Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals
Abstract
:1. Introduction
2. The Methodology of TEI Studies
2.1. Mutation
2.2. Social Transmission
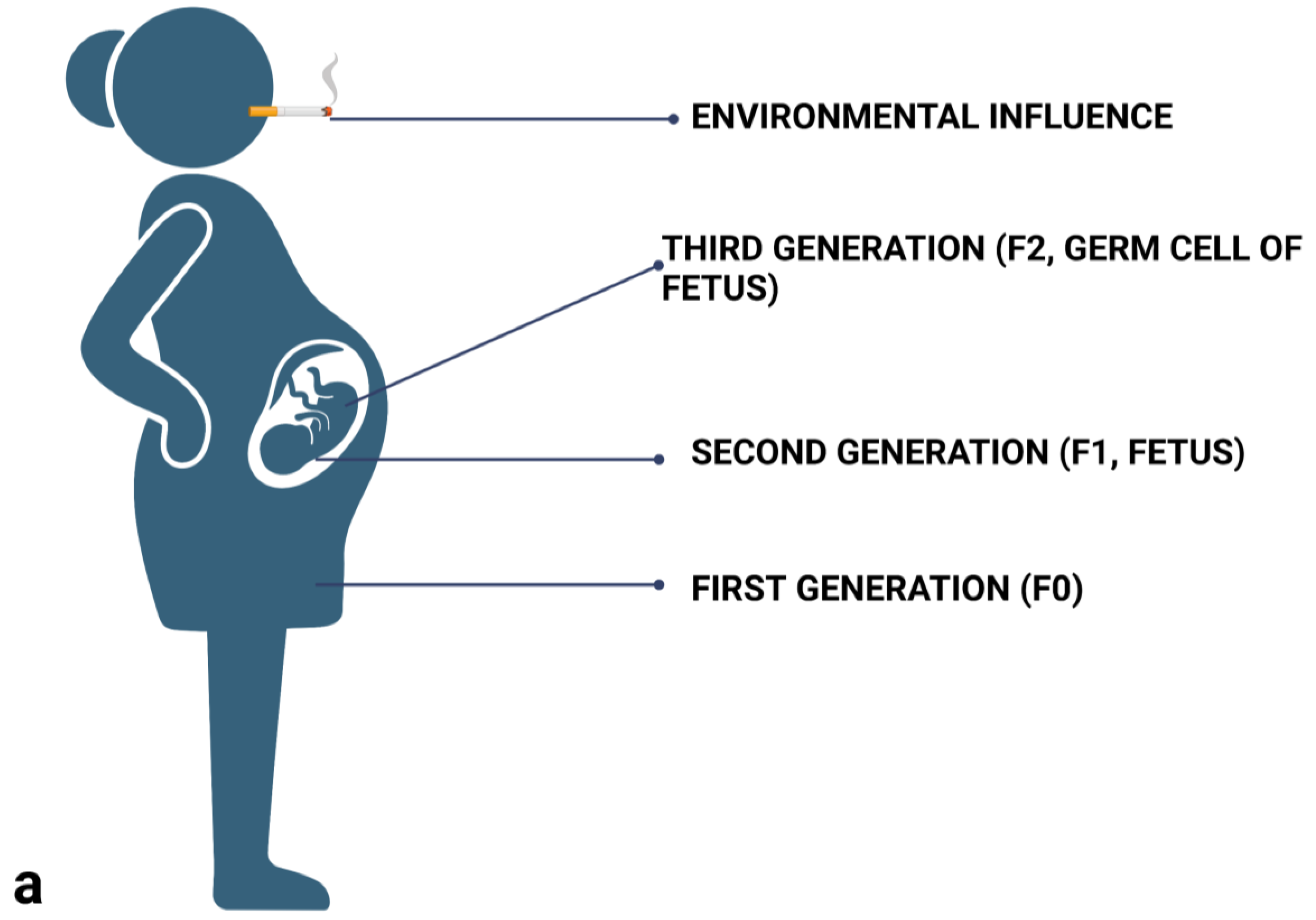
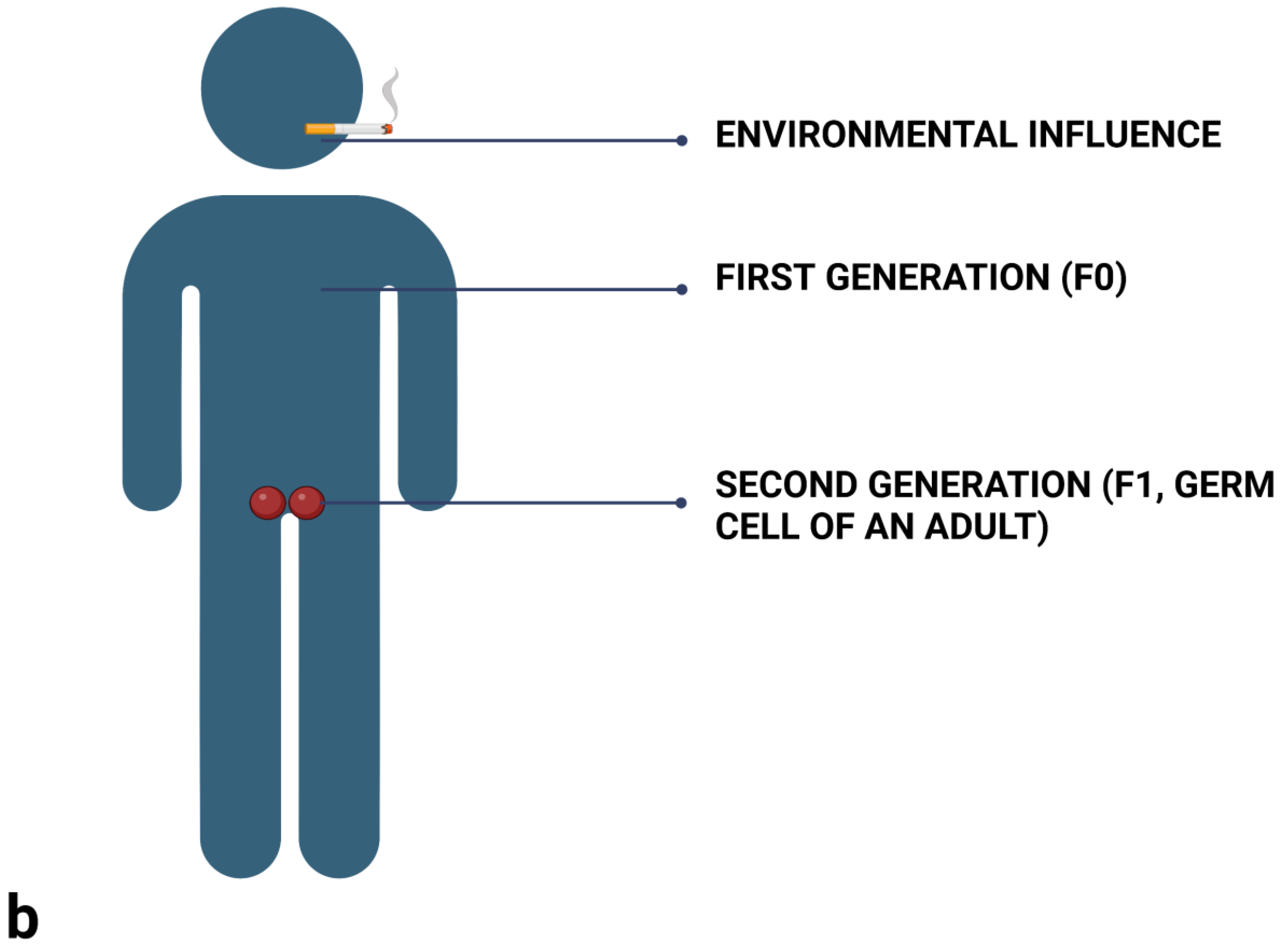
2.3. Differentiate between Prenatal (Maternal) Influences, and Intergenerational and Transgenerational Inheritance
2.4. Type of Epigenetic Process
2.5. Behavioural Tests
3. Traumatic Experience and Epigenetic Mediators: Intergenerational Studies
3.1. Stress, the HPA Axis, and Glucocorticoid Receptor (GR)
3.2. War Experience and Famine
4. Transgenerational Epigenetic Inheritance
4.1. Fearful Experiences
4.2. Separation Trauma
5. Back to Methodology: Difficulties with TEI Studies
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, S.A.; Forgacs, G.; Müller, G.B. Before programs: The physical origination of multicellular forms. Int. J. Dev. Biol. 2006, 50, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Brink, R.A. Paramutation. Annu. Rev. Genet. 1973, 7, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Lamb, M. Epigenetic Inheritance and Evolution: The Lamarckian Dimension; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Markoš, A.; Švorcová, J. Epigenetic Processes and the Evolution of Life; Taylor and Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Schmitz, R.J.; Ecker, J.R. Epigenetic and epigenomic variation in Arabidopsis thaliana. Trends Plant Sci. 2012, 17, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeven, K.J.F.; Jansen, J.J.; van Dijk, P.J.; Biere, A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol. 2010, 185, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [CrossRef]
- Yu, R.; Wang, X.; Moazed, D. Epigenetic inheritance mediated by coupling of RNAi and histone H3K9 methylation. Nature 2018, 558, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.-H.; Li, D.; Shimizu, H.; Nakamura, R.; Ishii, S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell 2011, 145, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Bantignies, F.; Grimaud, C.; Lavrov, S.; Gabut, M.; Cavalli, G. Inheritance of polycomb-dependent chromosomal interactions in Drosophila. Genes Dev. 2003, 17, 2406–2420. [Google Scholar] [CrossRef]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Rechavi, O.; Houri-Ze’evi, L.; Anava, S.; Goh, W.S.S.; Kerk, S.Y.; Hannon, G.J.; Hobert, O. Starvation-Induced Transgenerational Inheritance of Small RNAs in C. elegans. Cell 2014, 158, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.L.; Beese-Sims, S.E.; Brookes, E.; Spadafora, R.; Zhu, Y.; Rothbart, S.B.; Aristizábal-Corrales, D.; Chen, S.; Badeaux, A.I.; Jin, Q.; et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Rep. 2014, 7, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz-Orbach, L.; Zhang, C.; Sidoli, S.; Amin, R.; Kaur, D.; Zhebrun, A.; Ni, J.; Gu, S.G. Caenorhabditis elegans nuclear RNAi factor SET-32 deposits the transgenerational histone modification, H3K23me3. Elife 2020, 9, e54309. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.D.; Sutherland, H.; Martin, D.I.; Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999, 23, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [Green Version]
- Cropley, J.E.; Suter, C.M.; Beckman, K.B.; Martin, D.I.K. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc. Natl. Acad. Sci. USA 2006, 103, 17308–17312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blewitt, M.; Vickaryous, N.K.; Paldi, A.; Koseki, H.; Whitelaw, E. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006, 2, e49. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.L. Influence of maternal phenotype on metabolic differentiation of agouti locus mutants in the mouse. Genetics 1978, 88, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Flood, W.D.; Ruvinsky, A. Alternative splicing and expressivity of the Axin(Fu) allele in mice. Heredity 2001, 87, 146–152. [Google Scholar] [CrossRef]
- Waterland, R.A.; Dolinoy, D.C.; Lin, J.-R.; Smith, C.A.; Shi, X.; Tahiliani, K.G. Maternal Methyl Supplements Increase Offspring DNA methylation at Axin Fused. Genesis 2006, 44, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, T.M.; Ferguson-Smith, A.C. Metastable epialleles and their contribution to epigenetic inheritance in mammals. Semin. Cell Dev. Biol. 2020, 97, 93–105. [Google Scholar] [CrossRef]
- Weyrich, A.; Benz, S.; Karl, S.; Jeschek, M.; Jewgenow, K.; Fickel, J. Paternal heat exposure causes DNA methylation and gene expression changes of Stat3 in Wild guinea pig sons. Ecol. Evol. 2016, 6, 2657–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, T.; Marco, A.; Kisliouk, T.; Haron, A.; Shinder, D.; Druyan, S.; Meiri, N. Embryonic heat conditioning in chicks induces transgenerational heat/immunological resilience via methylation on regulatory elements. FASEB J. 2022, 36, e22406. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary Protein Restriction of Pregnant Rats Induces and Folic Acid Supplementation Prevents Epigenetic Modification of Hepatic Gene Expression in the Offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011, 152, 2228–2236. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, K.; Li, X.; Datta, J.; Bai, S.; Pogribny, I.; Pogribny, M.; Huang, Y.; Young, D.; Jacob, S.T. A Folate- and Methyl-Deficient Diet Alters the Expression of DNA Methyltransferases and Methyl CpG Binding Proteins Involved in Epigenetic Gene Silencing in Livers of F344 Rats1. J. Nutr. 2006, 136, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjöström, M.; Golding, J.; the ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 2006, 14, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Vassoler, F.M.; Johnson, N.L.; Byrnes, E.M. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. J. Psychopharmacol. 2013, 27, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Gangisetty, O.; Chaudhary, S.; Palagani, A.; Sarkar, D.K. Transgenerational inheritance of fetal alcohol effects on proopiomelanocortin gene expression and methylation, cortisol response to stress, and anxiety-like behaviors in offspring for three generations in rats: Evidence for male germline transmission. PLoS ONE 2022, 17, e0263340. [Google Scholar] [CrossRef]
- Uzumcu, M.; Suzuki, H.; Skinner, M.K. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod. Toxicol. 2004, 18, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Toxicology: Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anway, M.D.; Leathers, C.; Skinner, M.K. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 2006, 147, 5515–5523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, D.; Gore, A.C.; Hsu, T.S.; Dangleben, M.; Spinetta, N.L.; Schallert, T.; Anway, M.K.; Skinner, M.D. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. USA 2007, 104, 5942–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, A.; Skinner, M.K.; Yan, W. Ancestral vinclozolin exposure alters the epigenetic transgenerational inheritance of sperm small noncoding RNAs. Environ. Epigenetics 2016, 2, dvw001. [Google Scholar] [CrossRef]
- Iqbal, K.; Tran, D.A.; Li, A.X.; Warden, C.; Bai, A.Y.; Singh, P.; Wu, X.; Pfeifer, G.P.; Szabó, P.E. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol. 2015, 16, 59. [Google Scholar] [CrossRef] [Green Version]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009, 85, 742–752. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 2011, 31, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.; Larsen, G.; Manikkam, M.; Guerrero-Bosagna, C.; Savenkova, M.I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS ONE 2012, 7, e36129. [Google Scholar] [CrossRef] [Green Version]
- Mbiydzenyuy, N.E.; Hemmings, S.M.J.; Qulu, L. Prenatal maternal stress and offspring aggressive behavior: Intergenerational and transgenerational inheritance. Front. Behav. Neurosci. 2022, 16, 977416. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Huang, S.; Wang, X.; Zhu, Y.; Chen, Z.; Chen, D. N6-methyladenine functions as a potential epigenetic mark in eukaryotes. Bioessays 2015, 37, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Klungland, A.; Robertson, A.B. Oxidized C5-methyl cytosine bases in DNA: 5-Hydroxymethylcytosine; 5-formylcytosine; and 5-carboxycytosine. Free. Radic. Biol. Med. 2017, 107, 62–68. [Google Scholar] [CrossRef]
- Barlow, D.P.; Stöger, R.; Herrmann, B.G.; Saito, K.; Schweifer, N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Santini, L.; Halbritter, F.; Titz-Teixeira, F.; Suzuki, T.; Asami, M.; Ma, X.; Ramesmayer, J.; Lackner, A.; Warr, N.; Pauler, F.; et al. Genomic imprinting in mouse blastocysts is predominantly associated with H3K27me3. Nat. Commun. 2021, 12, 3804. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Imprinting disorders in humans: A review. Curr. Opin. Pediatr. 2020, 32, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasson, J.A.; Ruppersburg, C.C.; Katz, D.J. Restoring totipotency through epigenetic reprogramming. Brief. Funct. Genom. 2013, 12, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, V.; Isles, A.; Kelsey, G.; Ferguson-Smith, A.C.; the Erice Imprinting Group. Genomic Imprinting and Physiological Processes in Mammals. Cell 2019, 176, 952–965. [Google Scholar] [CrossRef] [Green Version]
- Lane, N.; Dean, W.; Erhardt, S.; Hajkova, P.; Surani, A.; Reik, W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 2003, 35, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.A.; Sengupta, R.; Zylicz, J.J.; Murakami, K.; Lee, C.; Down, T.A.; Surani, M.A. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 2013, 339, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The Dynamics of Genome-wide DNA Methylation Reprogramming in Mouse Primordial Germ Cells. Mol. Cell 2012, 48, 849–862. [Google Scholar] [CrossRef] [Green Version]
- Kremsky, I.; Corces, V.G. Protection from DNA re-methylation by transcription factors in primordial germ cells and pre-implantation embryos can explain trans-generational epigenetic inheritance. Genome Biol. 2020, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Liu, Y.-J.; Nakashima, H.; Umehara, H.; Inoue, K.; Matoba, S.; Tachibana, M.; Ogura, A.; Shinkai, Y.; Nakano, T. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 2012, 486, 415–419. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Zhang, Y.; Wang, S. Histone citrullination: A new target for tumors. Mol. Cancer 2021, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell 2019, 74, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, L.J.; Wang, W.; Strome, S. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science 2014, 345, 1515–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammoud, S.S.; Nix, D.A.; Zhang, H.; Purwar, J.; Carrell, D.T.; Cairns, B.R. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009, 460, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, A.M.; Nanni, P.; Mansuy, I.M. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 2014, 7, 2. [Google Scholar] [CrossRef]
- Long, J.; Walker, J.; She, W.; Aldridge, B.; Gao, H.; Deans, S.; Vickers, M.; Feng, X. Nurse cell –derived small RNAs define paternal epigenetic inheritance in Arabidopsis. Science 2021, 373, eabh0556. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shi, J.; Zhang, Y.; Zhang, H.; Liao, S.; Li, W.; Lei, L.; Han, C.; Ning, L.; Cao, Y.; et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012, 22, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, J.; Rassoulzadegan, M. Sperm RNA: Quo vadis? Semin. Cell Dev. Biol. 2020, 97, 123–130. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2015, 6780, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Remy, J.-J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, G.; Sun, W.; Rosenkranz, D.; Pelczar, P.; Opitz, L.; Efthymiou, V.; Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 10547–10556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, J.; Rassoulzadegan, M.; Tuorto, F.; Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 2019, 15, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Gapp, K.; Bohacek, J. Epigenetic germline inheritance in mammals: Looking to the past to understand the future. Genes Brain Behav. 2017, 17, e12407. [Google Scholar] [CrossRef]
- Cossetti, C.; Lugini, L.; Astrologo, L.; Saggio, I.; Fais, S.; Spadafora, C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: Possible transport by exosomes. PLoS ONE 2014, 9, e101629. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell 2018, 46, 481–494.e6. [Google Scholar] [CrossRef]
- Sharma, A. Transgenerational epigenetic inheritance: Focus on soma to germline information transfer. Prog. Biophys. Mol. Biol. 2013, 113, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Bhanu, N.V.; Garcia, B.A.; Bale, T.L. Epididymal glucocorticoid receptors promote intergenerational transmission of paternal stress. bioRxiv, 2018; preprint. [Google Scholar] [CrossRef]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Tu, S.; Reinberg, D. Molecular signals of epigenetic states. Science 2010, 330, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef] [Green Version]
- Bohacek, J.; Mansuy, J.B.I.M. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat. Methods 2017, 14, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E. Epigenetic inheritance and plasticity: The responsive germline. Prog. Biophys. Mol. Biol. 2013, 111, 99–107. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. 2008, 22, 1088. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, 3638. [Google Scholar] [CrossRef] [Green Version]
- Francis, D.D.; Meaney, M.J. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 1999, 9, 128–134. [Google Scholar] [CrossRef]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epi-genetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A general introduction to glucocorticoid biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babb, J.A.; Carini, L.M.; Spears, S.L.; Nephew, B.C. Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm. Behav. 2014, 65, 386–393. [Google Scholar] [CrossRef] [Green Version]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [Green Version]
- McCreary, J.K.; Truica, L.S.; Friesen, B.; Yao, Y.; Olson, D.M.; Kovalchuk, I.; Cross, A.R.; Metz, G.A. Altered brain morphology and functional connectivity reflect a vulnerable affective state after cumulative multigenerational stress in rats. Neuroscience 2016, 330, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, J.; Farinelli, M.; Mirante, O.; Steiner, G.; Gapp, K.; Coiret, G.; Ebeling, M.; Durán-Pacheco, G.; Iniguez, A.L.; Manuella, F.; et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol. Psychiatry 2015, 20, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Labonte, B.; Yerko, V.; Gross, J.; Mechawar, N.; Meaney, M.J.; Szyf, M.; Turecki, G. Differential glucocorticoid receptor exon 1 B, 1 C, and 1 H expression and methylation in suicide completers with a history of childhood abuse. Biol. Psychiatry 2012, 72, 41–48. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Chrousos, G.P.; Dorn, L.D.; Burke, L.; Helmers, K.; Kling, M.A.; Trickett, P.K.; Putnam, F.W. Hypothalamic-Pituitary-Adrenal Axis Dysregulation in Sexually abused Girls. J. Clin. Endocrinol. Metab. 1994, 78, 249–255. [Google Scholar]
- Vythilingam, M.; Heim, C.; Newport, D.J.; Miller, A.H.; Anderson, E.; Bronen, R.; Brummer, M.; Staib, L.; Vermetten, E.; Charney, D.S.; et al. Childhood Trauma Associated With Smaller Hippocampal Volume in Women With Major Depression. Am. J. Psychiatry 2002, 159, 2072–2080. [Google Scholar] [CrossRef] [Green Version]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Aoued, H.S.; Sannigrahi, S.; Hunter, S.C.; Doshi, N.; Sathi, Z.S.; Chan, A.W.S.; Walum, H.; Dias, B.G. Proximate causes and consequences of intergenerational influences of salient sensory experience. Genes Brain Behav. 2020, 19, e12638. [Google Scholar] [CrossRef]
- Gapp, K.; van Steenwyk, G.; Germain, P.L.; Matsushima, W.; Rudolph, K.L.M.; Manuella, F.; Roszkowski, M.; Vernaz, G.; Ghosh, T.; Pelczar, P.; et al. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol. Psychiatry 2020, 25, 2162–2174. [Google Scholar] [CrossRef] [Green Version]
- Roseboom, T.J.; van der Meulen, J.H.P.; Osmond, C.; Barker, D.J.P.; Ravelli, A.C.J.; Schroeder-Tanka, J.M.; van Montfrans, G.A.; Michels, R.P.J.; Bleker, O.P. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 2000, 84, 595–598. [Google Scholar] [CrossRef] [Green Version]
- Painter, R.C.; De Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine1–3. Am. J. Clin. Nutr. 2006, 84, 322–327. [Google Scholar] [CrossRef]
- Hoek, H.W.; Susser, E.; Buck, K.A.; Lumey, L.H.; Lin, S.P.; Gorman, J.M. Schizoid Personality Disorder After Prenatal Exposure to Famine. Am. J. Psychiatry 1996, 153, 1637–1639. [Google Scholar]
- Painter, R.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.; Roseboom, T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; van der Post, J.A.; Gluckman, P.D.; Hanson, M.A.; Roseboom, T.J. Transgenerational effects of prenatal exposure to the 1944–45 Dutch famine. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 548–554. [Google Scholar] [CrossRef]
- Rotar, O.; Moguchaia, E.; Boyarinova, M.; Kolesova, E.; Khromova, N.; Freylikhman, O.; Smolina, N.; Solntsev, V.; Kostareva, A.; Konradi, A.; et al. Seventy years after the siege of Leningrad: Does early life famine still affect cardiovascular risk and aging? J. Hypertens. 2015, 33, 1772–1779. [Google Scholar] [CrossRef]
- Lumey, L.; Stein, A.D.; Susser, E. Prenatal famine and adult health. Annu. Rev. Public Health 2011, 32, 237–262. [Google Scholar] [CrossRef]
- van den Berg, G.J.; Pinger, P.R. Transgenerational effects of childhood conditions on third generation health and education outcomes. Econ. Hum. Biol. 2016, 23, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Lehrner, A.; Yehuda, R. Trauma across generations and paths to adaptation and resilience. Psychol. Trauma Theory Res. Pract. Policy 2018, 10, 22–29. [Google Scholar] [CrossRef]
- Yehuda, R.; Schmeidler, J.; Wainberg, M.; Binder-Brynes, K.; Duvdevani, T. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am. J. Psychiatry 1998, 155, 1163–1171. [Google Scholar] [CrossRef]
- Yehuda, R.; Koenen, K.C.; Galea, S.; Flory, J.D. The role of genes in defining a molecular biology of PTSD. Dis. Markers 2011, 30, 67–76. [Google Scholar] [CrossRef]
- Lehrner, A.; Bierer, L.M.; Passarelli, V.; Pratchett, L.C.; Flory, J.D.; Bader, H.N.; Harris, I.R.; Bedi, A.; Daskalakis, N.P.; Makotkine, I.; et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology 2014, 40, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Kertes, D.A.; Kamin, H.S.; Hughes, D.A.; Rodney, N.C.; Bhatt, S.; Mulligan, C.J. Prenatal Maternal Stress Predicts Methylation of Genes Regulating the Hypothalamic–Pituitary–Adrenocortical System in Mothers and Newborns in the Democratic Republic of Congo. Child Dev. 2016, 87, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Rowland-Klein, D.; Dunlop, R. The Transmission of Trauma across Generations: Identification with Parental Trauma in Children of Holocaust Survivors. In Handbook of Stress, Trauma, and the Family; Routledge/Taylor & Francis Group: London, UK, 2013; pp. 117–136. [Google Scholar]
- Van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J.; Sagi-Schwartz, A. Are Children of Holocaust Survivors Less Well-Adapted? A Meta-Analytic Investigation of Secondary Traumatization. J. Trauma. Stress 2003, 16, 459–469. [Google Scholar] [CrossRef]
- Gapp, K.; Soldado-Magraner, S.; Alvarez-Sánchez, M.; Bohacek, J.; Vernaz, G.; Shu, H.; Franklin, T.; Wolfer, D.P.; Mansuy, I.M. Early life stress in fathers improves behavioural flexibility in their offspring. Nat. Commun. 2014, 5, 5466. [Google Scholar] [CrossRef] [Green Version]
- Levav, I.; Levinson, D.; Radomislensky, I.; Shemesh, A.A.; Kohn, R. Psychopathology and other health dimensions among the offspring of Holocaust survivors: Results from the Israel National Health Survey. Isr. J. Psychiatry Relat. Sci. 2007, 44, 144–151. [Google Scholar]
- Sagi-Schwartz, A.; Van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Does intergenerational transmission of trauma skip a generation? No meta-analytic evidence for tertiary traumatization with third generation of Holocaust survivors. Attach. Hum. Dev. 2008, 10, 105–121. [Google Scholar] [CrossRef]
- Dias, B.G.; Ressler, K. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Morrison, F.G.; Dias, B.G.; Ressler, K.J. Extinction reverses olfactory fear-conditioned increases in neuron number and glomerular size. Proc. Natl. Acad. Sci. USA 2015, 112, 12846–12851. [Google Scholar] [CrossRef] [Green Version]
- Aoued, H.S.; Sannigrahi, S.; Doshi, N.; Morrison, F.G.; Linsenbaum, H.; Hunter, S.C.; Walum, H.; Baman, J.; Yao, B.; Jin, P.; et al. Reversing Behavioral, Neuroanatomical, and Germline Influences of Intergenerational Stress. Biol. Psychiatry 2019, 85, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Kerimoglu, C.; Ramachandran, B.; Pena-Centeno, T.; Jain, G.; Stilling, R.M.; Islam, R.; Capece, V.; Zhou, Q.; Edbauer, D.; et al. RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep. 2018, 23, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, A.; Kunzi, M.; Mansoor, M.; Khan, Z.Y.; Abid, A.; Taha, M.; Rigotti, S.; Thumfart, K.; Faisal, S.; Chughtai, O.; et al. Distinct microRNA signature in human serum and germline after childhood trauma. medRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- Van Steenwyk, G.; Roszkowski, M.; Manuella, F.; Franklin, T.B.; Mansuy, I.M. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: Evidence in the 4th generation. Environ. Epigenetics 2018, 4, dvy023. [Google Scholar] [CrossRef] [PubMed]
- Ptashne, M. Epigenetics: Core misconcept. Proc. Natl. Acad. Sci. USA 2013, 110, 7101–7103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef] [Green Version]
- Grossniklaus, U.; Kelly, W.; Ferguson-Smith, A.; Pembrey, M.; Lindquist, S. Transgenerational epigenetic inheritance: How important is it? Nat. Rev. Genet. 2013, 14, 228–235. Available online: www.nature.com/reviews/genetics%0Ahttp://lindquistlab.wi.mit.edu/wp-content/uploads/2013/06/Grossniklaus2013NatRevGenet.pdf (accessed on 10 November 2022). [CrossRef] [PubMed] [Green Version]
- van Otterdijk, S.D.; Michels, K.B. Transgenerational epigenetic inheritance in mammals: How good is the evidence? FASEB J. 2016, 30, 2457–2465. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J. The Epigenetics Heretic. Science 2014, 343, 361–363. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Chéry, C.; Oussalah, A.; Nadaf, J.; Coelho, D.; Josse, T.; Flayac, J.; Robert, A.; Koscinski, I.; Gastin, I.; et al. APRDX1 mutant allele causes a MMACHC secondary epimutation in cblC patients. Nat. Commun. 2018, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Tufarelli, C.; Sloane-Stanley, J.A.; Garrick, D.; Sharpe, J.A.; Ayyub, H.; Wood, W.G.; Higgs, D.R. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat. Genet. 2003, 34, 157–165. [Google Scholar] [CrossRef]
- Mitchell, K. Calibrating Scientific Skepticism—A Wider Look at the Field of Transgenerational Epigenetics. 2018. Blog Entry. Available online: http://www.wiringthebrain.com/2018/07/calibrating-scientific-skepticism-wider.html (accessed on 10 November 2022).
- Uller, T.; English, S.; Pen, I. When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proc. R. Soc. B Boil. Sci. 2015, 282, 20150682. [Google Scholar] [CrossRef] [Green Version]
- Lachmann, M.; Jablonka, E. The inheritance of phenotypes: An adaptation to fluctuating environments. J. Theor. Biol. 1996, 181, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullston, T.; Teague, E.M.C.O.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef]
- Fullston, T.; Ohlsson-Teague, E.M.C.; Print, C.G.; Sandeman, L.Y.; Lane, M. Sperm microRNA content is altered in a mouse model of male obesity, but the same suite of microRNAs are not altered in offspring’s sperm. PLoS ONE 2016, 11, e0166076. [Google Scholar] [CrossRef]
- Portin, P. Does epigenetic inheritance revolutionize the foundations of the theory of evolution? Curr. Top. Genet. 2012, 5. Available online: http://www.researchtrends.net/tia/article_pdf.asp?in=0&vn=5&tid=45&aid=3652 (accessed on 14 November 2022).
- Laland, K.N.; Uller, T.; Feldman, M.W.; Sterelny, K.; Müller, G.B.; Moczek, A.P.; Jablonka, E.; Odling-Smee, F.J. The extended evolutionary synthesis: Its structure, assumptions and predictions. Proc. R. Soc. B Boil. Sci. 2015, 282, 20151019. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Baldwin, J.M. A New Factor in Evolution Published by: The University of Chicago Press for the American Society of Naturalists Stable. Am. Nat. 1896, 30, 441–451. Available online: http://www.jstor.org/stable/2453130 (accessed on 10 November 2022). [CrossRef] [Green Version]
- Waddington, C. Genetic Assimilation of an Acquired Character. Evolution 1952, 7, 118–126. [Google Scholar]
- Waddington, C.H. Genetic Assimilation of the Bithorax Phenotype. Evolution 1956, 10, 1–13. [Google Scholar] [CrossRef]
- Turner, B.M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3403–3418. [Google Scholar] [CrossRef] [Green Version]
- Hernando-Herraez, I.; Heyn, H.; Fernandez-Callejo, M.; Vidal, E.; Bellon, H.F.; Prado-Martinez, J.; Sharp, A.J.; Esteller, M.; Marques-Bonet, T. The interplay between DNA methylation and sequence divergence in recent human evolution. Nucleic Acids Res. 2015, 43, 8204–8214. [Google Scholar] [CrossRef] [PubMed]
- Makova, K.D.; Hardison, R. The effects of chromatin organization on variation in mutation rates in the genome. Nat. Rev. Genet. 2015, 16, 213–223. [Google Scholar] [CrossRef]
- Stotz, K. Extended evolutionary psychology: The importance of transgenerational developmental plasticity. Front. Psychol. 2014, 5, 908. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.E.; Barron, A.B. Epigenetics and the evolution of instincts: Instincts may evolve from learning and share the same cellular and molecular mechanisms. Science 2017, 356, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.K.M.; Katz, L.A. Epigenetics as Driver of Adaptation and Diversification in Microbial Eukaryotes. Front. Genet. 2021, 12, 642220. [Google Scholar] [CrossRef] [PubMed]
- Thorson, J.L.; Smithson, M.; Beck, D.; Sadler-Riggleman, I.; Nilsson, E.; Dybdahl, M.; Skinner, M.K. Epigenetics and adaptive phenotypic variation between habitats in an asexual snail. Sci. Rep. 2017, 7, 14139. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švorcová, J. Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals. Genes 2023, 14, 120. https://doi.org/10.3390/genes14010120
Švorcová J. Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals. Genes. 2023; 14(1):120. https://doi.org/10.3390/genes14010120
Chicago/Turabian StyleŠvorcová, Jana. 2023. "Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals" Genes 14, no. 1: 120. https://doi.org/10.3390/genes14010120
APA StyleŠvorcová, J. (2023). Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals. Genes, 14(1), 120. https://doi.org/10.3390/genes14010120



