.-L.; Chen, R.; Chen, L.; Yang, S.; Wang, Y.; Xue, Z.-F. An L314Q mutation in Map3k1 gene results in failure of eyelid fusion in the N-ethyl-N-nitrosourea-induced mutant line. Exp. Anim. 2021, 70, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Okuda, K.; Miura, I.; Motegi, H.; Wakana, S.; Ohno, T. A novel ENU-induced Cpox mutation causes microcytic hypochromic anemia in mice. Exp. Anim. 2022, 2022, 22-0032. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Walrant-Debray, O.; Nguyen, T.-M.; Esterle, L.; Garabédian, M.; Jehan, F. PHEX analysis in 118 pedigrees reveals new genetic clues in hypophosphatemic rickets. Hum. Genet. 2009, 125, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Carpenter, T.O.; Demay, M.B. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9637–9642. [Google Scholar] [CrossRef] [Green Version]
- Drezner, M.K. PHEX gene and hypophosphatemia. Kidney Int. 2000, 57, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenhouse, H.S.; Murer, H. Disorders of renal tubular phosphate transport. J. Am. Soc. Nephrol. JASN 2003, 14, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Segawa, H.; Kawakami, E.; Kaneko, I.; Kuwahata, M.; Ito, M.; Kusano, K.; Saito, H.; Fukushima, N.; Miyamoto, K.-I. Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflüg. Arch. Eur. J. Physiol. 2003, 446, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Tenenhouse, H.S. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu. Rev. Nutr. 2005, 25, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Bone–kidney axis in systemic phosphate turnover. Arch. Biochem. Biophys. 2014, 561, 154–158. [Google Scholar] [CrossRef]
- Penido, M.G.M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. Berl. Ger. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Feng, J.Q.; Bowman, S.; Liu, Y.; Blank, R.D.; Lindberg, I.; Drezner, M.K. Hexa-D-arginine treatment increases 7B2•PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, P.S.N. Regulation of bone-renal mineral and energy metabolism: The PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 2012, 22, 61–86. [Google Scholar] [CrossRef]
- Rowe, P.S. A unified model for bone-renal mineral and energy metabolism. Curr. Opin. Pharmacol. 2015, 22, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yoshiko, Y.; Yamamoto, R.; Minamizaki, T.; Kozai, K.; Tanne, K.; Aubin, J.E.; Maeda, N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 939–948. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.; Sun, W.; Bai, X.; Karaplis, A.C.; Goltzman, D.; Miao, D. Fibroblast growth factor 23 overexpression impacts negatively on dentin mineralization and dentinogenesis in mice. Clin. Exp. Pharmacol. Physiol. 2011, 38, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sitara, D.; Kim, S.; Razzaque, M.S.; Bergwitz, C.; Taguchi, T.; Schüler, C.; Erben, R.G.; Lanske, B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008, 4, e1000154. [Google Scholar] [CrossRef]
- Shalhoub, V.; Ward, S.C.; Sun, B.; Stevens, J.; Renshaw, L.; Hawkins, N.; Richards, W.G. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif. Tissue Int. 2011, 89, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Högler, W.; Linglart, A.; Padidela, R.; Van’t Hoff, W.; Mao, M.; Chen, C.-Y.; et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, Y.; Fukumoto, S. X-Linked Hypophosphatemia and FGF23-Related Hypophosphatemic Diseases: Prospect for New Treatment. Endocr. Rev. 2018, 39, 274–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aono, Y.; Yamazaki, Y.; Yasutake, J.; Kawata, T.; Hasegawa, H.; Urakawa, I.; Fujita, T.; Wada, M.; Yamashita, T.; Fukumoto, S.; et al. Therapeutic Effects of Anti-FGF23 Antibodies in Hypophosphatemic Rickets/Osteomalacia. J. Bone Miner. Res. 2009, 24, 1879–1888. [Google Scholar] [CrossRef]
- Wöhrle, S.; Henninger, C.; Bonny, O.; Thuery, A.; Beluch, N.; Hynes, N.E.; Guagnano, V.; Sellers, W.R.; Hofmann, F.; Kneissel, M.; et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 899–911. [Google Scholar] [CrossRef]
- Segawa, H.; Shiozaki, Y.; Kaneko, I.; Miyamoto, K. The Role of Sodium-Dependent Phosphate Transporter in Phosphate Homeostasis. J. Nutr. Sci. Vitaminol. 2015, 61, S119–S121. [Google Scholar] [CrossRef] [Green Version]
- Gowen, L.C.; Petersen, D.N.; Mansolf, A.L.; Qi, H.; Stock, J.L.; Tkalcevic, G.T.; Simmons, H.A.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.Z.; et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J. Biol. Chem. 2003, 278, 1998–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, V.; Martin, A.; Hedge, A.-M.; Rowe, P.S.N. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology 2009, 150, 4012–4023. [Google Scholar] [CrossRef] [Green Version]
- Rowe, P.S.N. The Wrickkened Pathways Of FGF23, MEPE and PHEX. Crit. Rev. Oral Biol. Med. 2004, 15, 264–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argiro, L.; Desbarats, M.; Glorieux, F.H.; Ecarot, B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics 2001, 74, 342–351. [Google Scholar] [CrossRef]
- Steiglitz, B.M.; Ayala, M.; Narayanan, K.; George, A.; Greenspan, D.S. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J. Biol. Chem. 2004, 279, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartaix, P.H.; Doulaverakis, M.; George, A.; Fisher, L.W.; Butler, W.T.; Qin, C.; Salih, E.; Tan, M.; Fujimoto, Y.; Spevak, L.; et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J. Biol. Chem. 2004, 279, 18115–18120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, I.; Qin, D.; Huang, B.; Sun, Y.; Mues, G.; Svoboda, K.; Bonewald, L.; Butler, W.T.; Feng, J.Q.; Qin, C. Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tissues Organs 2009, 189, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, Y.; Chen, L.; Guan, C.; Guo, L.; Qin, C. Expression and distribution of SIBLING proteins in the predentin/dentin and mandible of hyp mice. Oral Dis. 2010, 16, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, V.; Martin, A.; Hedge, A.-M.; Drezner, M.K.; Rowe, P.S.N. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am. J. Physiol. Ren. Physiol. 2011, 300, F783–F791. [Google Scholar] [CrossRef] [Green Version]
- Gundberg, C.M.; Clough, M.E.; Carpenter, T.O. Development and validation of a radioimmunoassay for mouse osteocalcin: Paradoxical response in the Hyp mouse. Endocrinology 1992, 130, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Gundberg, C.M. Osteocalcin abnormalities in Hyp mice reflect altered genetic expression and are not due to altered clearance, affinity for mineral, or ambient phosphorus levels. Endocrinology 1996, 137, 5213–5219. [Google Scholar] [CrossRef]
- Onishi, T.; Ogawa, T.; Hayashibara, T.; Hoshino, T.; Okawa, R.; Ooshima, T. Hyper-expression of osteocalcin mRNA in odontoblasts of Hyp mice. J. Dent. Res. 2005, 84, 84–88. [Google Scholar] [CrossRef]
- Atkins, G.J.; Rowe, P.S.; Lim, H.P.; Welldon, K.J.; Ormsby, R.; Wijenayaka, A.R.; Zelenchuk, L.; Evdokiou, A.; Findlay, D.M. Sclerostin Is a Locally Acting Regulator of Late-Osteoblast/Preosteocyte Differentiation and Regulates Mineralization Through a MEPE-ASARM-Dependent Mechanism. J. Bone Miner. Res. 2011, 26, 1425–1436. [Google Scholar] [CrossRef] [Green Version]
- Sabrautzki, S.; Rubio-Aliaga, I.; Hans, W.; Fuchs, H.; Rathkolb, B.; Calzada-Wack, J.; Cohrs, C.M.; Klaften, M.; Seedorf, H.; Eck, S.; et al. New mouse models for metabolic bone diseases generated by genome-wide ENU mutagenesis. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2012, 23, 416–430. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hakam, C.; Parenté, A.; Baraige, F.; Magnol, L.; Forestier, L.; Di Meo, F.; Blanquet, V. PHEXL222P Mutation Increases Phex Expression in a New ENU Mouse Model for XLH Disease. Genes 2022, 13, 1356. https://doi.org/10.3390/genes13081356
El Hakam C, Parenté A, Baraige F, Magnol L, Forestier L, Di Meo F, Blanquet V. PHEXL222P Mutation Increases Phex Expression in a New ENU Mouse Model for XLH Disease. Genes. 2022; 13(8):1356. https://doi.org/10.3390/genes13081356
Chicago/Turabian StyleEl Hakam, Carole, Alexis Parenté, Fabienne Baraige, Laetitia Magnol, Lionel Forestier, Florent Di Meo, and Véronique Blanquet. 2022. "PHEXL222P Mutation Increases Phex Expression in a New ENU Mouse Model for XLH Disease" Genes 13, no. 8: 1356. https://doi.org/10.3390/genes13081356
APA StyleEl Hakam, C., Parenté, A., Baraige, F., Magnol, L., Forestier, L., Di Meo, F., & Blanquet, V. (2022). PHEXL222P Mutation Increases Phex Expression in a New ENU Mouse Model for XLH Disease. Genes, 13(8), 1356. https://doi.org/10.3390/genes13081356





