MYOZ1 Gene Promotes Muscle Growth and Development in Meat Ducks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. DNA and Total RNA Extraction
2.3. cDNA Synthesis
2.4. Reverse Transcription-Quantitative Polymerase Chain Reaction (qRT-PCR)
2.5. Extraction of Sample Protein to Be Tested
2.6. Western Blot
2.7. Design of Primers for SNP Screening
2.8. Genotype Frequency and Gene Frequency
2.9. Statistical Analyses
3. Results
3.1. Feed Conversion Ratio (FCR, Feed Intake/Weight Gain) in the Highest (HF) and Lowest Feed (LF) Converter Groups
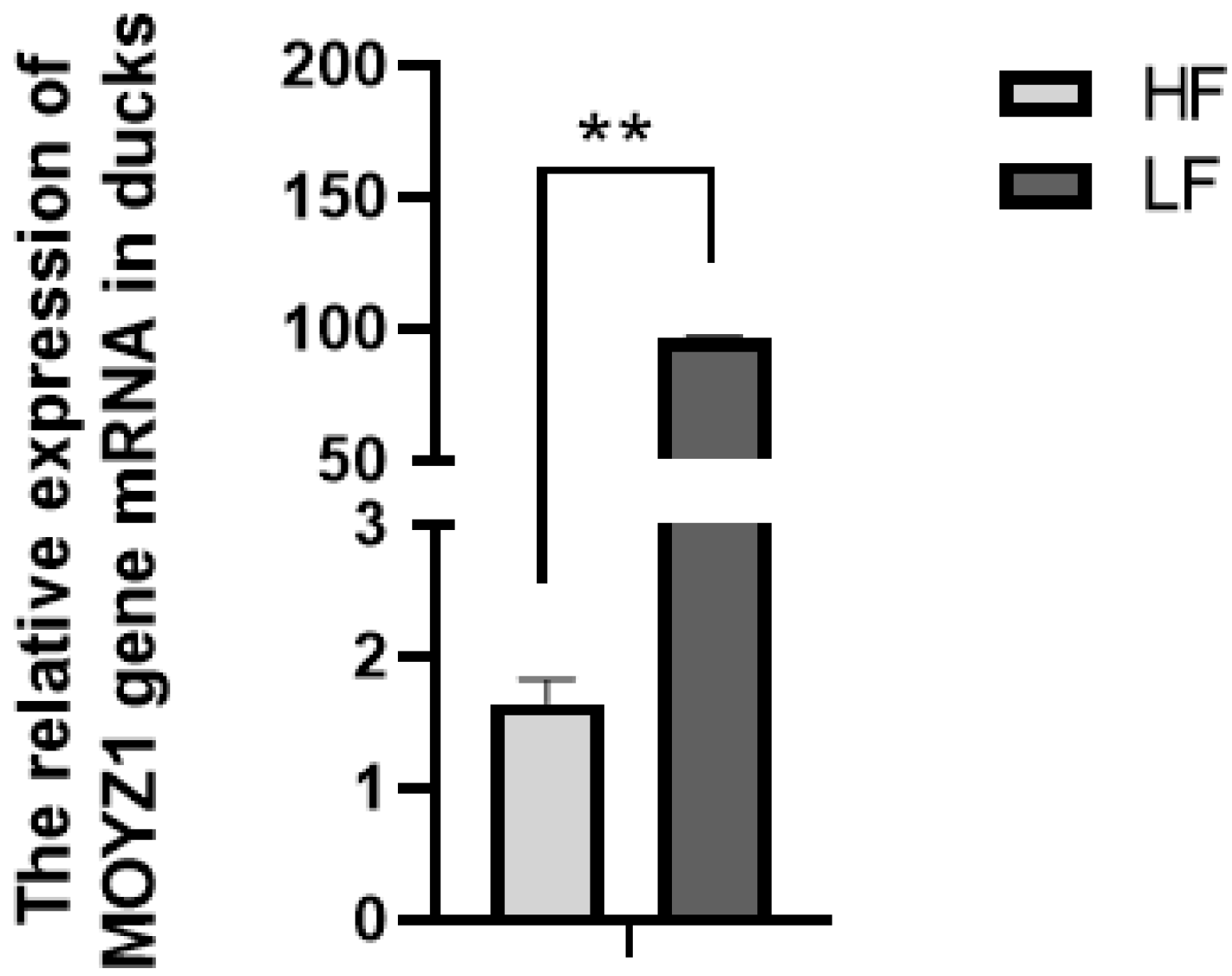
3.2. Relative Expression of MYOZ1 in the Different Feed Conversion Ratio Groups
3.3. Relative Expression of MYOZ1 Protein in HF and LF Groups
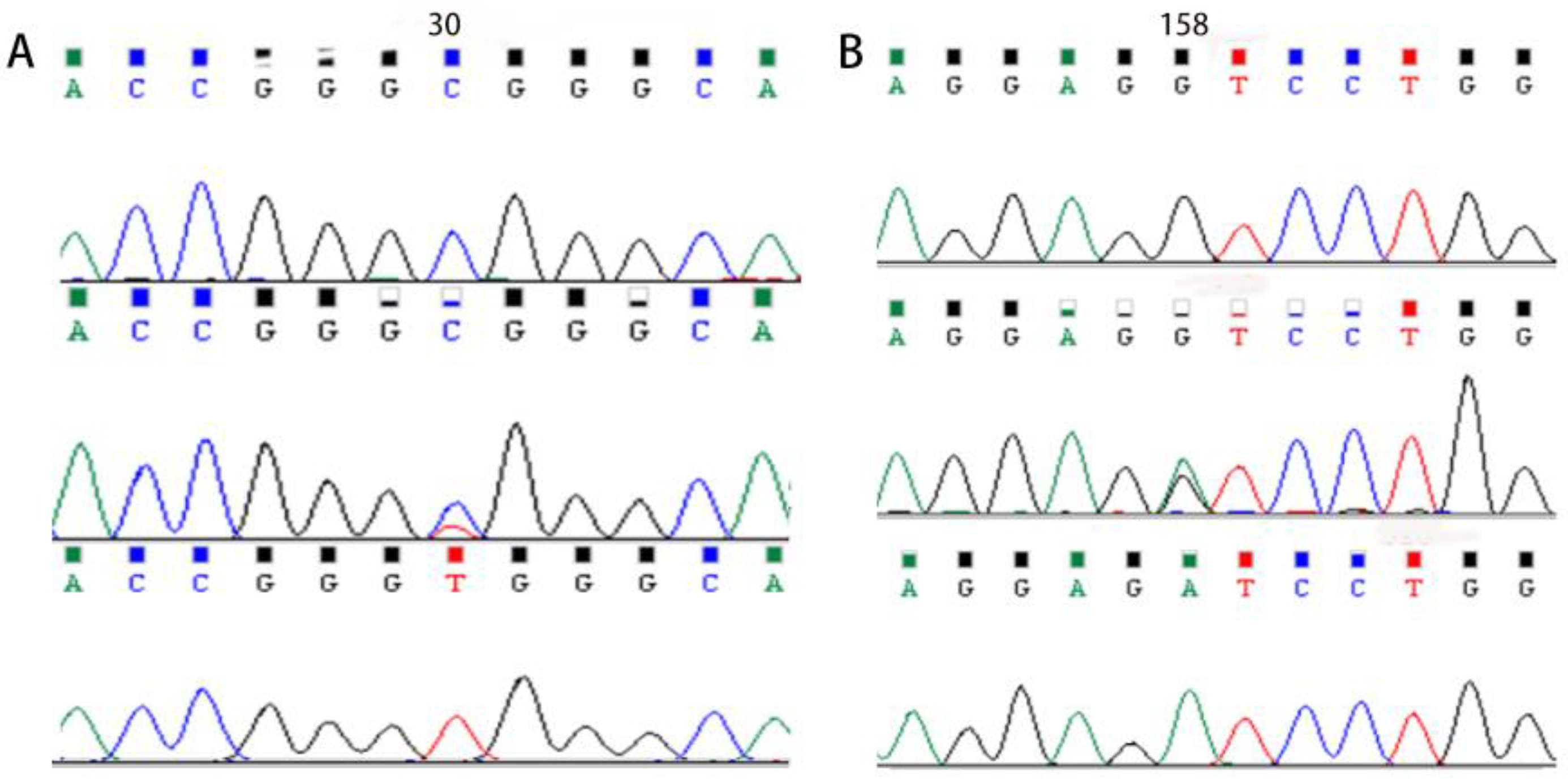
3.4. Analysis of Single Nucleotide Polymorphisms in MYOZ1
3.5. Genetic Diversity
3.6. Feed Conversion Ratio of Different Genotypes of Meat Ducks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Jia, Y.; Xu, L.; Ruan, Y. Research progress on molecular genetic regulation of skeletal muscle growth and development in beef cattle. China Cattle Sci. 2016, 42, 57–60. [Google Scholar]
- Chen, F.; Wu, P.; Shen, M.; He, M.; Chen, L.; Qiu, C.; Shi, H.; Zhang, T.; Wang, J.; Xie, K.; et al. Transcriptome analysis of differentially expressed genes related to the growth and development of the jinghai yellow chicken. Genes 2019, 10, 539. [Google Scholar] [CrossRef]
- Sharlo, K.; Paramonova, I.; Turtikova, O.; Tyganov, S.; Shenkman, B. Plantar mechanical stimulation prevents calcineurin-NFATc1 inactivation and slow-to-fast fiber type shift in rat soleus muscle under hindlimb unloading. J. Appl. Physiol. 2019, 126, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Transcriptome and Genome-Wide Methylation Analysis of Skeletal Muscle in Different Developmental Stages of Chinese and Foreign Pig Embryos. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Ou, X.; Li, X.; Zhong, Z.; Zhang, X. The relationship between muscle fiber characteristics and muscle quality in pigs and differences between breeds and genderbvns. Xinjiang Agric. Sci. 2019, 56, 195–202. [Google Scholar]
- Kawai, T.; Yu, M. The relationship between the thickness of pork muscle fibers and meat quality. Foreign Anim. Husb. 1983, 3, 51–54+64. [Google Scholar]
- Chen, B.; Qiu, L.; Wan, F.; Wang, H.; Zhang, Y.; Wang, Z.; Chen, G.; Chang, G. Comparative analysis of SNPs and INDELs of TAP1 gene in different chicken breeds. Chin. Poult. 2020, 42, 24–29. [Google Scholar]
- Frey, N.; Richardson, J.A.; Olson, E.N. Calsarcins, A novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 14632–14637. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, D.; Li, N.; Li, H.; Deng, X.; Wu, C. Association between single nucleotide polymorphisms of chicken Myostatin gene and skeletal muscle and fat growth. China Sci. C 2003, 33, 273–280. [Google Scholar]
- Xiang, L.; Sun, L.; Jiang, H. An overview of studies on the regulation of animal production performance by the myogenic determinant (MyoD) gene family. Mod. Anim. Husb. Vet. Med. 2016, 2, 54–58. [Google Scholar]
- Cheng, B.; Li, L.; Wang, L.; Zhang, H. Research progress of MEF2 gene family. China Anim. Husb. 2012, 48, 70–74. [Google Scholar]
- Wang, Z.; Yang, C.; Liang, W.; Song, Q.; Yan, D.; Tian, H.; Bai, H.; Jiang, Y.; Chang, G.; Chen, G. Research on the developmental characteristics of pectoral muscles in the Cherry Valley duck embryo. China Poult. 2021, 43, 103. [Google Scholar]
- Yu, C.; Jiang, X.; Du, H.; Li, Q.; Zhang, Z.; Qiu, M.; Yang, C. Research and Development Trends of Breeder Genetic Evaluation and Selection Technology in Broilers. China Poult. 2020, 42, 73–78. [Google Scholar]
- Zhao, X. A Preliminary Study on the Function of MYOZ1 Gene in Muscle Fiber Development. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2011. [Google Scholar]
- Ahmad, F.; Gonzalez, O.; Ramagli, L.; Xu, J.; Siciliano, M.J.; Bachinski, L.L.; Roberts, R. Identification and characterization of a novel gene (C4 or f5) located on human chromosome 4q with specific expression in cardiac and skeletal muscle. Genomics 2000, 70, 347–353. [Google Scholar] [CrossRef]
- Faulkner, G.; Pallavicini, A.; Comelli, A.; Salamon, M.; Bortoletto, G.; Ievolella, C.; Trevisan, S.; Kojić, S.; Dalla Vecchia, F.; Laveder, P.; et al. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J. Biol. Chem. 2000, 275, 41234–41242. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Xu, H.; Ma, E.; Ye, F.; Cui, C.; Zhu, Q.; Zhao, X.; Li, D.; Yin, H.; Wang, Y. Chicken MYOZ1 gene expression may be regulated by MuR F1. In Proceedings of the 19th Academic Symposium of the 2018 Annual Academic Conference of China Animal Husbandry and Veterinary Society Episode, Guangxi, China, 21 November 2018. [Google Scholar]
- Luo, B.; Ye, M.; Xu, H.; Ma, E.; Ye, F.; Cui, C.; Zhu, Q.; Zhao, X.; Yin, H.; Diyan, L.; et al. Expression analysis, single-nucleotide polymorphisms of the MYOZ1 gene and their association with carcase and meat quality traits in chickens. Ital. J. Anim. Sci. 2018, 17, 907–915. [Google Scholar] [CrossRef]
- Frey, N.; Frank, D.; Lippl, S.; Kuhn, C.; Kögler, H.; Barrientos, T.; Rohr, C.; Will, R.; Müller, O.J.; Weiler, H.; et al. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J. Clin. Investig. 2008, 118, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chai, J.; Shang, Y.; Li, Y.; Chen, R.; Jia, J.; Jiang, S.; Peng, J. Swine PPAR-γ2 expression upregulated in skeletal muscle of transgenic mice via the swine Myozenin-1 gene promoter. Transgenic Res. 2015, 24, 409–420. [Google Scholar] [CrossRef]
- Takada, F.; Woude, D.L.V.; Tong, H.Q.; Thompson, T.G.; Watkins, S.C.; Kunkel, L.M.; Beggs, A.H. Myozenin: An alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc. Natl. Acad. Sci. USA 2001, 98, 1595–1600. [Google Scholar] [CrossRef]
- Ren, R.M.; Liu, H.; Zhao, S.H.; Cao, J.H. Targeting of miR-432 to myozenin1 to regulate myoblast proliferation and differentiation. Genet. Mol. Res. GMR 2016, 15, gmr1504931. [Google Scholar] [CrossRef]
- Wang, M.; Li, A.; Zhao, Z.; Zhang, Y.; Duan, M.; Wang, Y.; Li, S.; Zan, L. Activity of bovine MYOZ1 gene promoter. J. Northwest A F Univ. (Nat. Sci. Ed.) 2016, 44, 1–9. [Google Scholar]
- Arola, A.M.; Sanchez, X.; Murphy, R.T.; Hasle, E.; Li, H.; Elliott, P.M.; McKenna, W.J.; Towbin, J.A.; Bowles, N.E. Mutations in PDLIM3 and MYOZ1 encoding myocyte Z line proteins are infrequently found in idiopathic dilated cardiomyopathy. Mol. Genet. Metab. 2007, 90, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Song, Q.; Dong, B.; Bi, Y.; Bai, H.; Jiang, Y.; Chang, G.; Chen, G. Transcriptome Sequencing to Identify Important Genes and lncRNAs Regulating Abdominal Fat Deposition in Ducks. Animals 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cao, J.; Liu, G.; Zhang, H.; Liu, X. Comparative Transcriptome Profiling of Skeletal Muscle from Black Muscovy Duck at Different Growth Stages Using RNA-seq. Genes 2020, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W. Screening of Porcine Calsarcin-2 Binding Protein by Yeast Two-Hybrid Technique. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2009. [Google Scholar]
- Su, Y.; Liu, D.; Song, H.; Cao, R.; Ba, C.; Wang, H. Cloning and tissue expression profiling of the coding region of porcine Calsarcin-2 gene. J. Liaoning Med. Coll. 2008, 29, 385–388. [Google Scholar]
- Luo, B. Identification of Polymorphic Loci of Chicken MYOZ1 Gene and Its Role in Muscle Atrophy. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2019. [Google Scholar]
- Yoshimoto, Y.; Ikemoto-Uezumi, M.; Hitachi, K.; Fukada, S.I.; Uezumi, A. Methods for Accurate Assessment of Myofiber Maturity During Skeletal Muscle Regeneration. Front. Cell Dev. Biol. 2020, 8, 267. [Google Scholar] [CrossRef] [Green Version]

| Name | Primers (5′→3′) | CT (°C) | Fragment Size (bp) |
|---|---|---|---|
| MYOZ1 | Forward primer CCTGAGAGCAAAGCAGAGAAC Reverse primer CTTGCAAATAGAAAGGGTTGAAAAG | 60 | 100 |
| GAPDH | Forward primer GGTTGTCTCCTGCGACTTCA Reverse primer TCCTTGGATGCCATGTGGAC | 60 | 165 |
| Name | Primers (5′→3′) | CT (°C) | Fragment Size (bp) |
|---|---|---|---|
| MYOZ1-1 | Forward primer GTTGCCCTTGCTGATGTTCTG Reverse primer CGGAAGAATGAGGTTGTTACTCAAA | 58 | 534 |
| Group | Live Weight (kg) | FCR (kg/kg) |
|---|---|---|
| HF | 2.17 ± 0.30 A | 3.25 ± 0.25 A |
| LF | 2.37 ± 0.36 B | 2.37 ± 0.17 B |
| Site 1 | Group | Sample Size | Genotype Frequency (N/Frequency) | X2 Value (p Value) | Allele Frequency | |||
| CC | CT | TT | C | T | ||||
| Exon4 C30T | LF | 50 | 35/0.70 | 14/0.28 | 1/0.02 | 0.09 (0.77) | 0.84 | 0.16 |
| HF | 50 | 47/0.94 | 3/0.06 | 0/0.00 | 0.05 (0.83) | 0.97 | 0.03 | |
| LF vs. HF | p = 0.001 | |||||||
| Site 2 | Group | Sample Size | Genotype Frequency (N/Frequency) | X2 Value (p Value) | Allele Frequency | |||
| AA | AG | GG | A | G | ||||
| Exon4 G158A | LF | 50 | 5/0.10 | 18/0.36 | 27/0.54 | 0.57 (0.45) | 0.28 | 0.72 |
| HF | 50 | 10/0.20 | 30/0.60 | 10/0.20 | 2.00 (0.16) | 0.50 | 0.50 | |
| LF vs. HF | p = 0.001 | |||||||
| Site | Group | Number of Individuals | FCR (kg/kg) | Effect Value (p Value) |
|---|---|---|---|---|
| Exon4 C30T | TT | 1 | 2.50 ± 0.00 A | 0.0.2450 (9.68 × 10−6) |
| CT | 17 | 2.51 ± 0.53 A | ||
| CC | 82 | 2.88 ± 0.50 B | ||
| Exon4 G158A | GG | 37 | 2.53 ± 0.38 A | 0.6014 (0.003905) |
| AG | 48 | 2.95 ± 0.45 B | ||
| AA | 15 | 3.06 ± 0.53 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Wu, Y.; Bi, Y.; Bai, H.; Jiang, Y.; Chen, G.; Chang, G.; Wang, Z. MYOZ1 Gene Promotes Muscle Growth and Development in Meat Ducks. Genes 2022, 13, 1574. https://doi.org/10.3390/genes13091574
Zhou T, Wu Y, Bi Y, Bai H, Jiang Y, Chen G, Chang G, Wang Z. MYOZ1 Gene Promotes Muscle Growth and Development in Meat Ducks. Genes. 2022; 13(9):1574. https://doi.org/10.3390/genes13091574
Chicago/Turabian StyleZhou, Tingting, Yijing Wu, Yulin Bi, Hao Bai, Yong Jiang, Guohong Chen, Guobin Chang, and Zhixiu Wang. 2022. "MYOZ1 Gene Promotes Muscle Growth and Development in Meat Ducks" Genes 13, no. 9: 1574. https://doi.org/10.3390/genes13091574






