Vasovagal Syncope Is Associated with Variants in Genes Involved in Neurohumoral Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DNA Extraction and Genotyping
2.3. Statistical Analysis
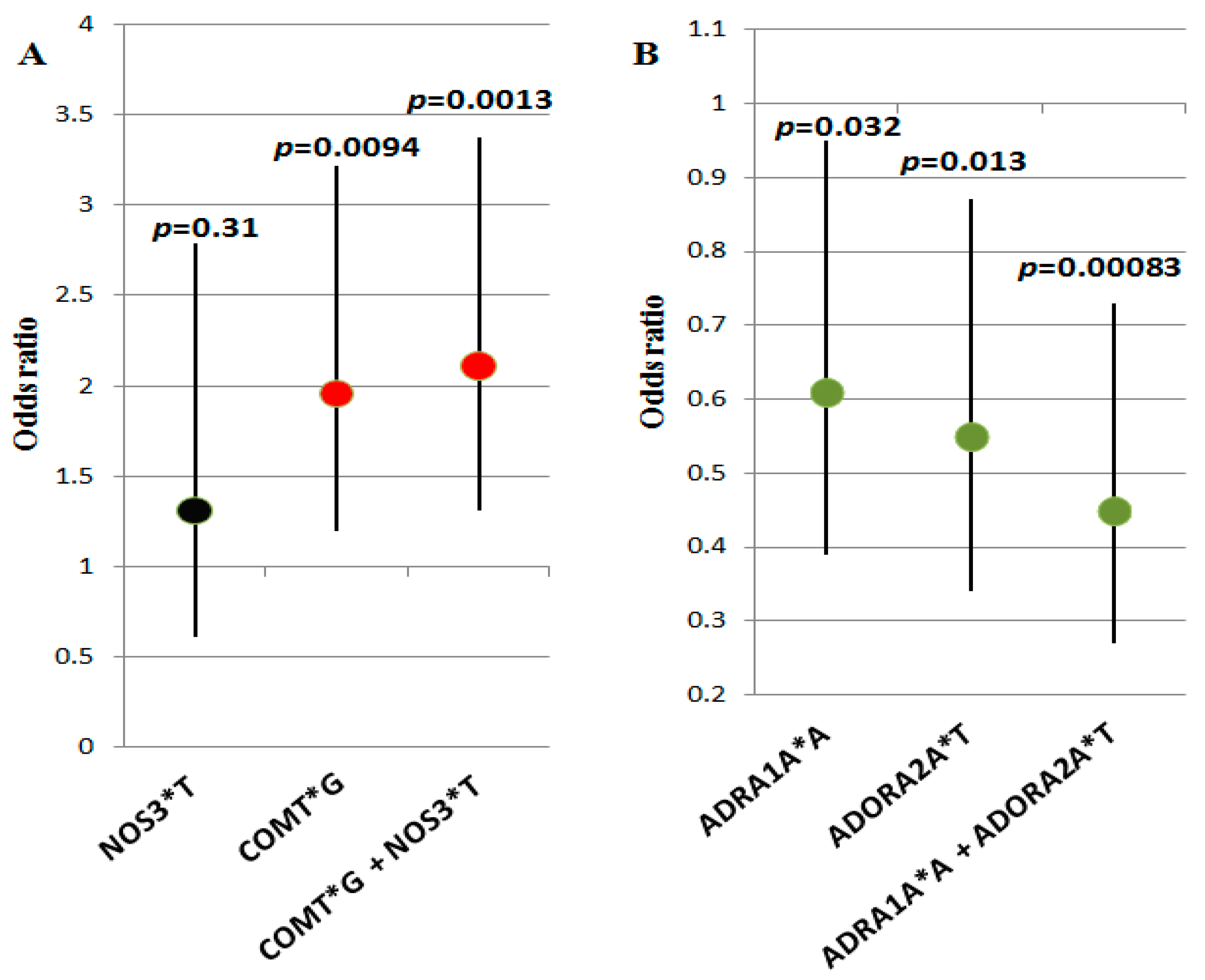
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brignole, M.; Moya, A.; De Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martínez, A.M.; et al. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, e43–e80. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, A.V.; Kuchinskaya, E.A.; Kiktev, V.G.; Kheimets, G.I. Treatment of Vasovagal Syncope Associated with Asystole: Literature Review and Case Report of Long-term Follow-up. Ration. Pharmacother. Cardiol. 2021, 17, 315–322. [Google Scholar] [CrossRef]
- Matveeva, N.; Titov, B.; Bazyleva, E.; Pevzner, A.; Favorova, O. Towards Understanding the Genetic Nature of Vasovagal Syncope. Int. J. Mol. Sci. 2021, 22, 10316. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Gerull, B. Genetic markers of vasovagal syncope. Auton. Neurosci. 2021, 235, 102871. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Sandhu, R.K. The Search for the Genes of Vasovagal Syncope. Front. Cardiovasc. Med. 2019, 6, 175. [Google Scholar] [CrossRef]
- Benditt, D.G.; Van Dijk, J.G.; Krishnappa, D.; Adkisson, W.O.; Sakaguchi, S. Neurohormones in the Pathophysiology of Vasovagal Syncope in Adults. Front. Cardiovasc. Med. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, A.V.; Kuchinskaia, E.A.; Vershuta, E.V.; Al’Bitskaia, K.V.; Kheĭmets, G.I.; Tripoten’, M.I.; Moiseeva, N.M.; Rogoza, A.; Golitsin, S.P. Long-term orthostatic and bicycle ergometry exercise tests in differential diagnosis of syncopal conditions of unclear origin. Ter. Arkh. 2004, 76, 23–27. [Google Scholar]
- Haploview 4.2. Available online: http://www.broad.mit.edu/mpg/haploview/index.php (accessed on 7 June 2022).
- GraphPad Instat. Available online: http://www.graphpad.com/quickcalcs/index.cfm (accessed on 16 June 2022).
- APSampler. Available online: http://apsampler.sourceforge.net/ (accessed on 12 April 2022).
- Favorov, A.V.; Andreewski, T.V.; Sudomoina, M.A.; Favorova, O.; Parmigiani, G.; Ochs, M.F. A Markov Chain Monte Carlo Technique for Identification of Combinations of Allelic Variants Underlying Complex Diseases in Humans. Genetics 2005, 171, 2113–2121. [Google Scholar] [CrossRef]
- Albert, P.R. Transcriptional regulation of the 5-HT1A receptor: Implications for mental illness. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2402–2415. [Google Scholar] [CrossRef]
- Augeri, A.L.; Tsongalis, G.J.; Van Heest, J.L.; Maresh, C.M.; Thompson, P.D.; Pescatello, L.S. The endothelial nitric oxide synthase −786 T>C polymorphism and the exercise-induced blood pressure and nitric oxide responses among men with elevated blood pressure. Atherosclerosis 2009, 204, e28–e34. [Google Scholar] [CrossRef]
- Ikenouchi-Sugita, A.; Yoshimura, R.; Kishi, T.; Umene-Nakano, W.; Hori, H.; Hayashi, K.; Katsuki, A.; Ueda, N.; Iwata, N.; Nakamura, J. Three polymorphisms of the eNOS gene and plasma levels of metabolites of nitric oxide in depressed Japanese patients: A preliminary report: eNOS and depression. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Hoehe, M.R.; Berrettini, W.H.; Schwinn, D.A.; Hsieh, W.-T. A two-allele Pstl RFLP for the α-1C adrenergic receptor gene (ADRA1C). Hum. Mol. Genet. 1992, 1, 349. [Google Scholar] [CrossRef] [PubMed]
- Ahles, A.; Engelhardt, S. Polymorphic Variants of Adrenoceptors: Pharmacology, Physiology, and Role in Disease. Pharmacol. Rev. 2014, 66, 598–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef]
- Alsene, K.; Deckert, J.; Sand, P.; de Wit, H. Association Between A2a Receptor Gene Polymorphisms and Caffeine-Induced Anxiety. Neuropsychopharmacology 2003, 28, 1694–1702. [Google Scholar] [CrossRef]
- Childs, E.; Hohoff, C.; Deckert, J.; Xu, K.; Badner, J.; De Wit, H. Association between ADORA2A and DRD2 Polymorphisms and Caffeine-Induced Anxiety. Neuropsychopharmacology 2008, 33, 2791–2800. [Google Scholar] [CrossRef]
- Hohoff, C.; Mullings, E.L.; Heatherley, S.V.; Freitag, C.M.; Neumann, L.C.; Domschke, K.; Krakowitzky, P.; Rothermundt, M.; Keck, M.E.; Erhardt, A.; et al. Adenosine A2A receptor gene: Evidence for association of risk variants with panic disorder and anxious personality. J. Psychiatr. Res. 2010, 44, 930–937. [Google Scholar] [CrossRef]
- Benarroch, E.E. The autonomic nervous system: Basic anatomy and physiology. Contin. Lifelong Learn. Neurol. 2007, 13, 13–32. [Google Scholar] [CrossRef]
- Dabire, H. Central 5-hydroxytryptamine (5-HT) receptors in blood pressure regulation. Therapies 1991, 46, 421–429. [Google Scholar]
- Vanhoutte, P.M. Nitric Oxide: From Good to Bad. Ann. Vasc. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Poblete, I.; Fernandez, R.; Pedemonte, C.; Cortés, V.; Huidobro-Toro, J.P. NO production and eNOS phosphorylation induced by epinephrine through the activation of β-adrenoceptors. Am. J. Physiol. Circ. Physiol. 2009, 297, H134–H143. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pacheco, G.; González-Hermosillo, A.; Murata, C.; Yescas, P.; Espínola-Zavaleta, N.; Martínez, M.; Serrano, H. Arg347Cys polymorphism of α1a-adrenergic receptor in vasovagal syncope. Case–control study in a Mexican population. Auton. Neurosci. 2014, 183, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Márquez, M.F.; Fragoso, J.M.; Pérez-Pérez, D.; Cázares-Campos, I.; Totomoch-Serra, A.; Gómez-Flores, J.R.; Vargas-Alarcón, G. Polymorphisms in B-Adrenergic Receptors Are Associated with Increased Risk to Have a Positive Head-Up Tilt Table Test in Patients with Vasovagal Syncope. Rev. Investig. Clín. 2019, 71, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.; Forleo, C.; Iacoviello, M.; Guida, P.; D’Andria, V.; Favale, S. Lack of association between genetic polymorphisms affecting sympathetic activity and tilt-induced vasovagal syncope. Auton. Neurosci. 2010, 155, 98–103. [Google Scholar] [CrossRef]
- Żelazowska, M.; Lelonek, M.; Fendler, W.; Pietrucha, T. Arg389Gly β1-adrenergic receptor polymorphism and susceptibility to syncope during tilt test. Aoms 2014, 2, 240–245. [Google Scholar] [CrossRef]
- Márquez, M.; Hernández-Pacheco, G.; Hermosillo, A.G.; Gómez, J.R.; Cárdenas, M.; Vargas-Alarcón, G. The Arg389Gly β1-adrenergic receptor gene polymorphism and susceptibility to faint during head-up tilt test. Europace 2007, 9, 585–588. [Google Scholar] [CrossRef]
- Hernández-Pacheco, G.; Serrano, H.; Márquez, M.F.; Hermosillo, A.G.; Pérez-Vielma, N.; Sotomayor, A.; Ferreira-Vidal, A.D.; Salas-Silva, E.; Cárdenas, M. Genetic study of the vasovagal syncope associated to the Arg389Gly polymorphism of the β1 adrenergic receptor. Arch Cardiol. Mex. 2008, 78, 134–138. [Google Scholar]
- Saadjian, A.Y.; Gerolami, V.; Giorgi, R.; Mercier, L.; Berge-Lefranc, J.-L.; Paganelli, F.; Ibrahim, Z.; By, Y.; Guéant, J.L.; Lévy, S.; et al. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009, 30, 1510–1515. [Google Scholar] [CrossRef]
- Mitro, P.; Habalova, V.; Evin, L.; Muller, E.; Simurda, M.; Slaba, E.; Murin, P.; Valocik, G. Gene Polymorphism of the Adenosine A2a Receptor in Patients with Vasovagal Syncope. Pacing Clin. Electrophysiol. 2016, 39, 330–337. [Google Scholar] [CrossRef]
- Lvovs, D.; Favorova, O.O.; Favorov, A.V. A Polygenic Approach to the Study of Polygenic Diseases. Acta Nat. 2012, 4, 59–71. [Google Scholar] [CrossRef]
- Mason, D.A.; Moore, J.D.; Green, S.A.; Liggett, S.B. A Gain-of-function Polymorphism in a G-protein Coupling Domain of the Human β1-Adrenergic Receptor. J. Biol. Chem. 1999, 274, 12670–12674. [Google Scholar] [CrossRef] [PubMed]
- La Rosée, K.; Huntgeburth, M.; Rosenkranz, S.; Böhm, M.; Schnabel, P. The Arg389Gly β1-adrenoceptor gene polymorphism determines contractile response to catecholamines. Pharmacogenetics 2004, 14, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Steinberg, S.F. S49G and R389G polymorphisms of the β1-adrenergic receptor influence signaling via the cAMP-PKA and ERK pathways. Physiol. Genom. 2013, 45, 1186–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissinen, E.; Männistö, P.T. Biochemistry and Pharmacology of Catechol-O-Methyltransferase Inhibitors. Int. Rev. Neurobiol. 2010, 95, 73–118. [Google Scholar]

| Gene and Its Chromosomal Localization | rs ID and Polymorphism (Amino Acid Substitution) | Encoded Protein | Known (Putative) Effects on Level/Activity of the Product | Bioactive Molecules Mediating Neurohumoral Signal Transduction Pathways |
|---|---|---|---|---|
| HTR1A 5q12.3 | rs6295 −1019 G>C | Serotonin 1A receptor | SNP blocks the function of specific repressors Hes1, Hes5 and Deaf1, resulting in increased 5-HT1A autoreceptor expression in animal models and humans [12]. | Serotonin |
| NOS3 (eNOS) 7q36.1 | rs2070744 −786 T>C | Endothelial nitric oxide synthase | SNP affects the NOS3 transcription [13] and the plasma NO level [14]. | Nitric oxide |
| ADRA1A 8p21.2 | rs1048101 1039 A>G (Cys347Arg) | α 1A-adrenergic receptor | Amino acid substitution Cys347Arg affects receptor interactions with G-proteins and intracellular signal transduction [15]. | Epinephrine, norepinephrine |
| ADRB1 10q25.3 | rs1801253 1165 G>C (Gly389Arg) | β 1-adrenergic receptor | Amino acid substitution Gly389Arg affects receptor interactions with G-proteins and intracellular signal transduction [16]. | Epinephrine, norepinephrine |
| COMT 22q11.21 | rs4680 472 G>A (Val158Met) | Catechol-O- methyltransferase | Amino acid substitution Val158Met affects the activity of catechol-O-methyltransferase [17]. | Epinephrine, norepinephrine, dopamine |
| ADORA2A 22q11.23 | rs5751876 1083 C>T (Tyr361Tyr) | Adenosine A2A receptor | SNP is associated with a level of anxiety after caffeine consumption [18,19] and incidence of panic attacks [20]. | Adenosine |
| Alleles and Genotypes | Patients, n (%) N = 157 | Controls, n (%) N = 161 | p Value (pcorr) | OR (95% CI) for Significant Differences |
|---|---|---|---|---|
| ADRA1A rs1048101 | ||||
| Allele frequency | ||||
| A | 64 (20) | 91 (28) | 0.021 | 0.65 (0.45–0.94) |
| G | 250 (80) | 231 (72) | 0.021 | 1.54 (1.07–2.22) |
| Allele and genotype carriage frequency | ||||
| A (A/A + A/G) | 60 (38) | 81(50) | 0.032 | 0.61 (0.39–0.95) |
| G (G/G + A/G) | 153 (98) | 151 (94) | NS | – |
| A/A | 4 (2) | 10 (6) | NS | – |
| A/G | 56 (36) | 71 (44) | NS | – |
| G/G | 97 (62) | 80 (50) | 0.032 | 1.64 (1.05–2.56) |
| ADRB1 rs1801253 | ||||
| Allele frequency | ||||
| C | 176 (56) | 204 (63) | NS | – |
| G | 138 (44) | 118 (37) | NS | – |
| Allele and genotype carriage frequency | ||||
| C (C/C + C/G) | 127 (81) | 136 (84) | NS | – |
| G (G/G + C/G) | 108 (68) | 93 (58) | 0.048 | 1.61 (1.02–2.55) |
| C/C | 49 (32) | 68 (42) | 0.048 | 0.62 (0.4–0.98) |
| C/G | 78 (49) | 68 (42) | NS | – |
| G/G | 30 (19) | 25 (16) | NS | – |
| ADORA2A rs5751876 | ||||
| Allele frequency | ||||
| C | 195 (62) | 173 (54) | 0.037 | 1.41 (1.03–1.94) |
| T | 119 (38) | 149 (46) | 0.037 | 0.7 (0.51–0.97) |
| Allele and genotype carriage frequency | ||||
| C (C/C + C/T) | 131 (84) | 129 (81) | NS | |
| T (T/T + C/T) | 93 (59) | 117 (70) | 0.013 | 0.55 (0.34–0.87) |
| C/C | 64 (41) | 44 (30) | 0.013 | 1.83 (1.14–2.93) |
| C/T | 67 (43) | 85 (51) | NS | |
| T/T | 26 (16) | 32 (19) | NS | |
| COMT rs4680 | ||||
| Allele frequency | ||||
| A | 140 (45) | 181 (56) | 0.0043 (0.026) | 0.63 (0.46–0.86) |
| G | 174 (55) | 141 (44) | 0.0043 (0.026) | 1.6 (1.17–2.18) |
| Allele and genotype carriage frequency | ||||
| A (A/A + A/G) | 105 (68) | 123 (76) | NS | – |
| G (G/G + A/G) | 122 (78) | 103 (64) | 0.0094 | 1.96 (1.2–3.22) |
| A/A | 35 (22) | 58 (36) | 0.0094 | 0.51 (0.31–0.84) |
| A/G | 70 (46) | 65 (40) | NS | – |
| G/G | 52 (32) | 38 (24) | NS | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titov, B.; Matveeva, N.; Kulakova, O.; Baulina, N.; Bazyleva, E.; Kheymets, G.; Rogoza, A.; Pevzner, A.; Favorova, O. Vasovagal Syncope Is Associated with Variants in Genes Involved in Neurohumoral Signaling Pathways. Genes 2022, 13, 1653. https://doi.org/10.3390/genes13091653
Titov B, Matveeva N, Kulakova O, Baulina N, Bazyleva E, Kheymets G, Rogoza A, Pevzner A, Favorova O. Vasovagal Syncope Is Associated with Variants in Genes Involved in Neurohumoral Signaling Pathways. Genes. 2022; 13(9):1653. https://doi.org/10.3390/genes13091653
Chicago/Turabian StyleTitov, Boris, Natalya Matveeva, Olga Kulakova, Natalia Baulina, Elizaveta Bazyleva, Grigory Kheymets, Anatolii Rogoza, Alexander Pevzner, and Olga Favorova. 2022. "Vasovagal Syncope Is Associated with Variants in Genes Involved in Neurohumoral Signaling Pathways" Genes 13, no. 9: 1653. https://doi.org/10.3390/genes13091653
APA StyleTitov, B., Matveeva, N., Kulakova, O., Baulina, N., Bazyleva, E., Kheymets, G., Rogoza, A., Pevzner, A., & Favorova, O. (2022). Vasovagal Syncope Is Associated with Variants in Genes Involved in Neurohumoral Signaling Pathways. Genes, 13(9), 1653. https://doi.org/10.3390/genes13091653






