Genome-Wide Association Study Identifies Candidate Genes for Stripe Pattern Feather Color of Rhode Island Red Chicks
Abstract
:1. Introduction
2. Materials and Methods
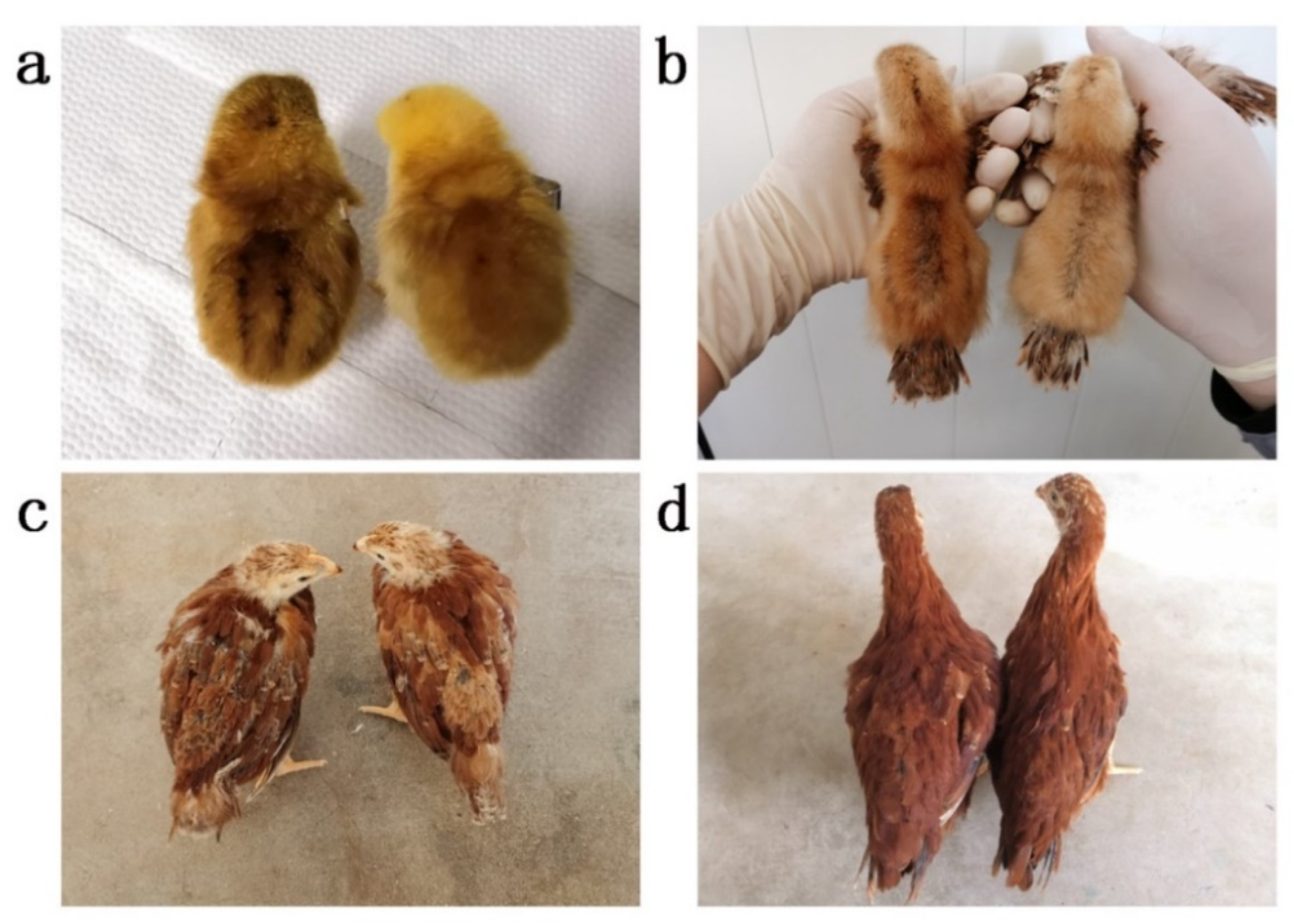
2.1. Animals and Sample Collection
2.2. Whole-Genome Sequencing and Variant Calling
2.3. Genome-Wide Association Studies
2.4. Variation Annotation and Candidate Gene Identification
2.5. Quantitative Real-Time PCR
3. Results
3.1. Overview of the Whole-Genome Sequencing Data
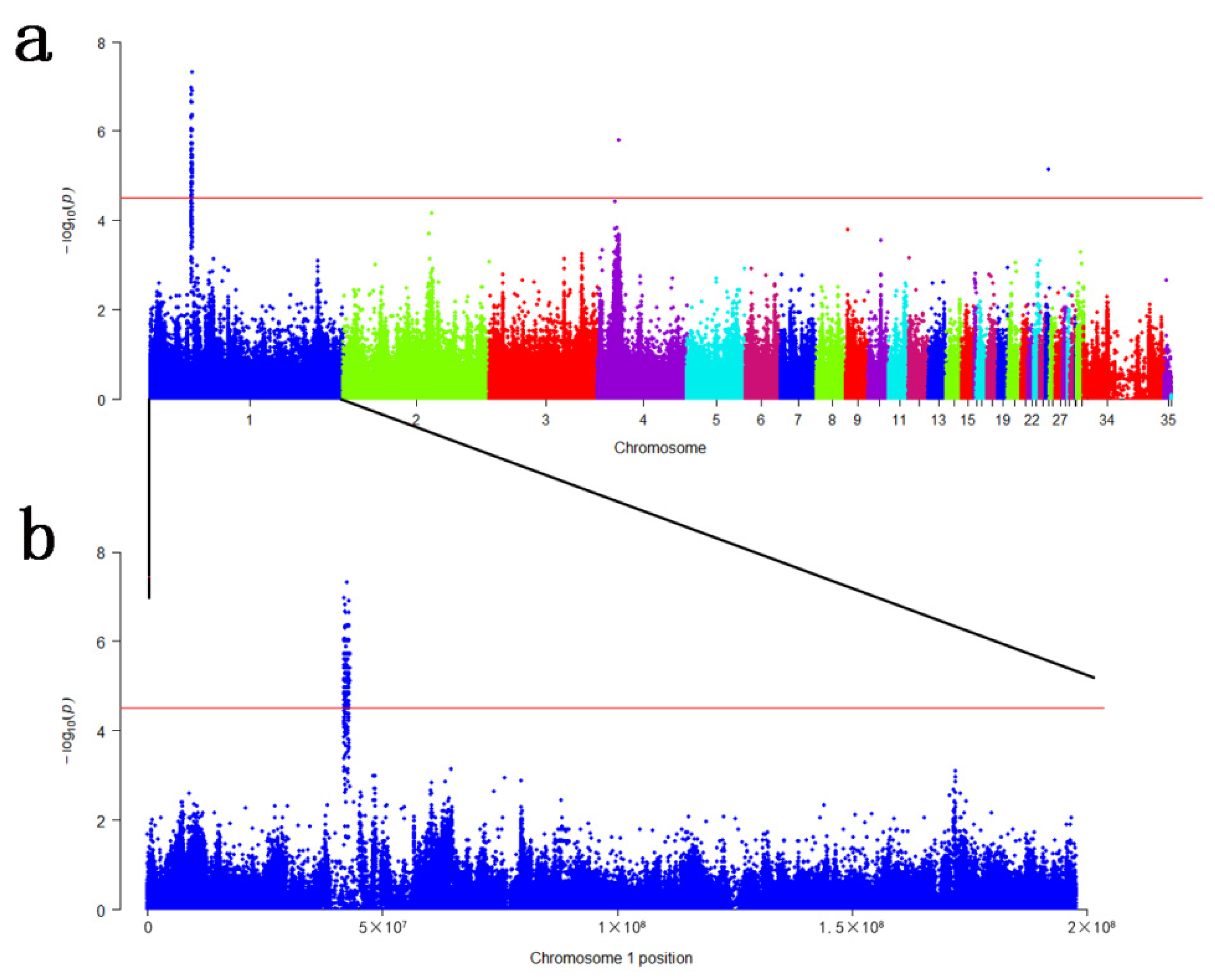
3.2. Genome-Wide Association Studies
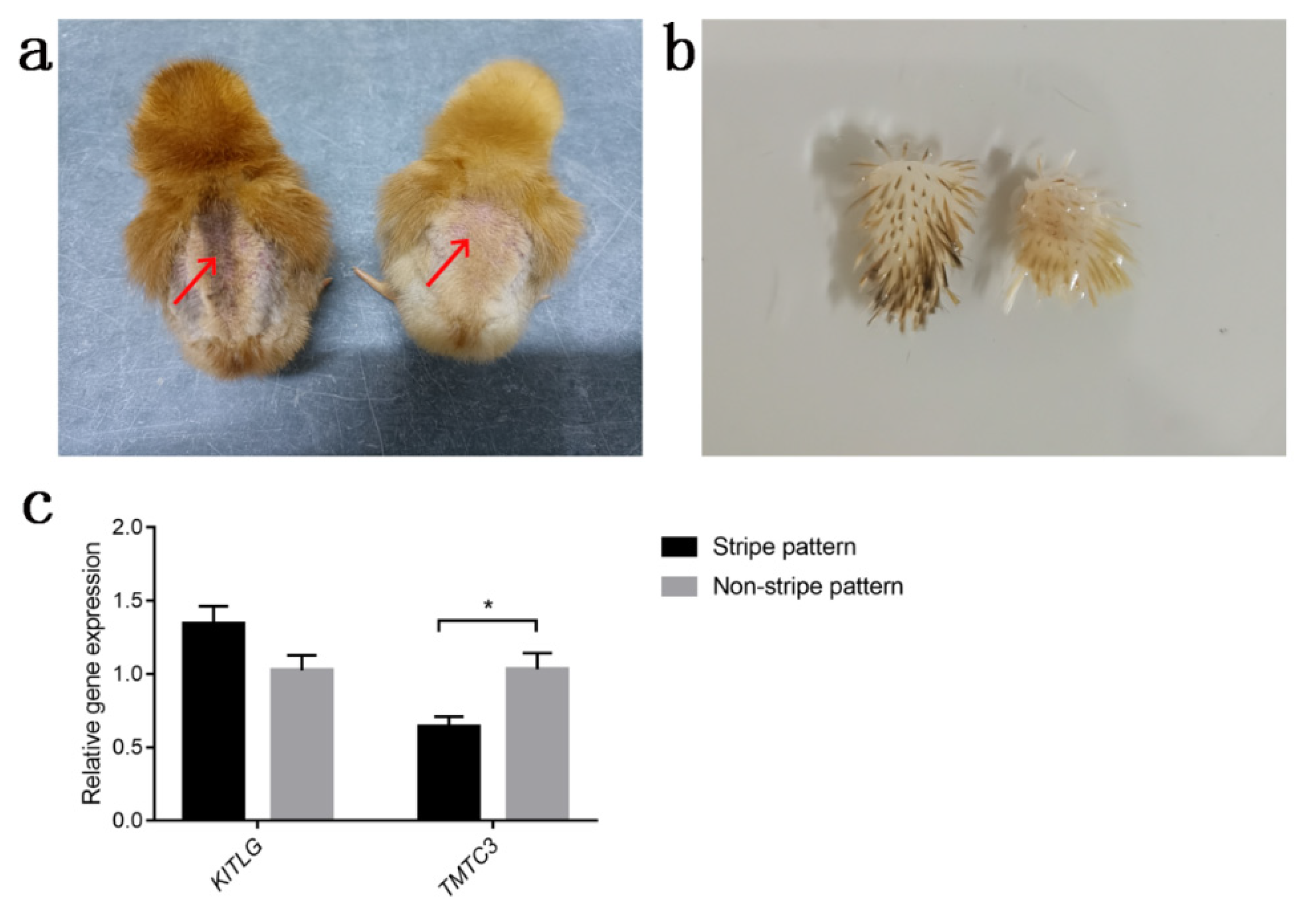
3.3. Quantitative Real-Time PCR
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cieslak, M.; Reissmann, M.; Hofreiter, M.; Ludwig, A. Colours of domestication. Biol. Rev. Camb. Philos. Soc. 2011, 86, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Chuong, C.M. Avian pigment pattern formation: Developmental control of macro- (across the body) and micro- (within a feather) level of pigment patterns. Front. Cell Dev. Biol. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Roulin, A.; Ducrest, A.L. Genetics of colouration in birds. Semin. Cell Dev. Biol. 2013, 24, 594–608. [Google Scholar] [CrossRef]
- Sheppy, A. The colour of domestication and the designer chicken. Opt. Laser Technol. 2011, 43, 295–301. [Google Scholar] [CrossRef]
- Roulin, A. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 2004, 79, 815–848. [Google Scholar] [CrossRef]
- Boswell, T.; Takeuchi, S. Recent developments in our understanding of the avian melanocortin system: Its involvement in the regulation of pigmentation and energy homeostasis. Peptides 2005, 26, 1733–1743. [Google Scholar] [CrossRef]
- Hoekstra, H.E. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 2006, 97, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Zi, J.; Yu, X.D.; Li, Y.Z.; Hu, X.H.; Xu, C.; Wang, X.J.; Liu, X.H.; Fu, R.T. Coloration strategies in peacock feathers. Proc. Natl. Acad. Sci. USA 2003, 100, 12576–12578. [Google Scholar] [CrossRef] [Green Version]
- Prum, R.O.; Torres, R.; Williamson, S.; Dyck, J. Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proc. R. Soc. B Boil. Sci. 1999, 266, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Prum, R.O.; Torres, R.H.; Williamson, S.; Dyck, J. Coherent light scattering by blue feather barbs. Nature 1998, 396, 28–29. [Google Scholar] [CrossRef]
- Kerje, S.; Lind, J.; Schutz, K.; Jensen, P.; Andersson, L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003, 34, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kerje, S.; Sharma, P.; Gunnarsson, U.; Kim, H.; Bagchi, S.; Fredriksson, R.; Schutz, K.; Jensen, P.; von Heijne, G.; Okimoto, R.; et al. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics 2004, 168, 1507–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.M.; Coville, J.L.; Coquerelle, G.; Gourichon, D.; Oulmouden, A.; Tixier-Boichard, M. Complete association between a retroviral insertion in the tyrosinase gene and the recessive white mutation in chickens. BMC Genom. 2006, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnarsson, U.; Hellstrom, A.R.; Tixier-Boichard, M.; Minvielle, F.; Bed’hom, B.; Ito, S.; Jensen, P.; Rattink, A.; Vereijken, A.; Andersson, L. Mutations in SLC45A2 cause plumage color variation in chicken and Japanese quail. Genetics 2007, 175, 867–877. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, D.S.; Ring, H.; Sundstrom, E.; Cao, X.F.; Larsson, M.; Kerje, S.; Hoglund, A.; Fogelholm, J.; Wright, D.; Jemth, P.; et al. The evolution of Sex-linked barring alleles in chickens involves both regulatory and coding changes in CDKN2A. PLoS Genet. 2017, 13, e1006665. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, U.; Kerje, S.; Bed’hom, B.; Sahlqvist, A.S.; Ekwall, O.; Tixier-Boichard, M.; Kampe, O.; Andersson, L. The dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell Melanoma Res. 2011, 24, 268–274. [Google Scholar] [CrossRef]
- Niwa, T.; Mochii, M.; Nakamura, A.; Shiojiri, N. Plumage pigmentation and expression of its regulatory genes during quail development-histochemical analysis using Bh (black at hatch) mutants. Mech. Dev. 2002, 118, 139–146. [Google Scholar] [CrossRef]
- Haupaix, N.; Curantz, C.; Bailleul, R.; Beck, S.; Robic, A.; Manceau, M. The periodic coloration in birds forms through a prepattern of somite origin. Science 2018, 361, eaar4777. [Google Scholar] [CrossRef] [Green Version]
- Haupaix, N.; Manceau, M. The embryonic origin of periodic color patterns. Dev. Biol. 2020, 460, 70–76. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. bioRxiv 2014. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Physiological and Genetic Analysis of the Formation of Blue Eggshell Pigments in Chicken; China Agricultural University: Beijing, China, 2008. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ng, C.S.; Li, W.H. Genetic and molecular basis of feather diversity in birds. Genome Biol. Evol. 2018, 10, 2572–2586. [Google Scholar] [CrossRef]
- Marchini, J.; Cardon, L.R.; Phillips, M.S.; Donnelly, P. The effects of human population structure on large genetic association studies. Nat. Genet. 2004, 36, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Besmer, P. The kit ligand encoded at the murine Steel locus: A pleiotropic growth and differentiation factor. Curr. Opin. Cell Biol. 1991, 3, 939–946. [Google Scholar] [CrossRef]
- Yoshida, H.; Kunisada, T.; Grimm, T.; Nishimura, E.K.; Nishioka, E.; Nishikawa, S.I. Review: Melanocyte migration and survival controlled by SCF/c-kit expression. J. Investig. Dermatol. Symp. Proc. 2001, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, M.A.F.; Jordan, S.A.; Budd, P.S.; Jackson, I.J. Activation of the receptor tyrosine kinase kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol. 1997, 192, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehrle-Haller, B. The role of kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, N.; Berx, G. From neural crest cells to melanocytes: Cellular plasticity during development and beyond. Cell. Mol. Life Sci. 2019, 76, 1919–1934. [Google Scholar] [CrossRef]
- Kunisada, T.; Yoshida, H.; Yamazaki, H.; Miyamoto, A.; Hemmi, H.; Nishimura, E.; Shultz, L.D.; Nishikawa, S.I.; Hayashi, S.I. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development 1998, 125, 2915–2923. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kono, M.; Akiyama, M. Pigmented macules in Waardenburg syndrome type 2 due to KITLG mutation. Pigment Cell Melanoma Res. 2017, 30, 501–504. [Google Scholar] [CrossRef]
- Amyere, M.; Vogt, T.; Hoo, J.; Brandrup, F.; Bygum, A.; Boon, L.; Vikkula, M. KITLG mutations cause familial progressive hyper- and hypopigmentation. J. Investig. Dermatol. 2011, 131, 1234–1239. [Google Scholar] [CrossRef]
- Cuell, A.; Bansal, N.; Cole, T.; Kaur, M.R.; Lee, J.; Loffeld, A.; Moss, C.; O’Donnell, M.; Takeichi, T.; Thind, C.K.; et al. Familial progressive hyper- and hypopigmentation and malignancy in two families with new mutations in KITLG. Clin. Exp. Dermatol. 2015, 40, 860–864. [Google Scholar] [CrossRef]
- Sulem, P.; Gudbjartsson, D.F.; Stacey, S.N.; Helgason, A.; Rafnar, T.; Magnusson, K.P.; Manolescu, A.; Karason, A.; Palsson, A.; Thorleifsson, G.; et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007, 39, 1443–1452. [Google Scholar] [CrossRef]
- Guenther, C.A.; Tasic, B.; Luo, L.Q.; Bedell, M.A.; Kingsley, D.M. A molecular basis for classic blond hair color in Europeans. Nat. Genet. 2014, 46, 748–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weich, K.; Affolter, V.; York, D.; Rebhun, R.; Grahn, R.; Kallenberg, A.; Bannasch, D. Pigment intensity in dogs is associated with a copy number variant upstream of KITLG. Genes 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talenti, A.; Bertolini, F.; Williams, J.; Moaeen-ud-Din, M.; Frattini, S.; Coizet, B.; Pagnacco, G.; Reecy, J.; Rothschild, M.F.; Crepaldi, P.; et al. Genomic analysis suggests KITLG is responsible for a roan pattern in two Pakistani goat breeds. J. Hered. 2018, 109, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Jerber, J.; Zaki, M.S.; Al-Aama, J.Y.; Rosti, R.O.; Ben-Omran, T.; Dikoglu, E.; Silhavy, J.L.; Caglar, C.; Musaev, D.; Albrecht, B.; et al. Biallelic mutations in TMTC3, encoding a transmembrane and TPR-Containing protein, lead to cobblestone lissencephaly. Am. J. Hum. Genet. 2016, 99, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.B.; Sunryd, J.C.; Mathavan, K.; Weir, E.; Larsen, I.S.B.; Halim, A.; Clausen, H.; Cousin, H.; Alfandari, D.; Hebert, D.N. Endoplasmic reticulum transmembrane protein TMTC3 contributes to O-mannosylation of E-cadherin, cellular adherence, and embryonic gastrulation. Mol. Biol. Cell 2020, 31, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tajima, A.; Kiguchi, M.; Kogusuri, S.; Fujii, A.; Sato, T.; Nozawa, S.; Yoshiike, M.; Mieno, M.; Kojo, K.; et al. Genome-wide association study of semen volume, sperm concentration, testis size, and plasma inhibin B levels. J. Hum. Genet. 2020, 65, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lamichhaney, S.; Berglund, J.; Almen, M.S.; Maqbool, K.; Grabherr, M.; Martinez-Barrio, A.; Promerova, M.; Rubin, C.J.; Wang, C.; Zamani, N.; et al. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 2015, 518, 371–375. [Google Scholar] [CrossRef]
- Yuan, J.; Ju, Q.Q.; Zhu, J.; Jiang, Y.; Yang, X.C.; Liu, X.Y.; Ma, J.Y.; Sun, C.; Shi, J.H. RASSF9 promotes NSCLC cell proliferation by activating the MEK/ERK axis. Cell Death Discov. 2021, 7, 199. [Google Scholar] [CrossRef]
- Shi, H.; Ju, Q.Q.; Mao, Y.T.; Wang, Y.J.; Ding, J.; Liu, X.Y.; Tang, X.; Sun, C. TAK1 phosphorylates RASSF9 and inhibits esophageal squamous tumor cell proliferation by targeting the RAS/MEK/ERK axis. Adv. Sci. 2021, 8, 2001575. [Google Scholar] [CrossRef]
- Lee, C.M.; Yang, P.L.; Chen, L.C.; Chen, C.C.; Wu, S.C.; Cheng, H.Y.; Chang, Y.S. A novel role of RASSF9 in maintaining epidermal homeostasis. PLoS ONE 2011, 6, e17867. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Long, X.; Zhang, L.; Chen, J.; Liu, P.; Li, H.; Wei, F.; Yu, W.; Ren, X.; Yu, J.J.O. NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 70303–70322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, E.; Sottile, G.; Sardina, M.T.; Criscione, A.; Bordonaro, S.; Sutera, A.M.; Zumbo, A.; Portolano, B.; Mastrangelo, S. Genome-wide analyses reveal the regions involved in the phenotypic diversity in Sicilian pigs. Anim. Genet. 2020, 51, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.R.D.; Santos, D.J.D.; Fernandes, G.A.; Aspilcueta-Borquis, R.R.; do Nascimento, A.V.; Seno, L.D.; Tonhati, H.; de Oliveira, H.N. Genome-wide association studies for growth traits in buffaloes using the single step genomic BLUP. J. Appl. Genet. 2020, 61, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Friis, T.E.; Stephenson, S.; Xiao, Y.; Whitehead, J.; Hutmacher, D.W. A polymerase chain reaction-based method for isolating clones from a complimentary DNA library in sheep. Tissue Eng. Part C Methods 2014, 20, 780–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, E.K.; Yoshida, H.; Kunisada, T.; Nishikawa, S.I. Regulation of E- and P-cadherin expression correlated with melanocyte migration and diversification. Dev. Biol. 1999, 215, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Li, J.Y.; Ma, T.; Li, J.P.; Li, Y.M.; Jiang, H.Z.; Zhang, Q.L. MiR-27a regulates WNT3A and KITLG expression in Cashmere goats with different coat colors. Anim. Biotechnol. 2021, 32, 205–212. [Google Scholar] [CrossRef]
- Song, X.C.; Xu, C.; Liu, Z.Y.; Yue, Z.G.; Liu, L.L.; Yang, T.G.; Cong, B.; Yang, F.H. Comparative transcriptome analysis of mink (Neovison vison) skin reveals the key genes involved in the melanogenesis of black and white coat colour. Sci. Rep. 2017, 7, 12461. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Li, J.; Zhao, F.; Zhou, M.; Wang, J.; Xiao, T. Transcriptome analysis of genes potentially associated with white and black plumage formation in Chinese indigenous ducks (Anas platyrhynchos). Br. Poult. Sci. 2022, 18, 1–9. [Google Scholar] [CrossRef]
| Gene | Primers | Sequence (5′–3′) | Size (bp) | Tm (°C) |
|---|---|---|---|---|
| TMTC3 | TMTC3-F | TTTGATTGTCTTCAGTCTCCG | 132 | 54 |
| TMTC3-R | CGTTCTGCTACCACAAATCCA | |||
| KITLG | KITLG-F | AAGAGGCACTTGGCTTCATTAG | 138 | 59 |
| KITLG-R | TTTCTGGTCTGGACTTAGGATG | |||
| GAPDH | GAPDH-F | ATACACAGAGGACCAGGTTG | 130 | 59 |
| GAPDH-R | AAACTCATTGTCATACCAGG |
| Chr. | Position (bp) | N_Sig a | Lead Variant b | p c | Genomic Location | Corresponding Genes |
|---|---|---|---|---|---|---|
| 1 | 41785264 | 1 | 41785264 | 7.83 × 10−6 | exon | TSPAN19 |
| 1 | 41799389–41889944 | 58 | 41847422 | 9.24 × 10−7 | intron; exon; downstream | ENSGALG00000044478 |
| 1 | 41892428 | 1 | 41892428 | 3.89 × 10−6 | Intergenic | ENSGALG00000044478-ALX1 |
| 1 | 41893987–41921738 | 18 | 41916556 | 1.06 × 10−7 | upstream; intron; exon; downstream | ALX1 |
| 1 | 41902222–41911298 | 7 | 41902973 | 3.89 × 10−6 | upstream; downstream | ENSGALG00000047575 |
| 1 | 41924948–42155127 | 180 | 42062678 | 1.57 × 10−7 | intergenic | ALX1-RASSF9 |
| 1 | 42156048–42190437 | 19 | 42156048 | 1.91 × 10−6 | upstream; exon; intron; downstream | RASSF9 |
| 1 | 42198934–42201800 | 3 | 42198934; 42200190 | 3.89 × 10−6 | intergenic | RASSF9-NTS |
| 1 | 42204316–42225096 | 13 | 42207440 | 9.81 × 10−7 | upstream; intron; downstream | NTS |
| 1 | 42226797–42241774 | 5 | 42232409 | 3.89 × 10−6 | intergenic | NTS-MGAT4C |
| 1 | 42247263–42362279 | 116 | 42305962; 42318478 | 2.25 × 10−7 | upstream; intron; downstream | MGAT4C |
| 1 | 42363559–42380754 | 11 | 42363559; 42372167 | 1.91 × 10−6 | intergenic | MGAT4C-ENSGALG00000045907 |
| 1 | 42387035–42392260 | 4 | 42387035 | 1.91 × 10−6 | upstream; downstream | ENSGALG00000045907 |
| 1 | 42395470–42402424 | 3 | 42402424 | 1.91 × 10−6 | intergenic | ENSGALG00000045907-ENSGALG00000053372 |
| 1 | 42417397–42483449 | 14 | 42466857 | 4.83 × 10−8 | exon; intron; upstream | ENSGALG00000053372 |
| 1 | 42484399–42808126 | 237 | 42484399 | 1.56 × 10−5 | intergenic | ENSGALG00000053372-C12orf50 |
| 1 | 42808720–42827406 | 22 | 42816606 | 1.91 × 10−6 | upstream; intron; exon; downstream | C12orf50 |
| 1 | 42828049–42836552 | 13 | 42835185 | 4.67 × 10−7 | upstream; intron; exon | C12orf29 |
| 1 | 42837178–42854277 | 17 | 42839207 | 4.73 × 10−7 | upstream; intron; exon | ENSGALG00000049176 |
| 1 | 42857947 | 1 | 42857947 | 2.14 × 10−5 | intergenic | ENSGALG00000049176-ENSGALG00000051263 |
| 1 | 42861965–42872432 | 10 | 42861965 | 9.24 × 10−7 | upstream; intron; downstream | ENSGALG00000051263 |
| 1 | 42872979–42883280 | 14 | 42877886 | 9.24 × 10−7 | exon; intron | ENSGALG00000011177 |
| 1 | 42884076–42950258 | 74 | 42905449; 42926288 | 9.24 × 10−7 | upstream; intron; exon; downstream | TMTC3 |
| 1 | 42953794–42977208 | 12 | 42973895 | 1.91 × 10−6 | intergenic | TMTC3-KITLG |
| 1 | 43028225–43047548 | 2 | 43047548 | 3.89 × 10−6 | intron | KITLG |
| 4 | 21698048 | 1 | 21698048 | 1.60 × 10−6 | intergenic | ENSGALG00000048717-GASK1B |
| 25 | 3002653 | 1 | 3002653 | 7.25 × 10−6 | upstream | KCNN3 |
| Association Genes | Position (bp) | Full Name | Biological Functions |
|---|---|---|---|
| KITLG | GGA1 43015486–43066975 | KIT ligand | Melanoblasts/melanocytes proliferation, differentiation, migration, colonization, melanin production, gametogenesis, and hematopoiesis [32,33,34,35,36,37]. |
| TMTC3 | GGA1 42888363–42945679 | Transmembrane and tetratricopeptide repeat containing 3 | Cellular adherence, cell migration, and embryogenesis [45,46]. |
| TSPAN19 | GGA1 41773256–41785441 | Tetraspanin 19 | Plasma inhibin B levels [47]. |
| ALX1 | GGA1 41898277–41919541 | ALX homeobox 1 | Effect craniofacial development and related to beak shape in Darwin’s finches [48]. |
| RASSF9 | GGA1 42160804–42190042 | Ras association domain family member 9 | Regulating tumor proliferation and maintainepidermal homeostasis [49,50,51]. |
| NTS | GGA1 42207171–42220099 | Neurotensin | Regulatory of the central nervous system and digestive system, and promoting tumor metastasis, etc. [52]. |
| MGAT4C | GGA1 42251047–42358204 | MGAT4 family member C | Related to animal growth traits [53,54]. |
| C12orf50 | GGA1 42813465–42822840 | C12orf50 homolog | Unclear |
| C12orf29 | GGA1 42829927–42836694 | C12orf29 homolog | Skeletal biology [55]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Zhou, J.; Li, J.; Zhao, X.; Zheng, L.; Bao, H.; Wu, C. Genome-Wide Association Study Identifies Candidate Genes for Stripe Pattern Feather Color of Rhode Island Red Chicks. Genes 2022, 13, 1511. https://doi.org/10.3390/genes13091511
Shen Q, Zhou J, Li J, Zhao X, Zheng L, Bao H, Wu C. Genome-Wide Association Study Identifies Candidate Genes for Stripe Pattern Feather Color of Rhode Island Red Chicks. Genes. 2022; 13(9):1511. https://doi.org/10.3390/genes13091511
Chicago/Turabian StyleShen, Qingmiao, Jieke Zhou, Junying Li, Xiaoyu Zhao, Lijie Zheng, Haigang Bao, and Changxin Wu. 2022. "Genome-Wide Association Study Identifies Candidate Genes for Stripe Pattern Feather Color of Rhode Island Red Chicks" Genes 13, no. 9: 1511. https://doi.org/10.3390/genes13091511
APA StyleShen, Q., Zhou, J., Li, J., Zhao, X., Zheng, L., Bao, H., & Wu, C. (2022). Genome-Wide Association Study Identifies Candidate Genes for Stripe Pattern Feather Color of Rhode Island Red Chicks. Genes, 13(9), 1511. https://doi.org/10.3390/genes13091511






