Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sequence Acquisition for Reproductive Efficiency Genes
2.3. Structural Variation Prediction, Retrotransposon Annotation, and Insertion Polymorphic Prediction
2.4. Animals for RIPs Verification and Genotyping
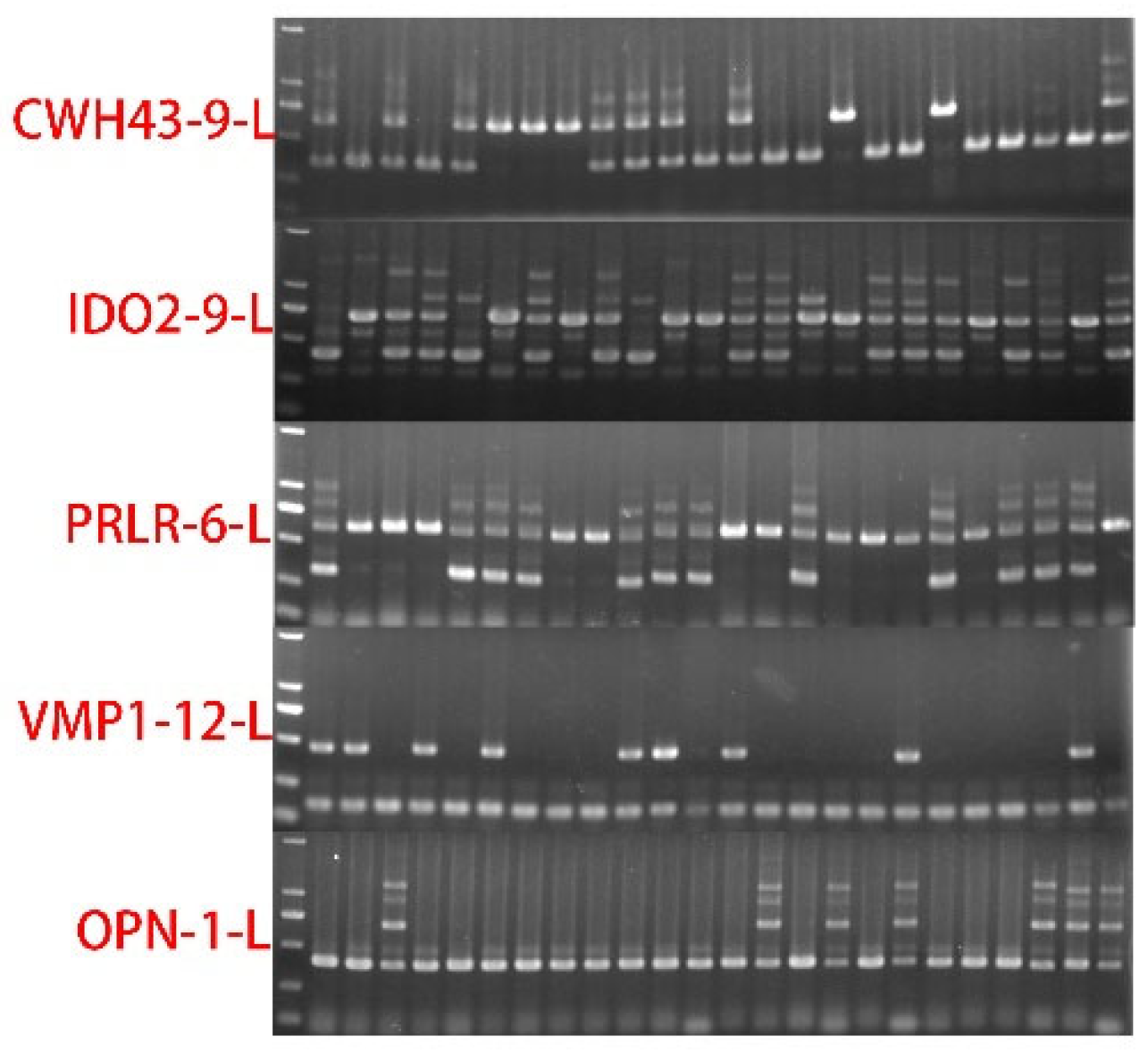
2.5. Samples Collection and PCR Analysis
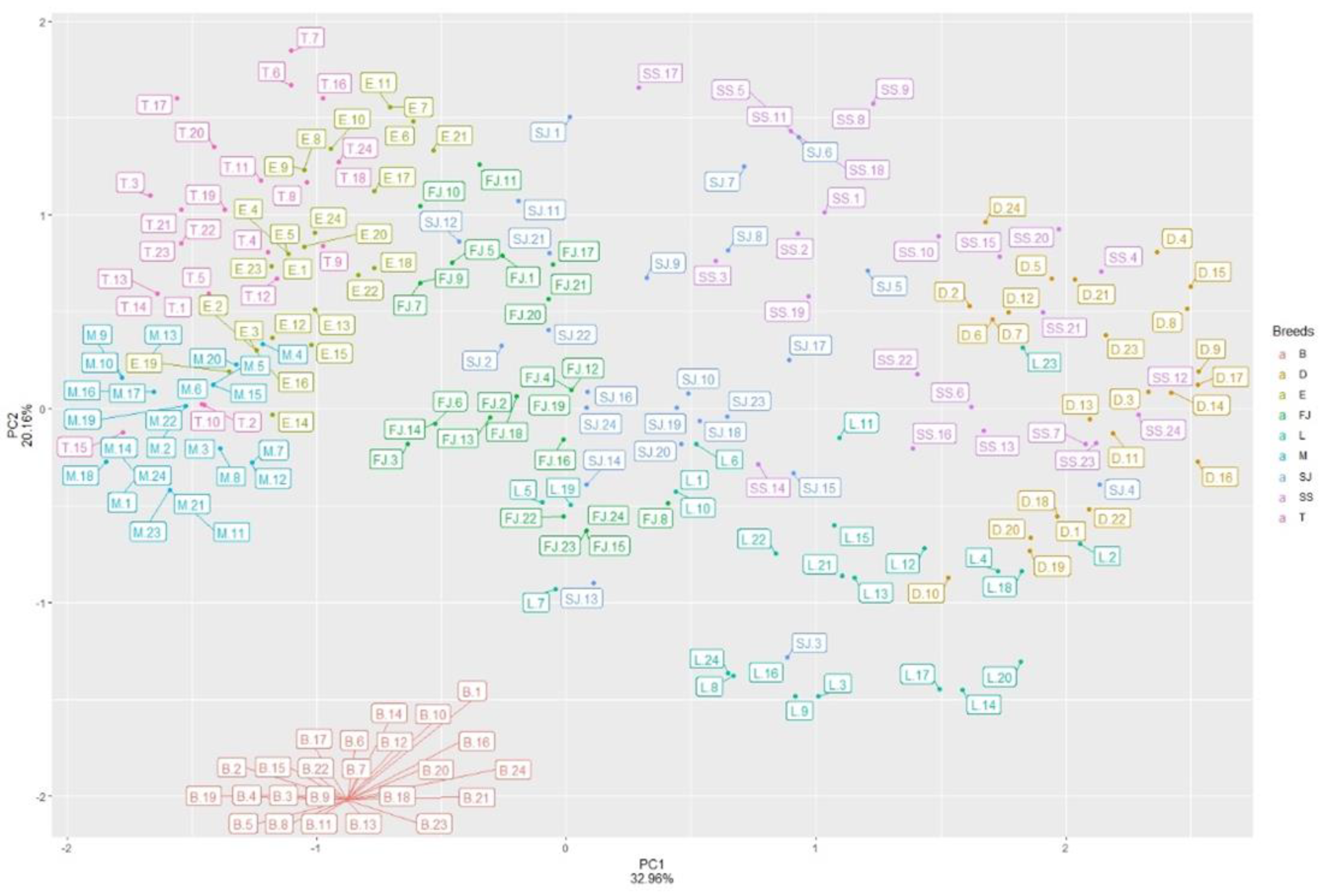
2.6. PCA and Cluster Analysis of the SINE RIPs
2.7. Traits Determinant
2.8. Statistical Analysis
3. Results
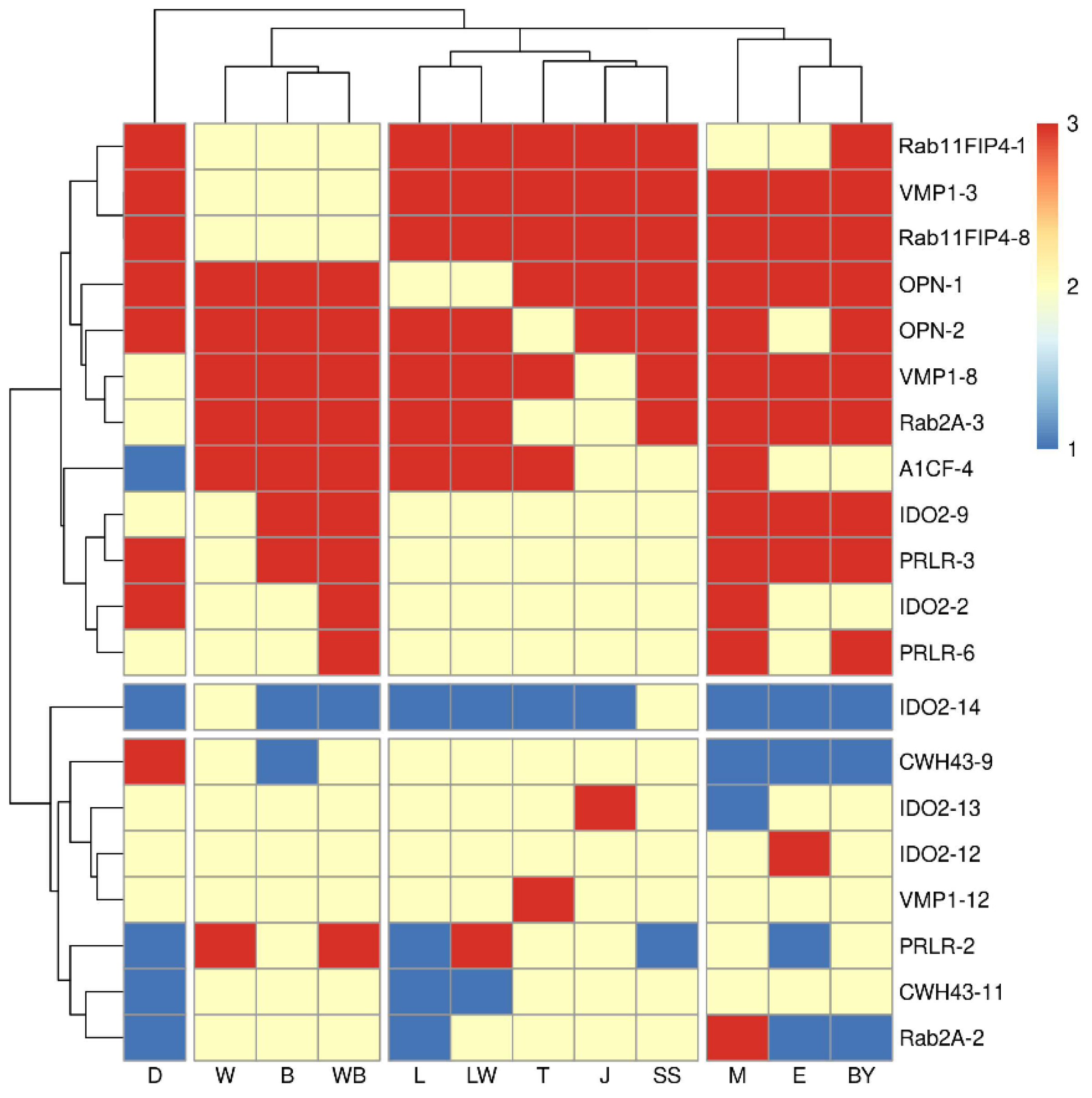
3.1. Reproductive Related Genes’ SVs Revelation and Detection by Pool-PCR in Different Pig Breeds
3.2. Statistical Analysis for the RIPs with the Reproductive Characteristic of Large White Pig
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finnegan, D.J. Retrotransposons. Curr. Biol. 2012, 22, R432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, R.N.; Vandewege, M.W.; Ray, D.A. Mammalian transposable elements and their impacts on genome evolution. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2018, 26, 25–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macas, J.; Kejnovský, E.; Neumann, P.; Novák, P.; Koblížková, A.; Vyskot, B. Next generation sequencing-based analysis of repetitive DNA in the model dioceous plant Silene latifolia. PLoS ONE 2011, 6, e27335. [Google Scholar] [CrossRef]
- Göke, J.; Ng, H.H. CTRL+ INSERT: Retrotransposons and their contribution to regulation and innovation of the transcriptome. EMBO Rep. 2016, 17, 1131. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, W.; Wang, X.; Shen, D.; Wang, S.; Wang, Y.; Gao, B.; Wimmers, K.; Mao, J.; Li, K. Retrotransposons evolution and impact on lncRNA and protein coding genes in pigs. Mob. DNA 2019, 10, 19. [Google Scholar] [CrossRef]
- Kalendar, R.N.; Aizharkyn, K.S.; Khapilina, O.N.; Amenov, A.A.; Tagimanova, D.S. Plant diversity and transcriptional variability assessed by retrotransposon-based molecular markers. Baвилoвcкий Жypнaл Гeнeтики И Ceлeкции 2017, 21, 128. [Google Scholar] [CrossRef] [Green Version]
- Kalendar, R.; Flavell, A.J.; Ellis, T.; Sjakste, T.; Moisy, C.; Schulman, A.H. Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 2011, 106, 520. [Google Scholar] [CrossRef] [Green Version]
- Seibt, K.M.; Wenke, T.; Wollrab, C.; Junghans, H.; Muders, K.; Dehmer, K.J.; Diekmann, K.; Schmidt, T. Development and application of SINE-based markers for genotyping of potato varieties. Theor. Appl. Genet. 2012, 125, 185. [Google Scholar] [CrossRef]
- Xu, Z.; Ramakrishna, W. Retrotransposon insertion polymorphisms in six rice genes and their evolutionary history. Gene 2008, 412, 50. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.J.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772. [Google Scholar] [CrossRef]
- Saenko, S.V.; Lamichhaney, S.; Barrio, A.M.; Rafati, N.; Andersson, L.; Milinkovitch, M.C. Amelanism in the corn snake is associated with the insertion of an LTR-retrotransposon in the OCA2 gene. Sci. Rep. 2015, 5, 17118. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Li, J.; Liu, S.; Hou, H.; Zhu, T.; Chen, J.; Liu, L.; Jia, Y.; Xiong, W. An L1 retrotransposon insertion–induced deafness mouse model for studying the development and function of the cochlear stria vascularis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107933118. [Google Scholar] [CrossRef]
- Yamamoto, G.; Miyabe, I.; Tanaka, K.; Kakuta, M.; Watanabe, M.; Kawakami, S.; Ishida, H.; Akagi, K. SVA retrotransposon insertion in exon of MMR genes results in aberrant RNA splicing and causes Lynch syndrome. Eur. J. Hum. Genet. 2021, 29, 680. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Zhang, Y.; Zhang, Q.; Huang, R. Progress in the physiological and genetic mechanisms underlying the high prolificacy of the Erhualian pig. Yi Chuan = Hereditas 2017, 39, 1016. [Google Scholar]
- Andersson, E.; Frössling, J.; Engblom, L.; Algers, B.; Gunnarsson, S. Impact of litter size on sow stayability in Swedish commercial piglet producing herds. Acta Vet. Scand. 2015, 58, 31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Z.; Ye, S.; He, Y.; Huang, S.; Yuan, X.; Chen, Z.; Zhang, H.; Li, J. Genome-wide association study for reproductive traits in a Duroc pig population. Animals 2019, 9, 732. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, Y.; Zhang, Z.; Zhao, W.; Zhang, Z.; Xiang, Y.; Wang, Q.; Pan, Y.; Guo, X.; Wang, Z. Genome-wide epistatic interactions of litter size at birth in Chinese indigenous pigs. Anim. Genet. 2021, 52, 739. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, A.; Munoz, M.; Fernandez, A.; Pena, R.N.; Tomas, A.; Noguera, J.L.; Ovilo, C.; Fernandez, A.I. Differential gene expression in ovaries of pregnant pigs with high and low prolificacy levels and identification of candidate genes for litter size. Biol. Reprod. 2011, 84, 299. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, D. RepeatMasker. Biotech Softw. Internet Rep. 2000, 1, 36. [Google Scholar] [CrossRef]
- Kolde, R. pheatmap: Pretty Heatmaps, R Package, Version 1.0.8; R Foundation for Statistical Computing: Vienna, Austria, 2015.
- Baumgartner, W.; Weiß, P.; Schindler, H. A nonparametric test for the general two-sample problem. Biometrics 1998, 54, 1129. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, W.; Fu, Y.; Fang, X.; Ren, S.; Ren, J. Genome-wide detection of genetic loci and candidate genes for teat number and body conformation traits at birth in Chinese Sushan pigs. Anim. Genet. 2019, 50, 753. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Structural variation: The genome’s hidden architecture. Nat. Methods 2012, 9, 133. [Google Scholar] [CrossRef]
- Garsed, D.W.; Marshall, O.J.; Corbin, V.D.A.; Hsu, A.; Di Stefano, L.; Schröder, J.; Li, J.; Feng, Z.; Kim, B.W.; Kowarsky, M. The architecture and evolution of cancer neochromosomes. Cancer Cell 2014, 26, 653. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Cai, C.; Wei, C.; Wang, X.; Wei, W.; Bo, G.; Wimmers, K.; Mao, J.; Song, C. Two new SINE insertion polymorphisms in pig Vertnin (VRTN) gene revealed by comparative genomic alignment. J. Integr. Agric. 2020, 19, 2514. [Google Scholar] [CrossRef]
- Gagnier, L.; Belancio, V.P.; Mager, D.L. Mouse germ line mutations due to retrotransposon insertions. Mob. DNA 2019, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Su, G.; Christensen, O.F.; Janss, L.; Lund, M.S. Genome-wide association analyses using a Bayesian approach for litter size and piglet mortality in Danish Landrace and Yorkshire pigs. BMC Genom. 2016, 17, 468. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Zhai, H.; Cheng, M.; Ma, J.; Cheng, S.; Ge, W.; Zhang, G.; Wang, J.; Zhang, R.; Wang, X. Whole-genome scanning for the litter size trait associated genes and SNPs under selection in dairy goat (Capra hircus). Sci. Rep. 2016, 6, 38096. [Google Scholar] [CrossRef]
- Chu, Q.; Zhou, B.; Xu, F.; Chen, R.; Shen, C.; Liang, T.; Li, Y.; Schinckel, A.P. Genome-wide differential mRNA expression profiles in follicles of two breeds and at two stages of estrus cycle of gilts. Sci. Rep. 2017, 7, 5052. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, L.; Wu, T.; Feng, Y.; Ding, Y.; Ye, P.; Yin, Z. Transcriptomic analysis of ovaries from pigs with high and low litter size. PLoS ONE 2015, 10, e0139514. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ran, X.; Niu, X.; Li, S.; Wang, J.; Zhang, Q. Insertion of 275-bp SINE into first intron of PDIA4 gene is associated with litter size in Xiang pigs. Anim. Reprod. Sci. 2018, 195, 16–23. [Google Scholar]
- Li, Z.; Wei, S.; Li, H.; Wu, K.; Cai, Z.; Li, D.; Wei, W.; Li, Q.; Chen, J.; Liu, H. Genome-wide genetic structure and differentially selected regions among Landrace, Erhualian, and Meishan pigs using specific-locus amplified fragment sequencing. Sci. Rep. 2017, 7, 10063. [Google Scholar] [CrossRef] [Green Version]
- Kwon, W.; Rahman, M.S.; Ryu, D.; Khatun, A.; Pang, M. Comparison of markers predicting litter size in different pig breeds. Andrology 2017, 5, 568. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, L.; Yong, L.I.; Hua, Y.; Zhao, K.; Wang, L. Erythropoietin Receptor Gene (EPOR) Polymorphisms are Associated with Sow Litter Sizes. Agric. Sci. China 2011, 10, 931. [Google Scholar] [CrossRef]
- Li, K.; Ren, J.; Xing, Y.; Zhang, Z.; Ma, J.; Guo, Y.; Huang, L. Quantitative trait loci for litter size and prenatal loss in a White Duroc × Chinese Erhualian resource population. Anim. Genet. 2009, 40, 963. [Google Scholar]
- Liu, C.; Li, P.; Zhou, W.; Ma, X.; Wang, X.; Xu, Y.; Jiang, N.; Zhao, M.; Zhou, T.; Yin, Y. Genome data uncover conservation status, historical relatedness and candidate genes under selection in Chinese indigenous pigs in the Taihu Lake region. Front. Genet. 2020, 11, 591. [Google Scholar] [CrossRef]
- Avalos, E.; Smith, C. Genetic improvement of litter size in pigs. Anim. Sci. 1987, 44, 153. [Google Scholar] [CrossRef]
- White, B.R.; McLaren, D.G.; Dziuk, P.J.; Wheeler, M.B. Age at puberty, ovulation rate, uterine length, prenatal survival and litter size in Chinese Meishan and Yorkshire females. Theriogenology 1993, 40, 85. [Google Scholar]
- Haley, C.S.; Lee, G.J. Genetic basis of prolificacy in Meishan pigs. J. Reprod. Fertil. Suppl. 1993, 1993, 247. [Google Scholar]
- Ran, X.; Pan, H.; Huang, S.; Liu, C.; Niu, X.; Li, S.; Wang, J. Copy number variations of MTHFSD gene across pig breeds and its association with litter size traits in Chinese indigenous Xiang pig. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1320. [Google Scholar] [CrossRef]
- Liu, C.; Ran, X.; Yu, C.; Xu, Q.; Niu, X.; Zhao, P.; Wang, J. Whole-genome analysis of structural variations between Xiang pigs with larger litter sizes and those with smaller litter sizes. Genomics 2019, 111, 310. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Ma, X.; Jiang, N.; Zhou, W.; Gao, C.; Zhao, M.; Niu, P.; Zhang, Z.; Li, Q. Association of rs339939442 in the AHR gene with litter size are inconsistent among Chinese indigenous pigs and western commercial pigs. Animals 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Sell-Kubiak, E.; Knol, E.F.; Lopes, M. Evaluation of the phenotypic and genomic background of variability based on litter size of Large White pigs. Genet. Sel. Evol. 2022, 54, 1. [Google Scholar] [CrossRef]
- Sell-Kubiak, E.; Duijvesteijn, N.; Lopes, M.S.; Janss, L.; Knol, E.F.; Bijma, P.; Mulder, H.A. Genome-wide association study reveals novel loci for litter size and its variability in a Large White pig population. BMC Genom. 2015, 16, 1049. [Google Scholar] [CrossRef] [Green Version]
- Terman, A. Effect of the polymorphism of prolactin receptor (PRLR) and leptin (LEP) genes on litter size in Polish pigs. J. Anim. Breed. Genet. = Zeitschrift Für Tierzüchtung Und Züchtungsbiologie 2005, 122, 400. [Google Scholar] [CrossRef]
- Niu, S.Y.; Wang, X.P.; Hao, F.G.; Zhao, R.X. Effect of the polymorphism of RBP4 and OPN genes on litter size in Tibet pigs. Acta Agric. Scand Sect. A 2008, 58, 10. [Google Scholar] [CrossRef]
- Kumchoo, T.; Mekchay, S. Association of non-synonymous SNPs of OPN gene with litter size traits in pigs. Arch. Anim. Breed. 2015, 58, 317. [Google Scholar] [CrossRef] [Green Version]
- Korwin-Kossakowska, A.; Pierzchała, D.; Lewczuk, D.; Faliszewska, G.; Pierzchała, M. Polymorphism of OPN and AREG Genes in Relation to Transcript Expression of a Panel of 12 Genes Controlling Reproduction Processes and Litter Size in Pigs. Ann. Anim. Sci. 2021, 21, 1315. [Google Scholar] [CrossRef]
- Diez-Roux, G.; Banfi, S.; Sultan, M.; Geffers, L.; Anand, S.; Rozado, D.; Magen, A.; Canidio, E.; Pagani, M.; Peluso, I. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011, 9, e1000582. [Google Scholar] [CrossRef]
- Cecati, M.; Emanuelli, M.; Giannubilo, S.R.; Quarona, V.; Senetta, R.; Malavasi, F.; Tranquilli, A.L.; Saccucci, F. Contribution of adenosine-producing ectoenzymes to the mechanisms underlying the mitigation of maternal-fetal conflicts. J. Biol. Regul. Homeost. Agents 2013, 27, 519. [Google Scholar] [PubMed]
- Wang, P.; Tang, M.; Gao, L.; Luo, H.; Wang, G.; Ma, X.; Duan, Y. Roles of I (f) and intracellular Ca2+ release in spontaneous activity of ventricular cardiomyocytes during murine embryonic development. J. Cell. Biochem. 2013, 114, 1852. [Google Scholar] [CrossRef] [PubMed]
- He, L.C.; Li, P.H.; Ma, X.; Sui, S.P.; Gao, S.; Kim, S.W.; Gu, Y.Q.; Huang, Y.; Ding, N.S.; Huang, R.H. Identification of new single nucleotide polymorphisms affecting total number born and candidate genes related to ovulation rate in Chinese Erhualian pigs. Anim. Genet. 2017, 48, 48. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Trejo, C.M.; Luna-Nevárez, G.; Reyna-Granados, J.R.; Zamorano-Algandar, R.; Romo-Rubio, J.A.; Sánchez-Castro, M.Á.; Mark Enns, R.; Speidel, S.E.; Thomas, M.G.; Luna-Nevárez, P. Polymorphisms associated with the number of live-born piglets in sows infected with the PRRS virus in southern Sonora Mexico. Revista Mexicana de Ciencias Pecuarias 2020, 11, 828. [Google Scholar] [CrossRef]
- Spinelli, P.; Latchney, S.E.; Reed, J.M.; Fields, A.; Baier, B.S.; Lu, X.; McCall, M.N.; Murphy, S.P.; Mak, W.; Susiarjo, M. Identification of the novel Ido1 imprinted locus and its potential epigenetic role in pregnancy loss. Hum. Mol. Genet. 2019, 28, 662. [Google Scholar] [CrossRef]
- Moraes, J.G.N.; Behura, S.K.; Geary, T.W.; Hansen, P.J.; Neibergs, H.L.; Spencer, T.E. Uterine influences on conceptus development in fertility-classified animals. Proc. Natl. Acad. Sci. USA 2018, 115, E1749. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zheng, X.; Zhao, Q.; Hu, Z.; Wang, H.; Zhou, L.; Liu, J. Analysis of structural variants reveal novel selective regions in the genome of Meishan pigs by whole genome sequencing. Front. Genet. 2021, 12, 99. [Google Scholar] [CrossRef]
| Gene Name | Predicted RIPs | Confirmed RIPs |
|---|---|---|
| BF_properdin | 1 | 0 |
| BRCA1 | 10 | 0 |
| EPOR | 1 | 0 |
| FSHB | 2 | 0 |
| FUT1 | 1 | 0 |
| GNRHR | 2 | 0 |
| IDO2 | 16 | 5 |
| LEP | 1 | 0 |
| OPN (SSP1) | 2 | 2 |
| PRLR | 6 | 3 |
| Rab2A | 3 | 2 |
| RBP4 | 1 | 0 |
| CASP6 | 5 | 0 |
| CWH43 | 9 | 2 |
| P2RX3 | 1 | 0 |
| ZNF518A | 2 | 0 |
| UCHL1 | 0 | 0 |
| VMP1 | 18 | 3 |
| RAB11FIP4 | 15 | 2 |
| A1CF | 4 | 1 |
| Total Numbers | 100 | 20 |
| Rip-Sites | Insertion Breeds | Deletion Breeds | Chr | Begin | End | Gene Structure | TE-Type | Length (bp) |
| A1CF-4 | Duroc, MS | The rest of the species | 5 | 98,976,497 | 98,976,792 | Intron-10 | ERVIII | 295 |
| CWH43-9 | MSBJ, Wuzhishan, Rongchang, Jinhua, Berkshire, Bama, Pietrain, Large White | The rest of the species | 8 | 38,940,696 | 38,940,699 | Intron-15 | SINEA | 281 |
| CWH43-11 | The rest of the species | Berkshire, Jinhua, MSBJ, MS, Bama, Wuzhishan, Large White | 8 | 38,945,310 | 38,945,752 | 3′flank | SINEA | 442 |
| IDO2-9 | D-Ninghe, Tibetan, Bamei, Landrace, Hampshire, Berkshire | The rest of the species | 17 | 9,326,173 | 9,326,195 | Intron-7 | SINEA | 304 |
| IDO2-14 | The rest of the species | MSBJ | 17 | 9,341,573 | 9,341,711 | Intron-9 | ERV I | 138 |
| VMP1-12 | Duroc, Rongchang, D-Ninghe, Bamei, Berkshire | The rest of the species | 12 | 35,992,635 | 35,992,950 | Intron-7 | SINEA | 315 |
| OPN-1 | Pietrain | The rest of the species | 8 | 131,078,869 | 131,078,870 | Intron-5 | SINEA | 312 |
| OPN-2 | MSBJ | The rest of the species | 8 | 131,076,528 | 131,076,529 | 3′flank | SINEA | 311 |
| PRLR-2 | MS, Cross-bred, Berkshire, Jinhua | The rest of the species | 16 | 20,643,376 | 20,643,377 | Intron-6 | SINEA | 284 |
| PRLR-3 | Hampshire, Landrace | The rest of the species | 16 | 20,642,748 | 20,642,943 | Intron-6 | SINEA | 322 |
| PRLR-6 | Bama, Hampshire, Landrace, Rongchang, Wuzhishan, Pietrain | The rest of the species | 16 | 20,630,123 | 20,630,134 | Intron-9 | SINEA | 290 |
| RAB11FIP4-1 | Bama | The rest of the species | 12 | 43,475,928 | 43,475,927 | 5′flank | SINEA | 299 |
| RIPs | Breed | Account | Genotype Frequency (%) | Allele Frequency (%) | Hard-Weinberg Equilibrium | PIC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | + | − | X2 | p | ||||
| CWH43-9 | Bama | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 |
| Tibetan | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| Duroc | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| Large White | 260 | 16.92 | 52.31 | 30.77 | 43.08 | 56.92 | 1.15 | 0.283 | 0.37 | |
| SuJiang | 24 | 54.17 | 41.67 | 4.16 | 75 | 25 | 0.624 | 0.43 | 0.282 | |
| SuShan | 24 | 0 | 41.67 | 58.33 | 20.83 | 79.17 | 18.857 | <0.01 | 0.117 | |
| Erhualian | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| Meishan | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| FengJing | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| IDO2-9 | Bama | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N |
| Tibetan | 24 | 0 | 54.17 | 45.83 | 27.08 | 72.92 | 28.091 | <0.01 | 0.141 | |
| Duroc | 24 | 12.5 | 54.17 | 33.33 | 39.58 | 60.42 | 22.403 | <0.01 | 0.227 | |
| Large White | 260 | 28.85 | 56.92 | 14.23 | 57.31 | 42.69 | 6.93 | 0.008 | 0.370 | |
| SuJiang | 24 | 20.83 | 29.17 | 50 | 35.42 | 64.58 | 28.735 | <0.01 | 0.241 | |
| SuShan | 24 | 58.33 | 41.67 | 0 | 79.17 | 20.83 | N | N | 0 | |
| Erhualian | 24 | 0 | 8.33 | 91.67 | 4.17 | 95.83 | 0.045 | 0.831 | 0.08 | |
| Meishan | 24 | 0 | 25 | 75 | 12.5 | 87.5 | 6.083 | 0.014 | 0.095 | |
| FengJing | 24 | 20.83 | 54.17 | 25 | 47.92 | 52.08 | 18.622 | <0.01 | 0.29 | |
| OPN-1 | Bama | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N |
| Tibetan | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| Duroc | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| Large White | 260 | 5.77 | 43.85 | 50.38 | 27.7 | 72.31 | 2.373 | 0.123 | 0.320 | |
| SuJiang | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| SuShan | 24 | 0 | 12.5 | 87.5 | 6.25 | 93.75 | 0.488 | 0.485 | 0.083 | |
| Erhualian | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 | |
| Meishan | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| FengJing | 24 | 0 | 100 | 0 | 50 | 50 | N | N | 0 | |
| PRLR-6 | Bama | 24 | 100 | 0 | 0 | 100 | 0 | N | N | 0 |
| Tibetan | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| Duroc | 24 | 66.67 | 33.33 | 0 | 83.33 | 16.67 | N | N | 0 | |
| Large White | 258 | 59.69 | 37.60 | 2.71 | 78.49 | 21.51 | 3.317 | 0.069 | 0.281 | |
| SuJiang | 24 | 37.5 | 50 | 12.5 | 62.5 | 37.5 | 5.695 | 0.017 | 0.373 | |
| SuShan | 24 | 20.83 | 54.17 | 25 | 47.92 | 52.08 | 18.622 | <0.01 | 0.29 | |
| Erhualian | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| Meishan | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| FengJing | 24 | 25 | 50 | 25 | 50 | 50 | 18.667 | <0.01 | 0.305 | |
| VMP1-12 | Bama | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N |
| Tibetan | 24 | 54.17 | 45.83 | 0 | 77.08 | 22.92 | N | N | 0 | |
| Duroc | 24 | 66.67 | 33.33 | 0 | 83.33 | 16.67 | N | N | 0 | |
| Large White | 247 | 12.55 | 27.13 | 60.32 | 29.12 | 73.89 | 38.695 | <0.01 | 0.277 | |
| SuJiang | 24 | 54.17 | 45.83 | 0 | 77.08 | 22.92 | N | N | 0 | |
| SuShan | 24 | 83.33 | 16.67 | 0 | 91.67 | 8.33 | N | N | 0 | |
| Erhualian | 24 | 58.33 | 41.67 | 0 | 79.17 | 20.83 | N | N | 0 | |
| Meishan | 24 | 0 | 0 | 100 | 0 | 100 | N | N | N | |
| FengJing | 24 | 25 | 75 | 0 | 62.5 | 37.5 | N | N | 0 | |
| RIP Name | Genotype | TNB | NBA | LW |
| CWH43-9 | SINE+/+ (n = 43) | 10.84 ± 3.14 A | 10.49 ± 2.95 A | 14.00 ± 4.12 A |
| SINE+/− (n = 131) | 9.61 ± 2.84 B | 9.15 ± 2.79 B | 12.42 ± 3.97 B | |
| SINE−/− (n = 78) | 9.88 ± 2.94 B | 9.47 ± 2.83 ab | 12.67 ± 3.71 B | |
| IDO2-9 | SINE+/+ (n = 72) | 9.51 ± 3.21 a | 9.08 ± 3.03 a | 11.99 ± 4.11 a |
| SINE+/− (n = 144) | 9.9 ± 2.86 a | 9.51 ± 2.79 a | 12.93 ± 3.89 ab | |
| SINE−/− (n = 36) | 10.58 ± 2.64 a | 10.11 ± 2.73 a | 12.77 ± 3.78 b | |
| PRLR-6 | SINE+/+ (n = 168) | 9.98 ± 2.91 a | 9.55 ± 2.84 a | 12.88 ± 4.07 a |
| SINE+/− (n = 77) | 9.79 ± 3.05 a | 9.36 ± 2.91 a | 12.55 ± 3.75 a | |
| SINE−/− (n = 7) | 9.92 ± 2.81 a | 8.86 ± 3.24 a | 12.41 ± 4.03 a | |
| VMP1-12 | SINE+/+ (n = 31) | 9.34 ± 2.64 a | 9.61 ± 2.59 a | 12.68 ± 3.57 a |
| SINE+/− (n = 65) | 10.34 ± 2.85 a | 9.92 ± 2.68 a | 13.19 ± 3.65 a | |
| SINE−/− (n = 145) | 9.68 ± 3.10 a | 9.24 ± 3.04 a | 12.57 ± 4.24 a | |
| OPN-1 | SINE+/+ (n = 15) | 9.80 ± 2.81 a | 9.67 ± 2.74 a | 12.54 ± 3.63 a |
| SINE+/− (n = 108) | 9.93 ± 2.94 a | 9.59 ± 2.84 a | 12.95 ± 4.02 a | |
| SINE−/− (n = 129) | 9.90 ± 2.98 a | 9.43 ± 2.91 a | 12.64 ± 3.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; D’Alessandro, E.; Asare, E.; Zheng, Y.; Wang, M.; Chen, C.; Wang, X.; Song, C. Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes. Genes 2022, 13, 1359. https://doi.org/10.3390/genes13081359
Du Z, D’Alessandro E, Asare E, Zheng Y, Wang M, Chen C, Wang X, Song C. Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes. Genes. 2022; 13(8):1359. https://doi.org/10.3390/genes13081359
Chicago/Turabian StyleDu, Zhanyu, Enrico D’Alessandro, Emmanuel Asare, Yao Zheng, Mengli Wang, Cai Chen, Xiaoyan Wang, and Chengyi Song. 2022. "Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes" Genes 13, no. 8: 1359. https://doi.org/10.3390/genes13081359
APA StyleDu, Z., D’Alessandro, E., Asare, E., Zheng, Y., Wang, M., Chen, C., Wang, X., & Song, C. (2022). Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes. Genes, 13(8), 1359. https://doi.org/10.3390/genes13081359








