Transcriptomic Analysis of the Porcine Gut in Response to Heat Stress and Dietary Soluble Fiber from Beet Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Collection
2.3. RNA Isolation
2.4. Library Preparation and Sequencing
2.5. Quality Analysis and Mapping of Reads
2.6. Bioinformatics Analysis
3. Results
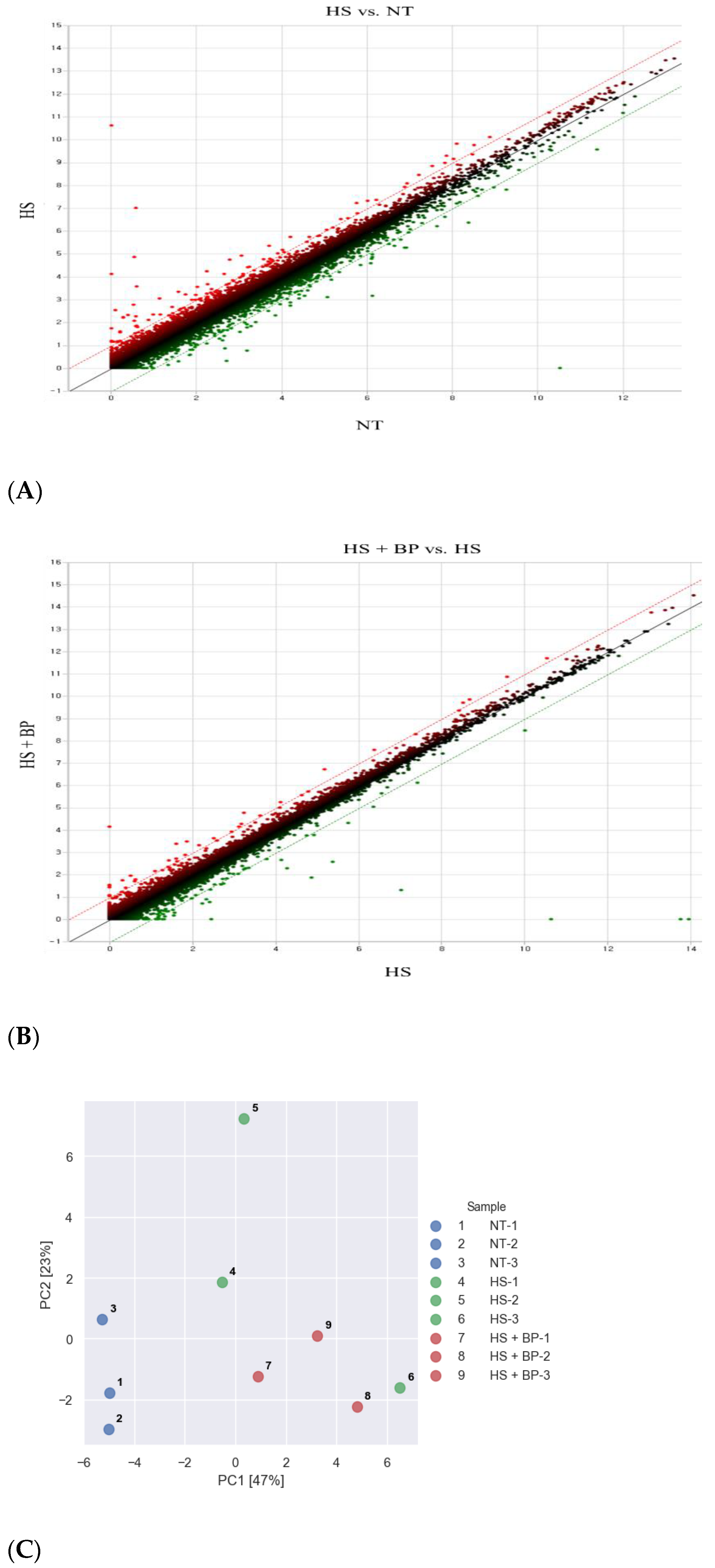
3.1. Screening and Clustering of the Gut Affected by HS and Dietary Supplemental BP
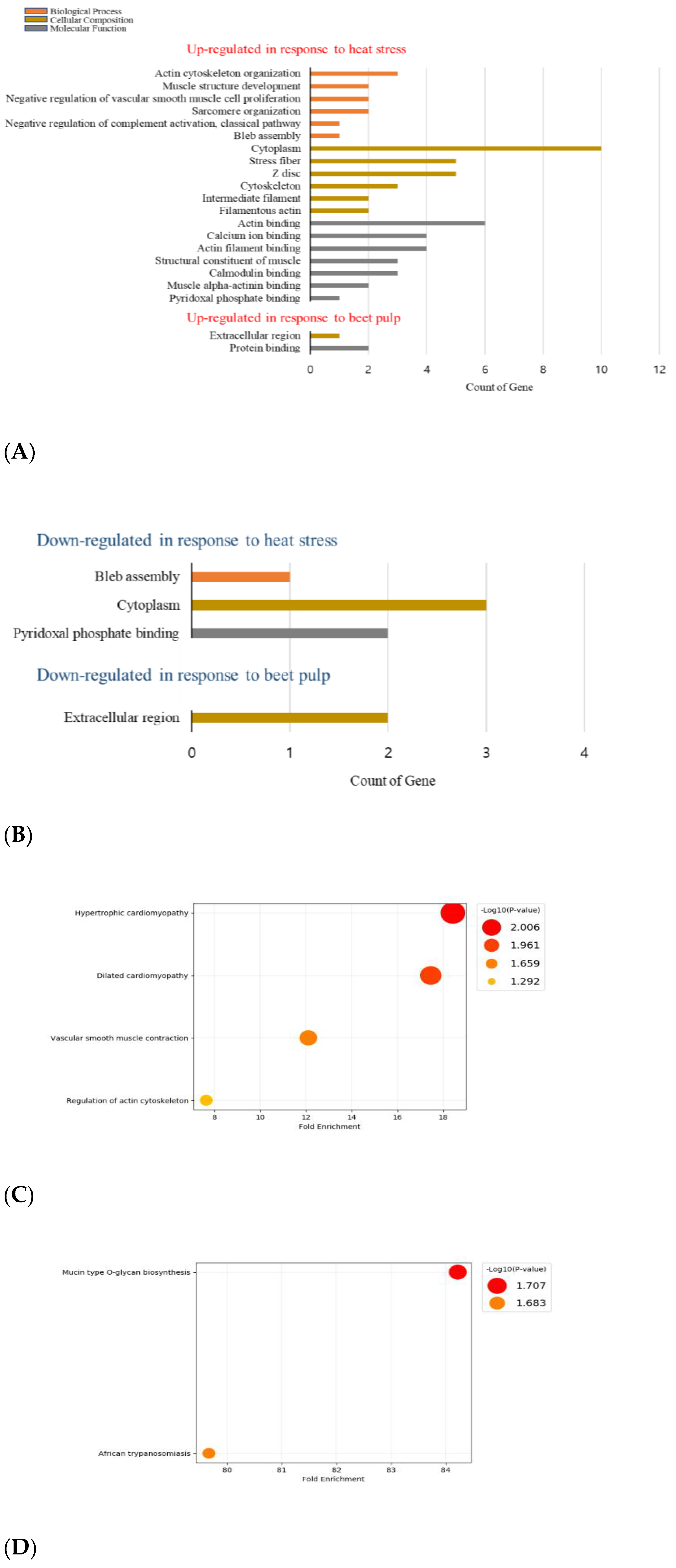
3.2. Porcine Gut Transcriptome Response to HS
3.3. Porcine Gut Transcriptome Response to BP under HS
4. Discussion
4.1. Transcriptome Regulation in Response to HS
4.2. Transcriptome Regulation in Response to BP under HS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, 52–77. [Google Scholar] [CrossRef]
- Ross, J.W.; Hale, B.J.; Gabler, N.K.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1381–1390. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012, 90, 257–259. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Cruzen, S.M.; Boddicker, R.L.; Graves, K.L.; Johnson, T.P.; Arkfeld, E.K.; Baumgard, L.H.; Ross, J.W.; Safranski, T.J.; Lucy, M.C.; Lonergan, S.M. Carcass composition of market weight pigs subjected to heat stress in utero and during finishing. J. Anim. Sci. 2015, 93, 2587–2596. [Google Scholar] [CrossRef]
- Johnson, J.S.; Fernandez, M.V.S.; Gutierrez, N.A.; Patience, J.F.; Ross, J.W.; Gabler, N.K.; Lucy, M.C.; Safranski, T.J.; Rhoads, R.P.; Baumgard, L.H. Effects of in utero heat stress on postnatal body composition in pigs: I. Growing phase. J. Anim. Sci. 2015, 93, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Hernandez, L.; Buenabad, L.; Avelar, E.; Bernal, H.; Baumgard, L.H.; Cervantes, M. Effect of heat stress on the endogenous intestinal loss of amino acids in growing pigs. J. Anim. Sci. 2016, 94, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wolp, R.C.; Rodrigues, N.E.B.; Zangeronimo, M.G.; Cantarelli, V.S.; Fialho, E.T.; Philomeno, R.; Alvarenga, R.R.; Rocha, L.F. Soybean oil and crude protein levels for growing pigs kept under heat stress conditions. Livest. Sci. 2012, 147, 148–153. [Google Scholar] [CrossRef]
- Fernandez, M.S.; Pearce, S.C.; Gabler, N.K.; Patience, J.F.; Wilson, M.E.; Socha, M.T.; Torrison, J.L.; Rhoads, R.P.; Baumgard, L.H. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 2014, 8, 43–50. [Google Scholar] [CrossRef]
- Pearce, S.C.; Fernandez, M.V.S.; Torrison, J.; Wilson, M.E.; Baumgard, L.H.; Gabler, N.K. Dietary organic zinc attenuates heat stress–induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 2015, 93, 4702–4713. [Google Scholar] [CrossRef]
- Kellner, T.A.; Baumgard, L.H.; Prusa, K.J.; Gabler, N.K.; Patience, J.F. Does heat stress alter the pig’s response to dietary fat? J. Anim. Sci. 2016, 94, 4688–4703. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801. [Google Scholar] [CrossRef]
- Rauw, W.M.; Mayorga, E.J.; Lei, S.M.; Dekkers, J.; Patience, J.F.; Gabler, N.K.; Lonergan, S.M.; Baumgard, L.H. Effects of diet and genetics on growth performance of pigs in response to repeated exposure to heat stress. Front. Genet. 2017, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, E.J.; Kvidera, S.K.; Horst, E.A.; Al-Qaisi, M.; Dickson, M.J.; Seibert, J.T.; Lei, S.; Keating, A.F.; Ross, J.W.; Rhoads, R.P.; et al. Effects of zinc amino acid complex on biomarkers of gut integrity and metabolism during and following heat stress or feed restriction in pigs. J. Anim. Sci. 2018, 96, 4173–4185. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef]
- Huang, S.; Wei, J.; Yu, H.; Hao, X.; Zuo, J.; Tan, C.; Deng, J. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals 2020, 10, 141. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Ross, J.W.; Keating, A.F.; Rhoads, R.P.; Baumgard, L.H. Biology of heat stress; the nexus between intestinal hyperpermeability and swine reproduction. Theriogenology 2020, 154, 73–83. [Google Scholar] [CrossRef]
- Liu, H.; Dicksved, J.; Lundh, T.; Lindberg, J.E. Heat shock proteins: Intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens 2014, 3, 187–210. [Google Scholar] [CrossRef]
- Naderi, N.; Ghorbani, G.R.; Sadeghi-Sefidmazgi, A.; Nasrollahi, S.M.; Beauchemin, K.A. Shredded beet pulp substituted for corn silage in diets fed to dairy cows under ambient heat stress: Feed intake, total-tract digestibility, plasma metabolites, and milk production. J. Dairy Sci. 2016, 99, 8847–8857. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Srikanth, K.; Park, J.E.; Ji, S.Y.; Kim, K.H.; Lee, Y.K.; Kumar, H.; Kim, M.; Baek, Y.C.; Kim, H.; Jang, G.W.; et al. Genome-wide transcriptome and metabolome analyses provide novel insights and suggest a sex-specific response to heat stress in pigs. Genes 2020, 11, 540. [Google Scholar] [CrossRef]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Pearce, S.C.; Lonergan, S.M.; Huff-Lonergan, E.; Baumgard, L.H.; Gabler, N.K. Acute heat stress and reduced nutrient intake alter intestinal proteomic profile and gene expression in pigs. PLoS ONE 2015, 10, e0143099. [Google Scholar] [CrossRef]
- Oladele, P.; Li, E.; Lu, H.; Cozannet, P.; Nakatsu, C.; Johnson, T.; Adeola, O.; Ajuwon, K.M. Effect of a carbohydrase admixture in growing pigs fed wheat-based diets in thermoneutral and heat stress conditions. J. Anim. Sci. 2021, 99, skab254. [Google Scholar] [CrossRef]
- Oh, S.; Hosseindoust, A.; Ha, S.; Moturi, J.; Mun, J.; Tajudeen, H.; Kim, J. Metabolic responses of dietary fiber during heat stress: Effects on reproductive performance and stress level of gestating sows. Metabolites 2022, 12, 280. [Google Scholar] [CrossRef]
- National Research Council (U.S.). Nutrient Requirements of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012; Volume 16, pp. 208–238. [Google Scholar]
- A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 April 2022).
- FASTX Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 15 April 2022).
- BBMap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 15 April 2022).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- Godyń, D.; Herbut, P.; Angrecka, S.; Vieira, F.M.C. Use of different cooling methods in pig facilities to alleviate the effects of heat stress-A review. Animals 2020, 10, 1459. [Google Scholar] [CrossRef]
- Gabler, N.K.; Pearce, S.C. The impact of heat stress on intestinal function and productivity in grow-finish pigs. Anim. Prod. Sci. 2015, 55, 1403–1410. [Google Scholar] [CrossRef]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Wallace, E.W.; Kear-Scott, J.L.; Pilipenko, E.V.; Schwartz, M.H.; Laskowski, P.R.; Rojek, A.E.; Katanski, C.D.; Riback, J.A.; Dion, M.F.; Franks, A.M.; et al. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 2015, 162, 1286–1298. [Google Scholar] [CrossRef]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D.A. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 2017, 168, 1028–1040. [Google Scholar] [CrossRef]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef]
- Shatov, V.M.; Gusev, N.B. Physico-chemical properties of two point mutants of small heat shock protein HspB6 (Hsp20) with abrogated cardioprotection. Biochimie 2020, 174, 126–135. [Google Scholar] [CrossRef]
- Lu, Z.; Chu, M.; Li, Q.; Jin, M.; Fei, X.; Ma, L.; Jin, M.; Fei, X.; Ma, L.; Zhang, L.; et al. Transcriptomic analysis provides novel insights into heat stress responses in sheep. Animals 2019, 9, 387. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D.X. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Chittoor-Vinod, V.G.; Lee, S.; Judge, S.M.; Notterpek, L. Inducible HSP70 is critical in preventing the aggregation and enhancing the processing of PMP22. ASN Neuro 2015, 7, 1–17. [Google Scholar] [CrossRef]
- Van Deursen, J.; Ruitenbeek, W.; Heerschap, A.; Jap, P.; Ter Laak, H.; Wieringa, B. Creatine kinase (CK) in skeletal muscle energy metabolism: A study of mouse mutants with graded reduction in muscle CK expression. Proc. Natl. Acad. Sci. USA 1994, 91, 9091–9095. [Google Scholar] [CrossRef]
- Kwasiborski, A.; Sayd, T.; Chambon, C.; Sante-Lhoutellier, V.; Rocha, D.; Terlouw, C. Pig Longissimus lumborum proteome: Part II: Relationships between protein content and meat quality. Meat Sci. 2008, 80, 982–996. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, G.; Xu, X.; Lundström, K.; Karlsson, A.; Lametsch, R. Phosphoproteome analysis of sarcoplasmic and myofibrillar proteins in bovine longissimus muscle in response to postmortem electrical stimulation. Food Chem. 2015, 175, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, L.; Shi, Z.; Chen, W.; Yang, X.; Hu, Y.; Zheng, C.; Jiang, Z. Mechanism of continuous high temperature affecting growth performance, meat quality, and muscle biochemical properties of finishing pigs. Genes Nutr. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, e2321. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Swelum, A.A.A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef]
- He, S.; Hou, X.; Xu, X.; Wan, C.; Yin, P.; Liu, X.; Chen, Y.; Shu, B.; Liu, F.; Xu, J. Quantitative proteomic analysis reveals heat stress-induced injury in rat small intestine via activation of the MAPK and NF-κB signaling pathways. Mol. Biosyst. 2015, 11, 826–834. [Google Scholar] [CrossRef]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Hou, X.; Wan, C.; He, S.; Chong, X.; Liu, M.; Li, H.; Liu, F. Microarray analysis of intestinal immune-related gene expression in heat-stressed rats. Int. J. Hyperth. 2014, 30, 324–327. [Google Scholar] [CrossRef]
- Greene, E.S.; Emami, N.K.; Dridi, S. Research Note: Phytobiotics modulate the expression profile of circulating inflammasome and cyto (chemo) kine in whole blood of broilers exposed to cyclic heat stress. Poult. Sci. 2021, 100, 100801. [Google Scholar] [CrossRef]
- Van Meulder, F.; Van Coppernolle, S.; Borloo, J.; Rinaldi, M.; Li, R.W.; Chiers, K.; Van den Broeck, W.; Vercruysse, J.; Claerebout, E.; Geldhof, P. Granule exocytosis of granulysin and granzyme B as a potential key mechanism in vaccine-induced immunity in cattle against the nematode Ostertagia ostertagi. Infect. Immun. 2013, 81, 1798–1809. [Google Scholar] [CrossRef]
- Schwerin, M.; Janczyk, P.; Ponsuksili, S.; Walz, C.; Souffrant, W.B. Effects of plant extract and natural substance food additives on stress and immune response in weaning piglets. Arch. Zootech. 2009, 12, 5–21. [Google Scholar]
- Malheiros, J.M.; Braga, C.P.; Grove, R.A.; Ribeiro, F.A.; Calkins, C.R.; Adamec, J.; Chardulo, L.A.L. Influence of oxidative damage to proteins on meat tenderness using a proteomics approach. Meat Sci. 2019, 148, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Shim, K.S. Proteomic changes in broiler liver by body weight differences under chronic heat stress. Poult. Sci. 2022, 101, 101794. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, J.R.; Zhang, Y.; Yang, P.G.; Feng, Y.J.; Cui, Y.J.; Yang, C.H.; Gu, X.H. The micro RNA expression profile in porcine skeletal muscle is changed by constant heat stress. Anim. Genet. 2016, 47, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gu, X. Proteomic changes of the porcine small intestine in response to chronic heat stress. J. Mol. Endocrinol. 2015, 55, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, Y.; Li, J.; Gao, Y.; Gu, X. Proteomic changes of the porcine skeletal muscle in response to chronic heat stress. J. Sci. Food Agric. 2018, 98, 3315–3323. [Google Scholar] [CrossRef]
- Cruzen, S.M.; Pearce, S.C.; Baumgard, L.H.; Gabler, N.K.; Huff-Lonergan, E.; Lonergan, S.M. Proteomic changes to the sarcoplasmic fraction of predominantly red or white muscle following acute heat stress. J. Proteom. 2015, 128, 141–153. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Zhang, Y.; Liu, L.; Wang, J.; Zhang, S. Effects of body weight and fiber sources on fiber digestibility and short chain fatty acid concentration in growing pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 1975. [Google Scholar] [CrossRef]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Heizmann, C.W. The multifunctional S100 protein family. Methods Mol. Biol. 2002, 172, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Woods, T.L.; Fu, J.; Zhang, T.; Stoll, S.W.; Elder, J.T. Biochemical characterization of S100A2 in human keratinocytes: Subcellular localization, dimerization, and oxidative cross-linking. J. Investig. Dermatol. 2000, 115, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.; Kerstens, H.; De Wit, A.; Smits, M.; Van Der Meulen, J.; Niewold, T. Early transcriptional response in the jejunum of germ-free piglets after oral infection with virulent rotavirus. Arch. Virol. 2008, 153, 1311–1322. [Google Scholar] [CrossRef]
- Connor, E.E.; Li, R.W.; Baldwin, R.L.; Li, C. Gene expression in the digestive tissues of ruminants and their relationships with feeding and digestive processes. Animal 2010, 4, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qin, Y.C.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. l-Glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway. Food Funct. 2020, 11, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.B.; Ye, L.; Pan, Z.Y.; Zhu, J.; Du, Z.D.; Zhu, G.Q.; Huang, X.G.; Wu, S.L. Microarray analysis of differential gene expression in sensitive and resistant pig to Escherichia coli F18. Anim. Genet. 2012, 43, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Priatel, J.J.; Chui, D.; Hiraoka, N.; Simmons, C.J.; Richardson, K.B.; Page, D.M.; Fukuda, M.; Varki, N.M.; Marth, J.D. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity 2000, 12, 273–283. [Google Scholar] [CrossRef]
- Xia, B.; Wu, W.; Zhang, L.; Wen, X.; Xie, J.; Zhang, H. Gut microbiota mediates the effects of inulin on enhancing sulfomucin production and mucosal barrier function in a pig model. Food Funct. 2021, 12, 10967–10982. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yin, D.; Mahmood, T.; Yuan, J. Transcriptome analysis reveals a molecular understanding of nicotinamide and butyrate sodium on meat quality of broilers under high stocking density. BMC Genom. 2020, 21, 412. [Google Scholar] [CrossRef]
- Olsen, H.G.; Skovgaard, K.; Nielsen, O.L.; Leifsson, P.S.; Jensen, H.E.; Iburg, T.; Heegaard, P.M. Organization and biology of the porcine serum amyloid A (SAA) gene cluster: Isoform specific responses to bacterial infection. PLoS ONE 2013, 8, e76695. [Google Scholar] [CrossRef]
- Sun, L.; Ye, R.D. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Huan, B.; Liu, K.; Li, Y.; Wei, J.; Shao, D.; Shi, Y.; Qiu, Y.; Li, B.; Ma, Z. Porcine serum amyloid A3 is expressed in extrahepatic tissues and facilitates viral replication during porcine respiratory and reproductive syndrome virus infection. Dev. Comp. Immunol. 2018, 79, 51–58. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Fold Change | Gene Expression Value | Gene Description | ||||
|---|---|---|---|---|---|---|---|
| HS/NT | HS + BP/NT | HS + BP/HS | Normalized Data (log2) | ||||
| NT | HS | HS + BP | |||||
| GPT2 | 0.472 | 0.452 | 0.957 | 4.658 | 3.575 | 3.511 | Glutamic-pyruvic transaminase 2 |
| HSPB6 | 2.191 | 2.629 | 1.200 | 6.224 | 7.356 | 7.618 | Heat shock protein family B (small) member 6 |
| PPP1R14A | 2.194 | 2.337 | 1.065 | 5.389 | 6.522 | 6.613 | Protein phosphatase 1 regulatory inhibitor subunit 14A |
| CKM | 3.128 | 4.095 | 1.309 | 3.706 | 5.352 | 5.740 | Creatine kinase, M-type |
| SYNM | 2.001 | 2.743 | 1.371 | 5.551 | 6.552 | 7.007 | Synemin |
| NEXN | 2.026 | 2.077 | 1.025 | 3.100 | 4.119 | 4.155 | Nexilin F-actin binding protein |
| TSPAN1 | 0.418 | 0.471 | 1.126 | 6.131 | 4.872 | 5.044 | Tetraspanin 1 |
| GSTA1 | 2.488 | 2.405 | 0.966 | 6.016 | 7.331 | 7.282 | Glutathione S-transferase α 1 |
| GZMB | 0.374 | 0.432 | 1.155 | 7.639 | 6.219 | 6.427 | Granzyme B |
| TAGLN | 2.363 | 2.987 | 1.264 | 8.874 | 10.115 | 10.453 | Transgelin |
| C4BPA | 2.145 | 2.244 | 1.046 | 4.646 | 5.747 | 5.812 | Complement component 4 binding protein, α |
| DDC | 0.407 | 0.494 | 1.213 | 5.162 | 3.866 | 4.145 | Dopa decarboxylase |
| CSRP1 | 2.047 | 2.352 | 1.149 | 6.886 | 7.919 | 8.120 | Cysteine and glycine rich protein 1 |
| TPM2 | 2.377 | 2.823 | 1.188 | 8.506 | 9.755 | 10.003 | Tropomyosin 2 (β) |
| CCL4 | 0.467 | 0.442 | 0.945 | 4.123 | 3.026 | 2.945 | C-C motif chemokine ligand 4 |
| ITGAE | 0.431 | 0.483 | 1.121 | 4.612 | 3.398 | 3.563 | Integrin subunit α E |
| PMP22 | 0.406 | 0.436 | 1.073 | 6.643 | 5.343 | 5.445 | Peripheral myelin protein 22 |
| MYLK | 2.216 | 2.703 | 1.220 | 6.927 | 8.074 | 8.361 | Myosin light chain kinase |
| SH3BGR | 2.097 | 2.377 | 1.133 | 3.716 | 4.784 | 4.965 | SH3 domain binding glutamate rich protein |
| PCP4 | 2.787 | 3.488 | 1.251 | 5.749 | 7.228 | 7.552 | Purkinje cell protein 4 |
| MX1 | 0.356 | 0.408 | 1.147 | 6.084 | 4.593 | 4.791 | MX dynamin like GTPase 1 |
| C14H10orf99 | 0.452 | 0.417 | 0.924 | 5.340 | 4.194 | 4.079 | Chromosome 14 C10orf99 homolog |
| PDLIM3 | 2.017 | 2.073 | 1.028 | 4.060 | 5.072 | 5.112 | PDZ and LIM domain 3 |
| DES | 2.696 | 3.604 | 1.337 | 7.855 | 9.286 | 9.705 | Desmin |
| MYL9 | 2.248 | 2.968 | 1.320 | 7.815 | 8.984 | 9.385 | Myosin light chain 9 |
| PYGM | 2.173 | 2.416 | 1.112 | 3.896 | 5.016 | 5.169 | Glycogen phosphorylase, muscle associated |
| CNN1 | 2.485 | 3.221 | 1.296 | 7.535 | 8.849 | 9.223 | Calponin 1 |
| PDLIM7 | 2.001 | 2.276 | 1.137 | 5.052 | 6.052 | 6.238 | PDZ and LIM domain 7 |
| GNLY | 0.317 | 0.356 | 1.123 | 6.921 | 5.265 | 5.432 | Granulysin |
| ACTG2 | 3.296 | 4.261 | 1.293 | 8.105 | 9.825 | 10.196 | Actin γ 2, smooth muscle |
| PLB1 | 0.468 | 0.407 | 0.869 | 5.122 | 4.028 | 3.825 | Phospholipase B1 |
| S100A2 | 2.226 | 6.477 | 2.910 | 4.019 | 5.173 | 6.714 | S100 calcium binding protein A2 |
| TPM1 | 2.190 | 2.943 | 1.344 | 8.031 | 9.162 | 9.588 | Tropomyosin 1 (α) |
| MGP | 2.374 | 2.155 | 0.908 | 7.204 | 8.451 | 8.311 | Matrix Gla protein |
| CYP2J34 | 0.837 | 1.703 | 2.034 | 3.882 | 3.626 | 4.650 | Cytochrome P450 family 2 subfamily J member 34 |
| HBB | 1.693 | 3.886 | 2.295 | 7.759 | 8.519 | 9.717 | Hemoglobin, β |
| SCGB1A1 | 1.291 | 0.327 | 0.253 | 3.898 | 4.266 | 2.286 | Secretoglobin family 1A member 1 |
| LOC396781 | 1.625 | 0.555 | 0.341 | 9.311 | 10.011 | 8.460 | IgG heavy chain |
| SAA3 | 85.820 | 1.642 | 0.019 | 0.588 | 7.012 | 1.304 | Serum amyloid A-3 protein |
| ST3GAL1 | 0.618 | 0.089 | 0.144 | 6.072 | 5.378 | 2.584 | ST3 β-galactoside α-2,3-sialyltransferase 1 |
| HSPA6 | 1.163 | 3.423 | 2.944 | 3.008 | 3.226 | 4.784 | Heat shock protein family A (Hsp70) member 6 |
| S100A2 | 2.226 | 6.477 | 2.910 | 4.019 | 5.173 | 6.714 | S100 calcium binding protein A2 |
| GCNT3 | 0.258 | 0.526 | 2.042 | 5.071 | 3.115 | 4.145 | Glucosaminyl (N-acetyl) transferase 3, mucin type |
| LYZ | 1.094 | 2.471 | 2.259 | 10.398 | 10.527 | 11.703 | Lysozyme |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Roura, E.; Choi, Y.; Kim, J. Transcriptomic Analysis of the Porcine Gut in Response to Heat Stress and Dietary Soluble Fiber from Beet Pulp. Genes 2022, 13, 1456. https://doi.org/10.3390/genes13081456
Kim M, Roura E, Choi Y, Kim J. Transcriptomic Analysis of the Porcine Gut in Response to Heat Stress and Dietary Soluble Fiber from Beet Pulp. Genes. 2022; 13(8):1456. https://doi.org/10.3390/genes13081456
Chicago/Turabian StyleKim, Minju, Eugeni Roura, Yohan Choi, and Joeun Kim. 2022. "Transcriptomic Analysis of the Porcine Gut in Response to Heat Stress and Dietary Soluble Fiber from Beet Pulp" Genes 13, no. 8: 1456. https://doi.org/10.3390/genes13081456
APA StyleKim, M., Roura, E., Choi, Y., & Kim, J. (2022). Transcriptomic Analysis of the Porcine Gut in Response to Heat Stress and Dietary Soluble Fiber from Beet Pulp. Genes, 13(8), 1456. https://doi.org/10.3390/genes13081456







