Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update
Abstract
:1. Introduction
2. Urinary Tract Infections and Uroptahogenic E. coli
2.1. Epidemiology and Burden of UTI
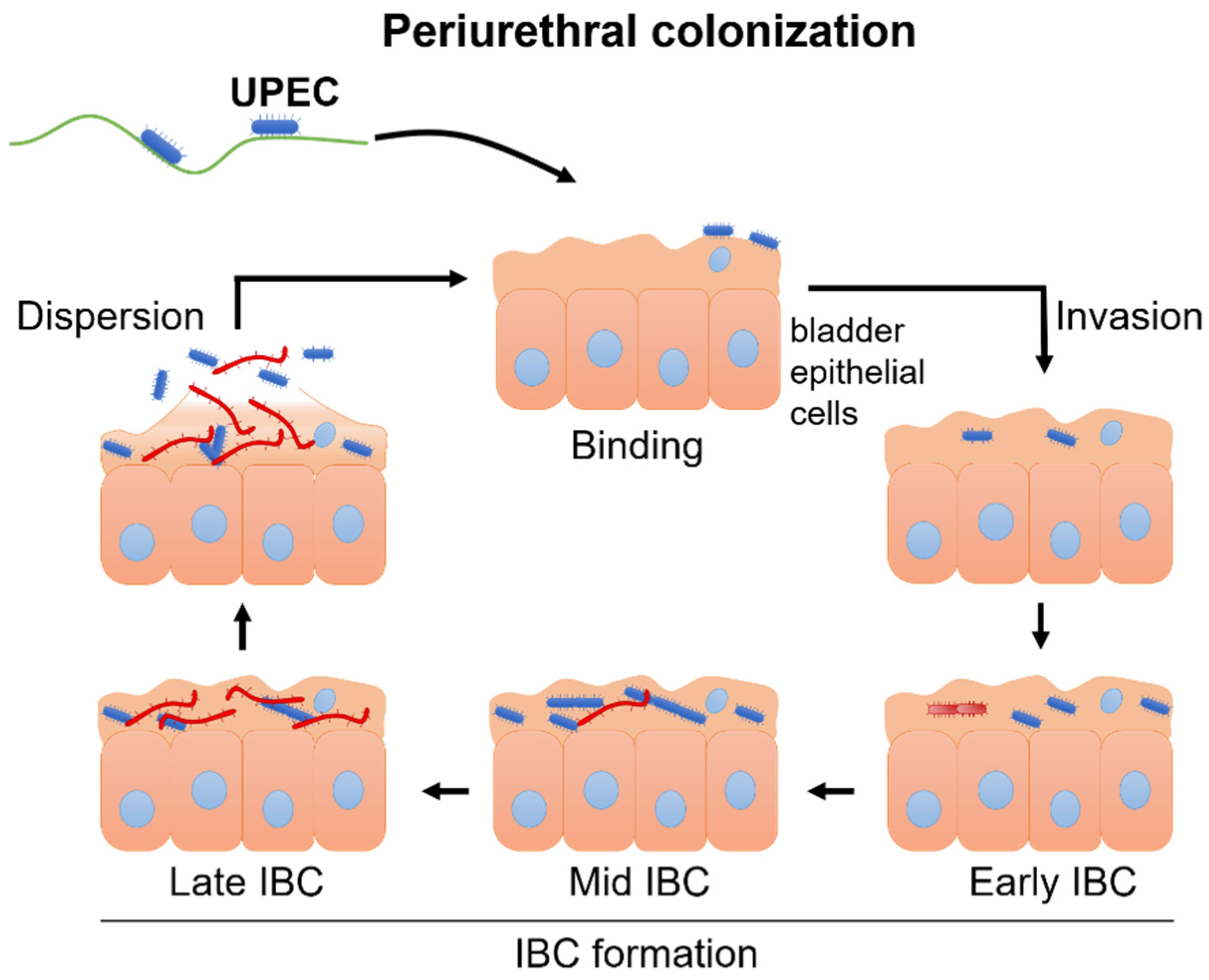
2.2. Pathomechanism of UTI
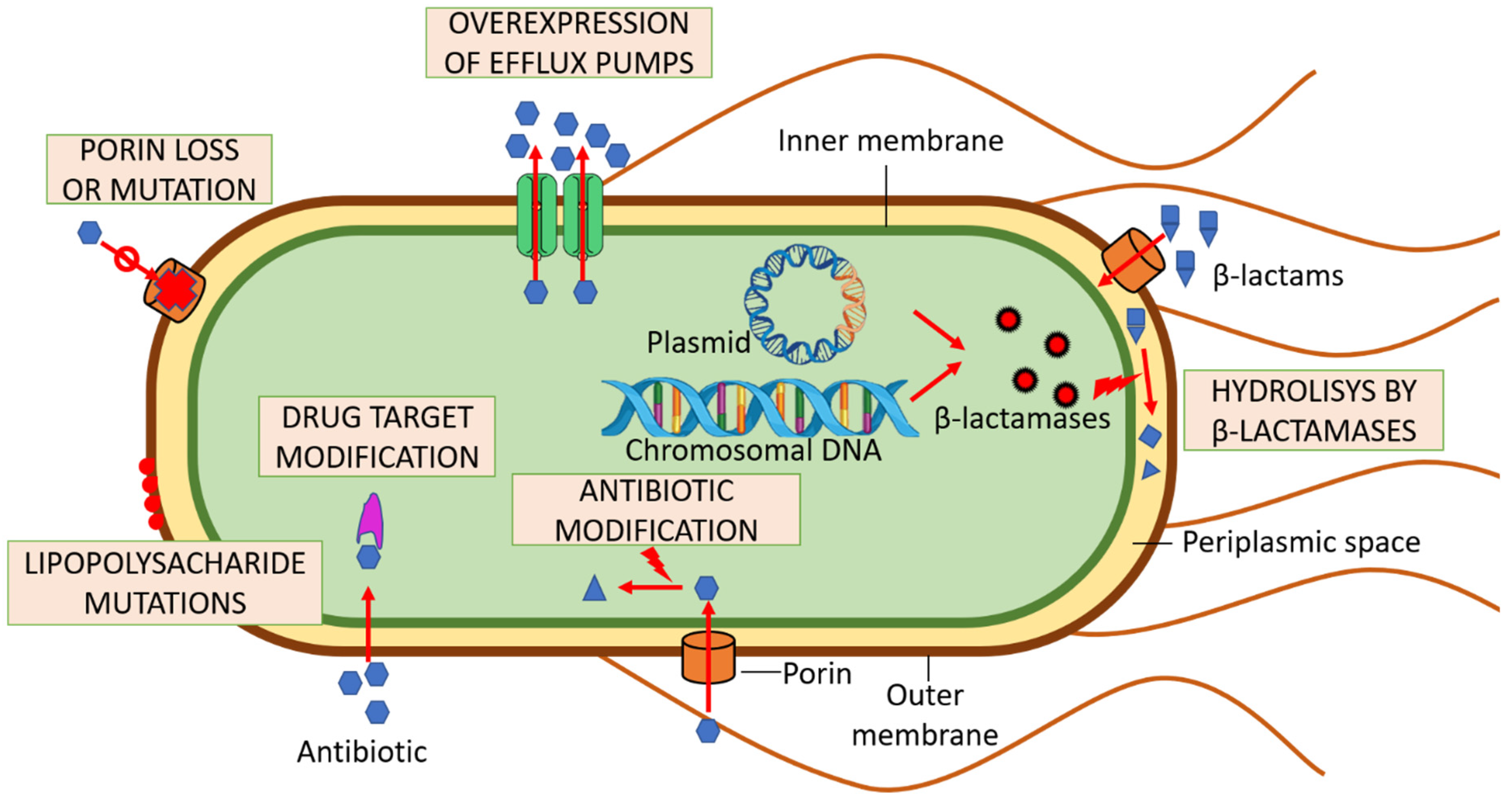
3. Multi-Drug Resistance and UPEC
3.1. Bacterial Efflux Pumps
3.1.1. The ABC Transporters
3.1.2. The MacAB–TolC Pump Complex
3.1.3. The AcrAB–TolC and MexAB–OprM Pump Complexes
3.2. Enzymatic Decomposition of Antibiotics
3.2.1. β-lactams
3.2.2. Carbapenems
3.3. Drug Target Modifications
3.4. Antibiotic Molecule Modification
4. Mobile Genetic Elements in Antibiotic Resistance
4.1. Transposons
4.2. Integrons
4.3. Plasmids
5. Obstacles in UTI Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ABC | ATP-binding cassette |
| CNF-1 | Cytotoxic Necrotizing Factor type I |
| DAEC | Diffusely adherent Escherichia coli |
| dsDNA | Double-stranded DNA |
| dsRNA | Double-stranded RNA |
| EAggEC | Enteroaggregative Escherichia coli |
| EHEC | Enterohaemorrhagic Escherichia coli |
| EIEC | Enteroinvasive Escherichia coli |
| EPEC | Enteropathogenic Escherichia coli |
| ESBL | Extended spectrum β-lactamase |
| ETEC | Enterotoxigenic Escherichia coli |
| ExPEC | Extraintestinal Escherichia coli |
| HGT | Horizontal gene transfer |
| IBC | Intracellular bacterial communities |
| IS | Insertion sequence |
| KTP | Kindey transplant patients |
| MATE | Multidrug and toxic compound extrusion |
| MDR | Multi drug resistant |
| MGE | Mobile genetic element |
| MIC | Minimum inhibitory concentration |
| MPF | Membrane fusion protein |
| NBD | Nucleotide-binding domain |
| NGS | Next Generation Sequencing |
| NMEC | Neonatal Meningitis Escherichia coli |
| PMQR | Plasmid-mediated quinolone resistance |
| Qnr | Quinolone resistance |
| ROS | Reactive oxygen species |
| SMR | Small multidrug resistance |
| ssDNA | Single-stranded DNA |
| TE | Transposable element |
| TMD | Transmembrane domain |
| UPEC | Uropathogenic Escherichia coli |
| UTI | Urinary tract infection |
References
- Hacker, J.; Blum-Oehler, G. In appreciation of Theodor Escherich. Nat. Rev. Microbiol. 2007, 5, 902. [Google Scholar] [CrossRef] [Green Version]
- Blount, Z.D. The unexhausted potential of E. coli. Elife 2015, 4, e05826. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Pinto, L.; Silva, C.G.; Lopes Dde, O.; Machado-Silva, A.; Machado, C.R. Escherichia coli as a model system to study DNA repair genes of eukaryotic organisms. Genet. Mol. Res. 2003, 2, 77–91. [Google Scholar] [PubMed]
- Evans, D.J., Jr.; Evans, D.G. Classification of pathogenic Escherichia coli according to serotype and the production of virulence factors, with special reference to colonization-factor antigens. Rev. Infect. Dis. 1983, 5, S692–S701. [Google Scholar] [CrossRef] [PubMed]
- Stenutz, R.; Weintraub, A.; Widmalm, G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 2006, 30, 382–403. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, C.; Williams, D.M.; Kelly, S.D. Lipopolysaccharide O-antigens-bacterial glycans made to measure. J. Biol. Chem. 2020, 295, 10593–10609. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-PLoSkonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Babiker, A.; Master, R.N.; Luu, T.; Mathur, A.; Bordon, J. Antibiotic Resistance among Urinary Isolates from Female Outpatients in the United States in 2003 and 2012. Antimicrob. Agents Chemother. 2016, 60, 2680–2683. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis. Mon. 2003, 49, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooton, T.M.; Scholes, D.; Hughes, J.P.; Winter, C.; Roberts, P.L.; Stapleton, A.E.; Stergachis, A.; Stamm, W.E. A prospective study of risk factors for symptomatic urinary tract infection in young women. N. Engl. J. Med. 1996, 335, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Yun, K.W.; Kim, D.S.; Kim, W.; Lim, I.S. Molecular typing of uropathogenic Escherichia coli isolated from Korean children with urinary tract infection. Korean J. Pediatr. 2015, 58, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Lara, F.B.; Nery, D.R.; de Oliveira, P.M.; Araujo, M.L.; Carvalho, F.R.; Messias-Silva, L.C.; Ferreira, L.B.; Faria-Junior, C.; Pereira, A.L. Virulence Markers and Phylogenetic Analysis of Escherichia coli Strains with Hybrid EAEC/UPEC Genotypes Recovered from Sporadic Cases of Extraintestinal Infections. Front. Microbiol. 2017, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Dadi, B.R.; Abebe, T.; Zhang, L.; Mihret, A.; Abebe, W.; Amogne, W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 108. [Google Scholar] [CrossRef] [Green Version]
- Halaji, M.; Shahidi, S.; Atapour, A.; Ataei, B.; Feizi, A.; Havaei, S.A. Characterization of Extended-Spectrum beta-Lactamase-Producing Uropathogenic Escherichia coli Among Iranian Kidney Transplant Patients. Infect. Drug Resist. 2020, 13, 1429–1437. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, T.; Wu, D.; Wan, Q. Epidemiology, susceptibility, and risk factors for acquisition of MDR/XDR Gram-negative bacteria among kidney transplant recipients with urinary tract infections. Infect. Drug Resist. 2018, 11, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwin, M.S.; Saigal, C.S.; Yano, E.M.; Avila, C.; Geschwind, S.A.; Hanley, J.M.; Joyce, G.F.; Madison, R.; Pace, J.; Polich, S.M.; et al. Urologic diseases in America Project: Analytical methods and principal findings. J. Urol. 2005, 173, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Justice, S.S.; Hung, C.; Theriot, J.A.; Fletcher, D.A.; Anderson, G.G.; Footer, M.J.; Hultgren, S.J. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlowski, A.C.; Wang, W.; Koteva, K.; Barton, H.A.; McArthur, A.G.; Wright, G.D. A diverse intrinsic antibiotic resistome from a cave bacterium. Nat. Commun. 2016, 7, 13803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Liu, P.; Chen, Y.; Lv, Q.; Wang, Z.; Huang, W.; Jiang, H.; Zheng, Y.; Jiang, Y.; Sun, L. Dictamnine Inhibits the Adhesion to and Invasion of Uropathogenic Escherichia coli (UPEC) to Urothelial Cells. Molecules 2022, 27, 272. [Google Scholar] [CrossRef]
- Scharf, B.; Schmidt, T.J.; Rabbani, S.; Stork, C.; Dobrindt, U.; Sendker, J.; Ernst, B.; Hensel, A. Antiadhesive natural products against uropathogenic E. coli: What can we learn from cranberry extract? J. Ethnopharmacol. 2020, 257, 112889. [Google Scholar] [CrossRef]
- Loubet, P.; Ranfaing, J.; Dinh, A.; Dunyach-Remy, C.; Bernard, L.; Bruyere, F.; Lavigne, J.P.; Sotto, A. Alternative Therapeutic Options to Antibiotics for the Treatment of Urinary Tract Infections. Front. Microbiol. 2020, 11, 1509. [Google Scholar] [CrossRef]
- Hutt, P.; Shchepetova, J.; Loivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Rishabh, R.S.S. Significance of Regional Antibiogram and MDR of ESBL Producing Uropathogens Infecting Non-hospitalized Patients. Gurugram. Int. J. Curr. Microbiol. 2018, 7, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Javed, S.; Mirani, Z.A.; Pirzada, Z.A. Study of class 1 integrons and plasmid profile among multiple drug resistant uropathogenic Escherichia coli. Pak. J. Pharm. Sci. 2020, 33, 2643–2649. [Google Scholar] [PubMed]
- Ali, I.; Rafaque, Z.; Ahmed, S.; Malik, S.; Dasti, J.I. Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac. J.Trop. Biomed. 2016, 6, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Alvarado, L.M.; Zavala-Vega, S.; Cruz-Cordova, A.; Reyes-Grajeda, J.P.; Escalona-Venegas, G.; Flores, V.; Alcazar-Lopez, V.; Arellano-Galindo, J.; Hernandez-Castro, R.; Castro-Escarpulli, G.; et al. Molecular Epidemiology of Multidrug-Resistant Uropathogenic Escherichia coli O25b Strains Associated with Complicated Urinary Tract Infection in Children. Microorganisms 2021, 9, 2299. [Google Scholar] [CrossRef]
- Hadifar, S.; Moghoofei, M.; Nematollahi, S.; Ramazanzadeh, R.; Sedighi, M.; Salehi-Abargouei, A.; Miri, A. Epidemiology of Multidrug Resistant Uropathogenic Escherichia coli in Iran: A Systematic Review and Meta-Analysis. Jpn. J. Infect. Dis. 2017, 70, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-Gonzalez, F.J.; Marquez-Diaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- Moeinizadeh, H.; Shaheli, M. Frequency of hlyA, hlyB, hlyC and hlyD genes in uropathogenic Escherichia coli isolated from UTI patients in Shiraz. GMS Hyg. Infect. Control 2021, 16, 25. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillier, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- Colomb-Cotinat, M.; Lacoste, J.; Brun-Buisson, C.; Jarlier, V.; Coignard, B.; Vaux, S. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), France, 2012. Antimicrob. Resist. Infect. Control 2016, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Paun, V.I.; Lavin, P.; Chifiriuc, M.C.; Purcarea, C. First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core. Sci. Rep. 2021, 11, 514. [Google Scholar] [CrossRef]
- Sommer, M.O.A.; Dantas, G.; Church, G.M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 2009, 325, 1128–1131. [Google Scholar] [CrossRef] [Green Version]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Rosenberg, E.Y.; Foulds, J. Porin channels in Escherichia coli: Studies with beta-lactams in intact cells. J. Bacteriol. 1983, 153, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitsaz, M.; Brown, M.H. The role played by drug efflux pumps in bacterial multidrug resistance. Essays Biochem. 2017, 61, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Veen, H.W.; Margolles, A.; Muller, M.; Higgins, C.F.; Konings, W.N. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 2000, 19, 2503–2514. [Google Scholar] [CrossRef] [Green Version]
- Veen, H.W.; Venema, K.; Bolhuis, H.; Oussenko, I.; Kok, J.; Poolman, B.; Driessen, A.J.; Konings, W.N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 1996, 93, 10668–10672. [Google Scholar] [CrossRef] [Green Version]
- Ughachukwu, P.; Unekwe, P. Efflux pump-mediated resistance in chemotherapy. Ann. Med. Health Sci. Res. 2012, 2, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Reuter, G.; Janvilisri, T.; Venter, H.; Shahi, S.; Balakrishnan, L.; van Veen, H.W. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J. Biol. Chem. 2003, 278, 35193–35198. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.; Miu, A.; Alexander, M.K.; Garcia, N.K.; Oh, A.; Zilberleyb, I.; Reichelt, M.; Austin, C.D.; Tam, C.; Shriver, S.; et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 2018, 557, 196–201. [Google Scholar] [CrossRef]
- Lu, S.; Zgurskaya, H.I. MacA, a periplasmic membrane fusion protein of the macrolide transporter MacAB-TolC, binds lipopolysaccharide core specifically and with high affinity. J. Bacteriol. 2013, 195, 4865–4872. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, N.; Suhani, S.; Purkaystha, A.; Begum, M.K.; Raihan, T.; Alam, M.J.; Islam, K.; Azad, A.K. Identification of AcrAB-TolC Efflux Pump Genes and Detection of Mutation in Efflux Repressor AcrR from Omeprazole Responsive Multidrug-Resistant Escherichia coli Isolates Causing Urinary Tract Infections. Microbiol. Insights 2019, 12, 1178636119889629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetri, S.; Bhowmik, D.; Paul, D.; Pandey, P.; Chanda, D.D.; Chakravarty, A.; Bora, D.; Bhattacharjee, A. AcrAB-TolC efflux pump system plays a role in carbapenem non-susceptibility in Escherichia coli. BMC Microbiol. 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. beta-Lactamases and beta-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Buettcher, M.; Trueck, J.; Niederer-Loher, A.; Heininger, U.; Agyeman, P.; Asner, S.; Berger, C.; Bielicki, J.; Kahlert, C.; Kottanattu, L.; et al. Swiss consensus recommendations on urinary tract infections in children. Eur. J. Pediatr. 2021, 180, 663–674. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, X.; Kong, N.; Zhang, L.; Cao, M.; Sun, M.; Wei, Q.; Liu, W. Characterization of Beta-Lactamases in Bloodstream-Infection Escherichia coli: Dissemination of blaADC–162 and blaCMY-2 Among Bacteria via an IncF Plasmid. Front. Microbiol. 2019, 10, 2175. [Google Scholar] [CrossRef]
- Gatya Al-Mayahie, S.M.; Al-Guranie, D.R.T.; Hussein, A.A.; Bachai, Z.A. Prevalence of common carbapenemase genes and multidrug resistance among uropathogenic Escherichia coli phylogroup B2 isolates from outpatients in Wasit Province/Iraq. PLoS ONE 2022, 17, e0262984. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Gizatullina, J.S. Epidemiological characteristics of uropatogenic iso-lates of Escherichia coli in hospitals. Russ. Clin. Lab. Diagn. 2021, 66, 248–256. [Google Scholar] [CrossRef]
- Pan, Y.; Zeng, J.; Li, L.; Yang, J.; Tang, Z.; Xiong, W.; Li, Y.; Chen, S.; Zeng, Z. Coexistence of Antibiotic Resistance Genes and Virulence Factors Deciphered by Large-Scale Complete Genome Analysis. mSystems 2020, 5, e00821-19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Zhou, X.; Hu, X.; Zhou, Y.; Liu, D.; Maxwell, A.; Mi, K. The plasmid-borne quinolone resistance protein QnrB, a novel DnaA-binding protein, increases the bacterial mutation rate by triggering DNA replication stress. Mol. Microbiol. 2019, 111, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Montero, C.; Mateu, G.; Rodriguez, R.; Takiff, H. Intrinsic resistance of Mycobacterium smegmatis to fluoroquinolones may be influenced by new pentapeptide protein MfpA. Antimicrob. Agents Chemother. 2001, 45, 3387–3392. [Google Scholar] [CrossRef] [Green Version]
- Merens, A.; Matrat, S.; Aubry, A.; Lascols, C.; Jarlier, V.; Soussy, C.J.; Cavallo, J.D.; Cambau, E. The pentapeptide repeat proteins MfpAMt and QnrB4 exhibit opposite effects on DNA gyrase catalytic reactions and on the ternary gyrase-DNA-quinolone complex. J. Bacteriol. 2009, 191, 1587–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinue, L.; Corcoran, M.A.; Hooper, D.C.; Jacoby, G.A. Mutations That Enhance the Ciprofloxacin Resistance of Escherichia coli with qnrA1. Antimicrob. Agents Chemother. 2015, 60, 1537–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cag, Y.; Caskurlu, H.; Fan, Y.; Cao, B.; Vahaboglu, H. Resistance mechanisms. Ann. Transl. Med. 2016, 4, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, S.A.; Cruz-Cordova, A.; Luna-Pineda, V.M.; Reyes-Grajeda, J.P.; Cazares-Dominguez, V.; Escalona, G.; Sepulveda-Gonzalez, M.E.; Lopez-Montiel, F.; Arellano-Galindo, J.; Lopez-Martinez, B.; et al. Multidrug- and Extensively Drug-Resistant Uropathogenic Escherichia coli Clinical Strains: Phylogenetic Groups Widely Associated with Integrons Maintain High Genetic Diversity. Front. Microbiol. 2016, 7, 2042. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, R.A.; Poirel, L.; Carattoli, A.; Nordmann, P. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS ONE 2012, 7, e34752. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Miller, S.F.; Strauss, C.; Zhao, C.; Cheng, L.; Ye, Z.; Griffin, K.; Te, R.; Lee, H.; Chen, C.C.; et al. Antibiotic treatment enhances the genome-wide mutation rate of target cells. Proc. Natl Acad. Sci. USA 2016, 113, e2498–e2505. [Google Scholar] [CrossRef] [Green Version]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijavec, M.; Starcic Erjavec, M.; Ambrozic Avgustin, J.; Reissbrodt, R.; Fruth, A.; Krizan-Hergouth, V.; Zgur-Bertok, D. High prevalence of multidrug resistance and random distribution of mobile genetic elements among uropathogenic Escherichia coli (UPEC) of the four major phylogenetic groups. Curr. Microbiol. 2006, 53, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deatherage, D.E.; Barrick, J.E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 2014, 1151, 165–188. [Google Scholar] [CrossRef] [Green Version]
- Barrick, J.E.; Colburn, G.; Deatherage, D.E.; Traverse, C.C.; Strand, M.D.; Borges, J.J.; Knoester, D.B.; Reba, A.; Meyer, A.G. Identifying structural variation in haploid microbial genomes from short-read resequencing data using breseq. BMC Genom. 2014, 15, 1039. [Google Scholar] [CrossRef]
- Durrant, M.G.; Li, M.M.; Siranosian, B.A.; Montgomery, S.B.; Bhatt, A.S. A Bioinformatic Analysis of Integrative Mobile Genetic Elements Highlights Their Role in Bacterial Adaptation. Cell Host Microbe 2020, 27, 140–153. [Google Scholar] [CrossRef]
- Sousa, A.; Bourgard, C.; Wahl, L.M.; Gordo, I. Rates of transposition in Escherichia coli. Biol. Lett. 2013, 9, 20130838. [Google Scholar] [CrossRef] [Green Version]
- Finnegan, D. Eukaryotic transposable elements and genome evolution. Trends Genetics 1989, 5, 103–107. [Google Scholar] [CrossRef]
- Babakhani, S.; Oloomi, M. Transposons: The agents of antibiotic resistance in bacteria. J. Basic Microbiol. 2018, 58, 905–917. [Google Scholar] [CrossRef]
- Carvalho, R.; Aburjaile, F.; Canario, M.; Nascimento, A.M.A.; Chartone-Souza, E.; Jesus, L.; Zamyatnin, A.A., Jr.; Brenig, B.; Barh, D.; Ghosh, P.; et al. Genomic Characterization of Multidrug-Resistant Escherichia coli BH100 Sub-strains. Front. Microbiol. 2020, 11, 549254. [Google Scholar] [CrossRef]
- Liebert, C.A.; Hall, R.M.; Summers, A.O. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 1999, 63, 507–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, E.; Lambin, M.; Dandoy, D.; Galloy, C.; Nguyen, N.; Oger, C.A.; Hallet, B. The Tn3-family of Replicative Transposons. Microbiol. Spectr. 2015, 3, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffron, F.; McCarthy, B.J.; Ohtsubo, H.; Ohtsubo, E. DNA sequence analysis of the transposon Tn3: Three genes and three sites involved in transposition of Tn3. Cell 1979, 18, 1153–1163. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Oliveira Alvarenga, D.; Ross, K.; Hallet, B.; Van Melderen, L.; Varani, A.M.; Chandler, M. Toxin-Antitoxin Gene Pairs Found in Tn3 Family Transposons Appear To Be an Integral Part of the Transposition Module. mBio 2020, 11, e00452-20. [Google Scholar] [CrossRef] [Green Version]
- Roy Chowdhury, P.; McKinnon, J.; Liu, M.; Djordjevic, S.P. Multidrug Resistant Uropathogenic Escherichia coli ST405 With a Novel, Composite IS26 Transposon in a Unique Chromosomal Location. Front. Microbiol. 2018, 9, 3212. [Google Scholar] [CrossRef] [Green Version]
- Varani, A.; He, S.; Siguier, P.; Ross, K.; Chandler, M. The IS6 family, a clinically important group of insertion sequences including IS26. Mob. DNA 2021, 12, 11. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Zhu, J.H.; Cai, R.M.; Zheng, X.R.; Zhang, L.J.; Chang, M.X.; Lu, Y.W.; Fang, L.X.; Sun, J.; Jiang, H.X. IS26 Is Responsible for the Evolution and Transmission of blaNDM-Harboring Plasmids in Escherichia coli of Poultry Origin in China. mSystems 2021, 6, e0064621. [Google Scholar] [CrossRef]
- Rafaque, Z.; Dasti, J.I.; Andrews, S.C. Draft genome sequence of a uropathogenic Escherichia coli isolate (ST38 O1:H15) from Pakistan, an emerging multidrug-resistant sequence type with a high virulence profile. New Microbes New Infect. 2019, 27, 1–2. [Google Scholar] [CrossRef]
- Rafaque, Z.; Dasti, J.I.; Andrews, S.C. Draft genome sequence of a multidrug-resistant CTX-M-15 beta-lactamase-producing uropathogenic Escherichia coli isolate (ST131-O25b-H30) from Pakistan exhibiting high potential virulence. J. Glob. Antimicrob. Resist. 2018, 15, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, K.; Chan, E.W.; Chen, S. Characterization of the stability and dynamics of Tn6330 in an Escherichia coli strain by nanopore long reads. J. Antimicrob. Chemother. 2019, 74, 1807–1811. [Google Scholar] [CrossRef]
- Kaushik, M.; Kumar, S.; Kapoor, R.K.; Virdi, J.S.; Gulati, P. Integrons in Enterobacteriaceae: Diversity, distribution and epidemiology. Int. J. Antimicrob. Agents 2018, 51, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.F.; Gaze, W.H.; Kuroda, M.; et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Messier, N.; Roy, P.H. Integron integrases possess a unique additional domain necessary for activity. J. Bacteriol. 2001, 183, 6699–6706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le-Vo, H.N.; Tran, P.T.; Le, L.; Matsumoto, Y.; Motooka, D.; Nakamura, S.; Jones, J.W.; Iida, T.; Cao, V. Complex Class 1 Integron in a Clinical Escherichia coli Strain From Vietnam Carrying Both mcr-1 and bla NDM-1. Front. Microbiol. 2019, 10, 2472. [Google Scholar] [CrossRef]
- Shams, F.; Hasani, A.; Rezaee, M.A.; Nahaie, M.R.; Hasani, A.; Haghi, M.H.S.B.; Pormohammad, A.; Arbatan, A.E. Carriage of Class 1 and 2 Integrons in Quinolone, Extended-Spectrum-beta-Lactamase-Producing and Multi Drug Resistant, E.coli and K.pneumoniae: High Burden of Antibiotic Resistance. Adv. Pharm. Bull. 2015, 5, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Poey, M.E.; Lavina, M. Horizontal transfer of class 1 integrons from uropathogenic Escherichia coli to E. coli K12. Microb. Pathog. 2018, 117, 16–22. [Google Scholar] [CrossRef]
- Poey, M.E.; Lavina, M. Integrons in uropathogenic Escherichia coli and their relationship with phylogeny and virulence. Microb. Pathog. 2014, 77, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, C.; Diamantino, C.; Oliveira, P.L.; Reis, M.P.; Costa, P.S.; Paiva, M.C.; Nardi, R.M.D.; Magalhaes, P.P.; Chartone-Souza, E.; Nascimento, A.M.A. Occurrence and characterization of class 1 integrons in Escherichia coli from healthy individuals and those with urinary infection. J. Med. Microbiol. 2017, 66, 577–583. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, E.; Ribas-Aparicio, R.M.; Belmont-Monroy, L.; Aparicio-Ozores, G.; Manjarrez-Hernandez, H.A.; Gavilanes-Parra, S.; Balcazar, J.L.; Molina-Lopez, J. Identification and characterization of class 1 integrons among multidrug-resistant uropathogenic Escherichia coli strains in Mexico. Microb. Pathog. 2022, 162, 105348. [Google Scholar] [CrossRef]
- Mirnezami, M.; Ranjbar, R.; Niakan, M.; Ahmadi, M.H. Frequency of Antimicrobial Resistance and Class 1 and 2 Integrons in Escherichia coli Strains Isolated from Urinary Tract Infections. Iran. J. Pharm. Res. 2020, 19, 282–287. [Google Scholar] [CrossRef]
- Xicohtencatl-Cortes, J.; Cruz-Cordova, A.; Cazares-Dominguez, V.; Escalona-Venegas, G.; Zavala-Vega, S.; Arellano-Galindo, J.; Romo-Castillo, M.; Hernandez-Castro, R.; Ochoa, S.A.; Luna-Pineda, V.M. Uropathogenic Escherichia coli strains harboring tosA gene were associated to high virulence genes and a multidrug-resistant profile. Microb. Pathog. 2019, 134, 103593. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarlton, N.J.; Moritz, C.; Adams-Sapper, S.; Riley, L.W. Genotypic analysis of uropathogenic Escherichia coli to understand factors that impact the prevalence of beta-lactam-resistant urinary tract infections in a community. J. Glob. Antimicrob. Resist. 2019, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.D.; Irshad, P.; Anusree, M.; Rekha, I.; Shailaja, S.; Suresh, J.; Aishwarya, G.; Shrestha, S.; Shome, B.R. Whole genome global insight of antibiotic resistance gene repertoire and virulome of high–risk multidrug-resistant Uropathogenic Escherichiacoli. Microb. Pathog. 2021, 161, 105256. [Google Scholar] [CrossRef] [PubMed]
- Muriuki, C.W.; Ogonda, L.A.; Kyanya, C.; Matano, D.; Masakhwe, C.; Odoyo, E.; Musila, L. Phenotypic and Genotypic Characteristics of Uropathogenic Escherichia coli Isolates from Kenya. Microb. Drug Resist. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Sadeghi, A.; Halaji, M.; Fayyazi, A.; Havaei, S.A. Characterization of Plasmid-Mediated Quinolone Resistance and Serogroup Distributions of Uropathogenic Escherichia coli among Iranian Kidney Transplant Patients. Biomed. Res. Int. 2020, 2020, 2850183. [Google Scholar] [CrossRef]
- Rahman, Z.; Islam, A.; Rashid, M.U.; Johura, F.T.; Monira, S.; Watanabe, H.; Ahmed, N.; Camilli, A.; Alam, M. Existence of a novel qepA variant in quinolone resistant Escherichia coli from aquatic habitats of Bangladesh. Gut Pathog. 2017, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- El-Badawy, M.F.; Tawakol, W.M.; El-Far, S.W.; Maghrabi, I.A.; Al-Ghamdi, S.A.; Mansy, M.S.; Ashour, M.S.; Shohayeb, M.M. Molecular Identification of Aminoglycoside-Modifying Enzymes and Plasmid-Mediated Quinolone Resistance Genes among Klebsiella pneumoniae Clinical Isolates Recovered from Egyptian Patients. Int. J. Microbiol. 2017, 2017, 8050432. [Google Scholar] [CrossRef] [Green Version]
- Paniagua-Contreras, G.L.; Monroy-Perez, E.; Bautista, A.; Reyes, R.; Vicente, A.; Vaca-Paniagua, F.; Diaz, C.E.; Martinez, S.; Dominguez, P.; Garcia, L.R.; et al. Multiple antibiotic resistances and virulence markers of uropathogenic Escherichia coli from Mexico. Pathog. Glob. Health 2018, 112, 415–420. [Google Scholar] [CrossRef]
- Basu, S.; Mukherjee, M. Incidence and risk of co-transmission of plasmid-mediated quinolone resistance and extended-spectrum beta-lactamase genes in fluoroquinolone-resistant uropathogenic Escherichia coli: A first study from Kolkata, India. J. Glob. Antimicrob. Resist. 2018, 14, 217–223. [Google Scholar] [CrossRef]
- Nuesch-Inderbinen, M.T.; Baschera, M.; Zurfluh, K.; Hachler, H.; Nuesch, H.; Stephan, R. Clonal Diversity, Virulence Potential and Antimicrobial Resistance of Escherichia coli Causing Community Acquired Urinary Tract Infection in Switzerland. Front. Microbiol. 2017, 8, 2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Klug, D.M.; Idiris, F.I.M.; Blaskovich, M.A.T.; von Delft, F.; Dowson, C.G.; Kirchhelle, C.; Roberts, A.P.; Singer, A.C.; Todd, M.H. There is no market for new antibiotics: This allows an open approach to research and development. Wellcome Open Res. 2021, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.W.; McKinney, J.D. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. Elife 2021, 10, e66481. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Da Cunda, P.; Notejane, M.; Zunino, P.; Scavone, P.; Robino, L. Fosfomycin tromethamine activity on biofilm and intracellular bacterial communities produced by uropathogenic Escherichia coli isolated from patients with urinary tract infection. Pathog. Dis. 2019, 77, ftz022. [Google Scholar] [CrossRef]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Cusumano, C.K.; Pinkner, J.S.; Han, Z.; Greene, S.E.; Ford, B.A.; Crowley, J.R.; Henderson, J.P.; Janetka, J.W.; Hultgren, S.J. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl. Med. 2011, 3, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, D.; Mukherjee, M. Combination of bactericidal antibiotics and inhibitors of Universal stress protein A (UspA): A potential therapeutic alternative against multidrug resistant Escherichia coli in urinary tract infections. J. Antibiot. 2022, 75, 21–28. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef] [Green Version]
- Gawel, D.; Seed, P.C. Urinary tract infection drives genome instability in uropathogenic Escherichia coli and necessitates translesion synthesis DNA polymerase IV for virulence. Virulence 2011, 2, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Yakimov, A.; Bakhlanova, I.; Baitin, D. Targeting evolution of antibiotic resistance by SOS response inhibition. Comput. Struct. Biotechnol. J. 2021, 19, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Alvarado, C.L.; Sutton, M.D. Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo. Antimicrob. Agents Chemother. 2021, 65, e0001321. [Google Scholar] [CrossRef] [PubMed]
- Adamus-Bialek, W.; Wawszczak, M.; Arabski, M.; Majchrzak, M.; Gulba, M.; Jarych, D.; Parniewski, P.; Gluszek, S. Ciprofloxacin, amoxicillin, and aminoglycosides stimulate genetic and phenotypic changes in uropathogenic Escherichia coli strains. Virulence 2019, 10, 260–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocquet, D.; Llanes, C.; Thouverez, M.; Kulasekara, H.D.; Bertrand, X.; Plesiat, P.; Mazel, D.; Miller, S.I. Evidence for induction of integron-based antibiotic resistance by the SOS response in a clinical setting. PLoS Pathog. 2012, 8, e1002778. [Google Scholar] [CrossRef] [Green Version]
- Memar, M.Y.; Yekani, M.; Celenza, G.; Poortahmasebi, V.; Naghili, B.; Bellio, P.; Baghi, H.B. The central role of the SOS DNA repair system in antibiotics resistance: A new target for a new infectious treatment strategy. Life Sci. 2020, 262, 118562. [Google Scholar] [CrossRef]
- Bunnell, B.E.; Escobar, J.F.; Bair, K.L.; Sutton, M.D.; Crane, J.K. Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli. PLoS ONE 2017, 12, e0178303. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Rosado, A.I.; Valencia, E.Y.; Rodriguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Rodriguez-Beltran, J.; Blazquez, J. N-acetylcysteine blocks SOS induction and mutagenesis produced by fluoroquinolones in Escherichia coli. J. Antimicrob. Chemother. 2019, 74, 2188–2196. [Google Scholar] [CrossRef]
- Recacha, E.; Machuca, J.; Diaz Alba, P.; Ramos-Guelfo, M.; Docobo-Perez, F.; Rodriguez-Beltran, J.; Blazquez, J.; Pascual, A.; Rodriguez-Martinez, J.M. Quinolone Resistance Reversion by Targeting the SOS Response. mBio 2017, 8, e00971-17. [Google Scholar] [CrossRef] [Green Version]


| Antimicrobial Class/Agent | ARG | Mechanism of Resistance |
|---|---|---|
| β-lactams | blaTEM | hydrolysis of the antibiotic molecule |
| blaOXA | ||
| blaCTX-M | ||
| blaSHV | ||
| blaVEB | ||
| blaPER | ||
| blaKPC | ||
| blaVIM | ||
| ampC | ||
| Aminoglycosides | aadA | antibiotic molecule modification (adenylyltransferase) |
| armA | drug target modification (methylase) | |
| rmtB | ||
| aaC(3)—IIa | antibiotic molecule modification (acetyltransferase) | |
| aacA2 | ||
| aacA4 | ||
| Quinolones | qnrA | drug target modification |
| qnrB | ||
| qnrC | ||
| qnrD | ||
| qnrS | ||
| mfpA | ||
| qepA | efflux pump | |
| oqxAB | ||
| Tetracyclines | tet(a) | efflux pump |
| tet(b) | ||
| Sulfonamides | dfrA1 | drug target modification |
| sul1 | ||
| sul2 | ||
| Macrolides | ere(2) | hydrolysis of the antibiotic molecule |
| acrB | efflux pump | |
| acrA | ||
| macB | ||
| mph(A) | Antibiotic molecule modification (phosphotransferase) | |
| Vancomycin | vanA | drug target modification |
| Colistin | mcr-1 | drug target modification (phosphatidylethanolamine transferase) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozwadowski, M.; Gawel, D. Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes 2022, 13, 1397. https://doi.org/10.3390/genes13081397
Rozwadowski M, Gawel D. Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes. 2022; 13(8):1397. https://doi.org/10.3390/genes13081397
Chicago/Turabian StyleRozwadowski, Marcin, and Damian Gawel. 2022. "Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update" Genes 13, no. 8: 1397. https://doi.org/10.3390/genes13081397
APA StyleRozwadowski, M., & Gawel, D. (2022). Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes, 13(8), 1397. https://doi.org/10.3390/genes13081397






