Molecular Characterization of Tropomyosin and Its Potential Involvement in Muscle Contraction in Pacific Abalone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Sample Collection
2.2. Tissues Collection for Gene Cloning and Expression Analysis
2.3. Fertilization and Sample Collection from the Embryonic and Larval Development (ELD) Stages
2.4. Tissue Samples from Heat-Stress-Treated Pacific Abalones
2.5. Tissue Samples from Different Growth Types of Pacific Abalone
2.6. Tissue Samples from Starved Pacific Abalones
2.7. Seasonal Tissue Sample Collection from Pacific Abalones
2.8. Extraction of RNA and cDNA Synthesis
2.9. Cloning of the Full-Length Tropomyosin Sequence in Pacific Abalone
2.9.1. Partial Sequence Cloning
2.9.2. RACE (5′- and 3′-) Sequence Cloning
2.10. Sequence Analysis of Cloned H. discus hannai Tropomyosin (Hdh-TPM)
2.11. Orthology Analysis
2.12. Homology Modeling of Hdh-TPM
2.13. Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.14. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.15. Statistical Analysis
3. Results
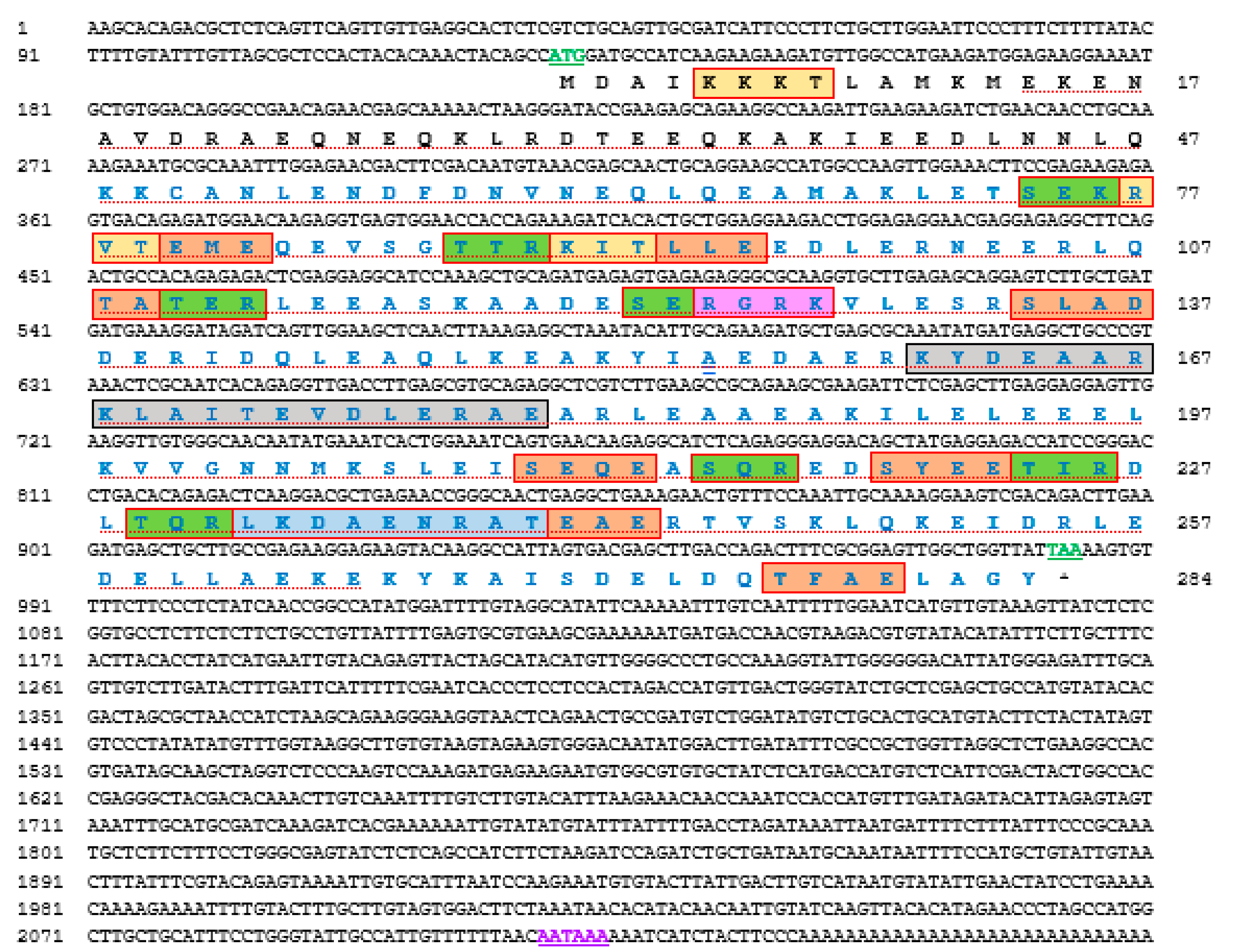
3.1. H. discus hannai Tropomyosin (Hdh-TPM) Sequence
3.2. Structure of the Hdh-TPM Protein
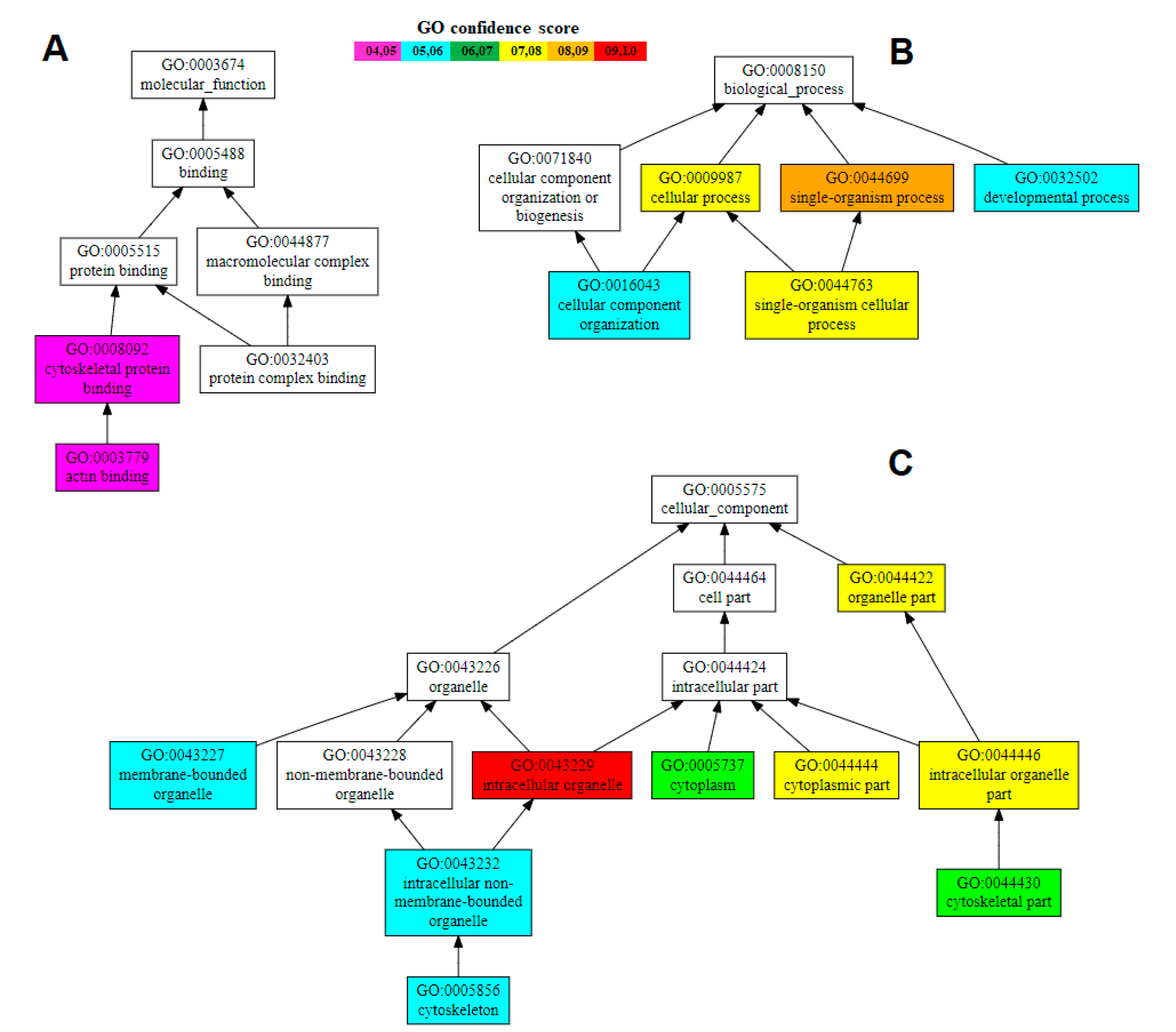
3.3. Properties and Gene Ontology of the Hdh-TPM Amino Acid Sequence
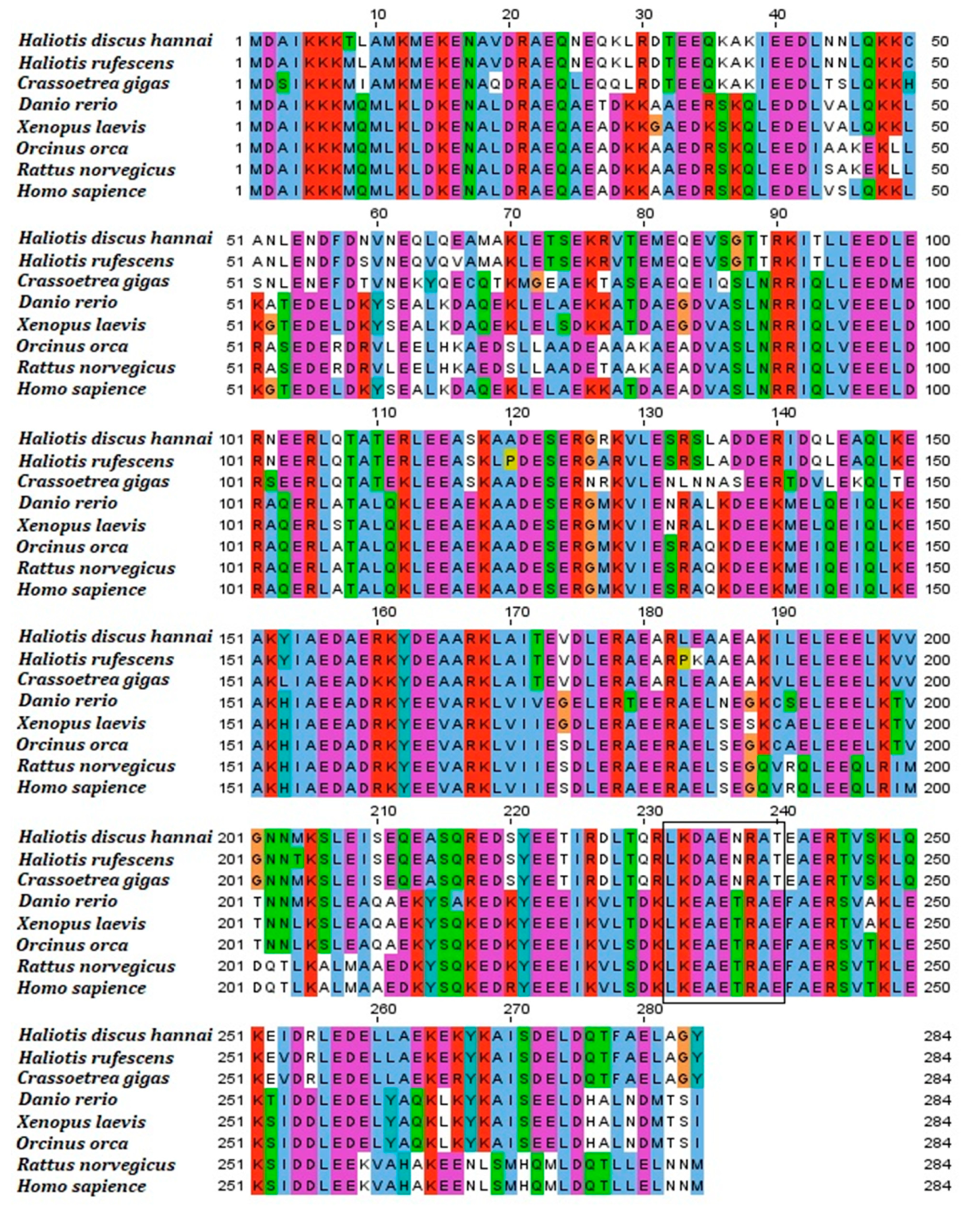
3.4. Orthology Assessment
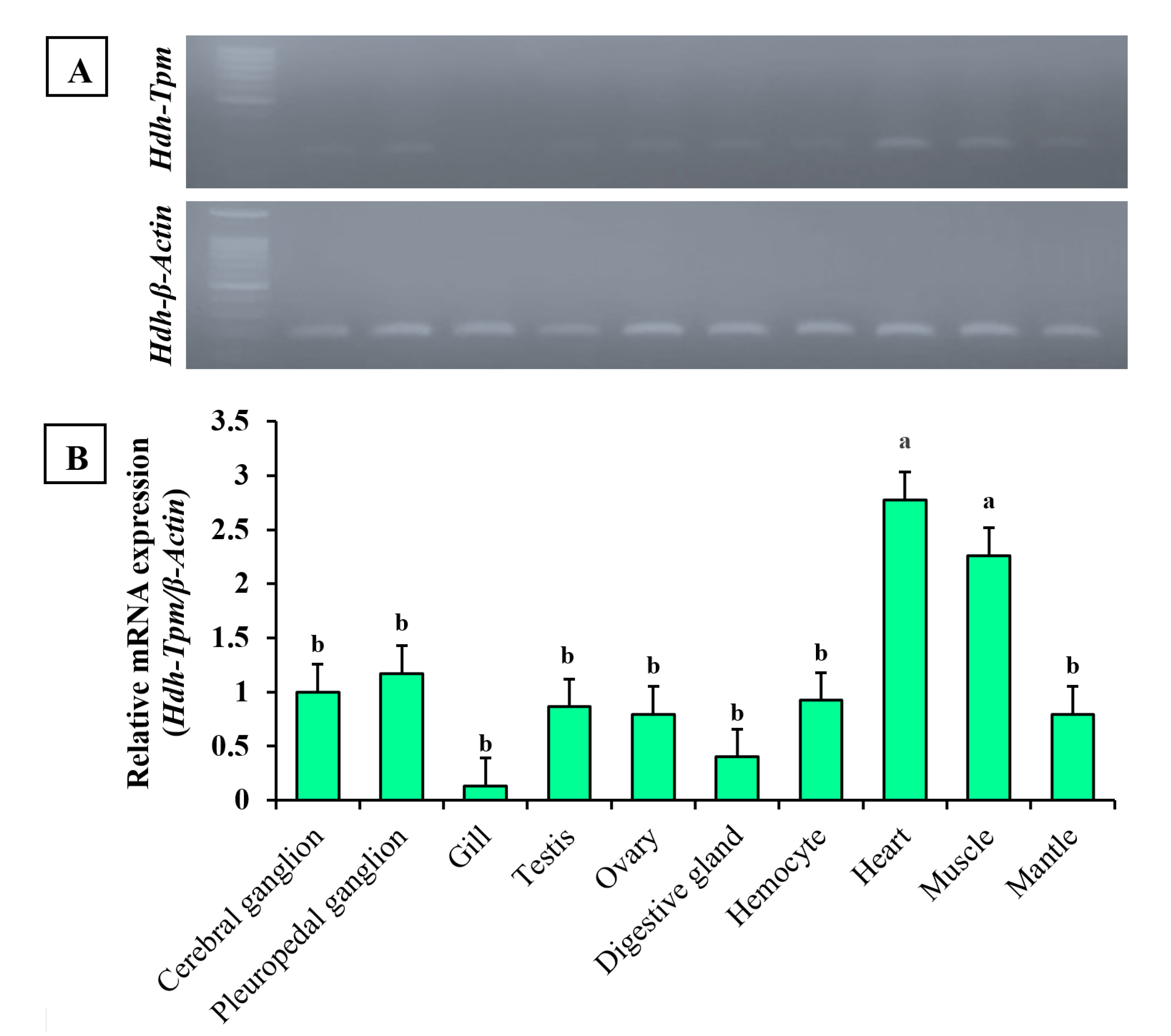
3.5. Expression of Hdh-TPM in Different Tissues
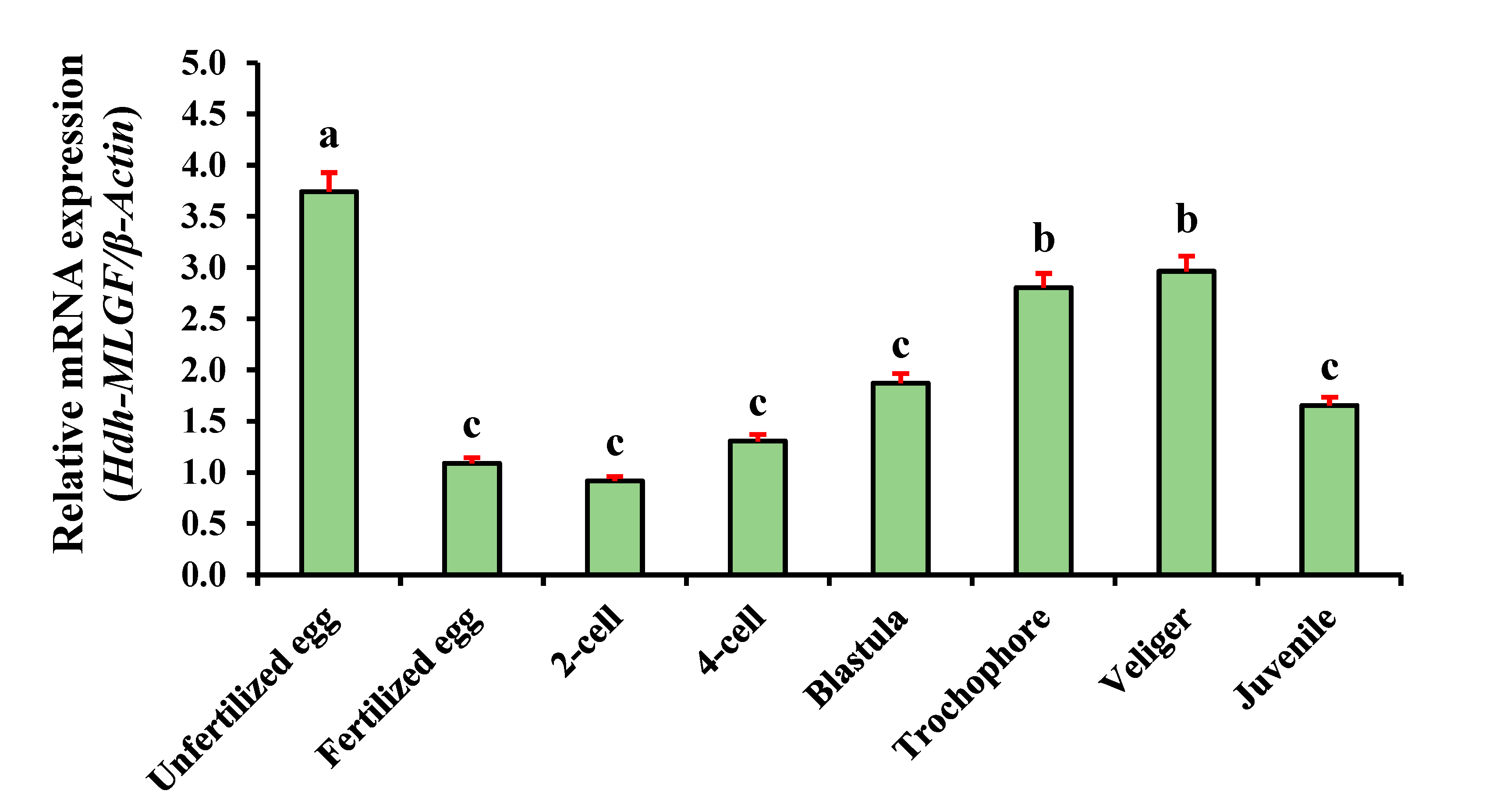
3.6. Hdh-TPM Expression at Different Stages of Embryonic and Larval Development (ELD)
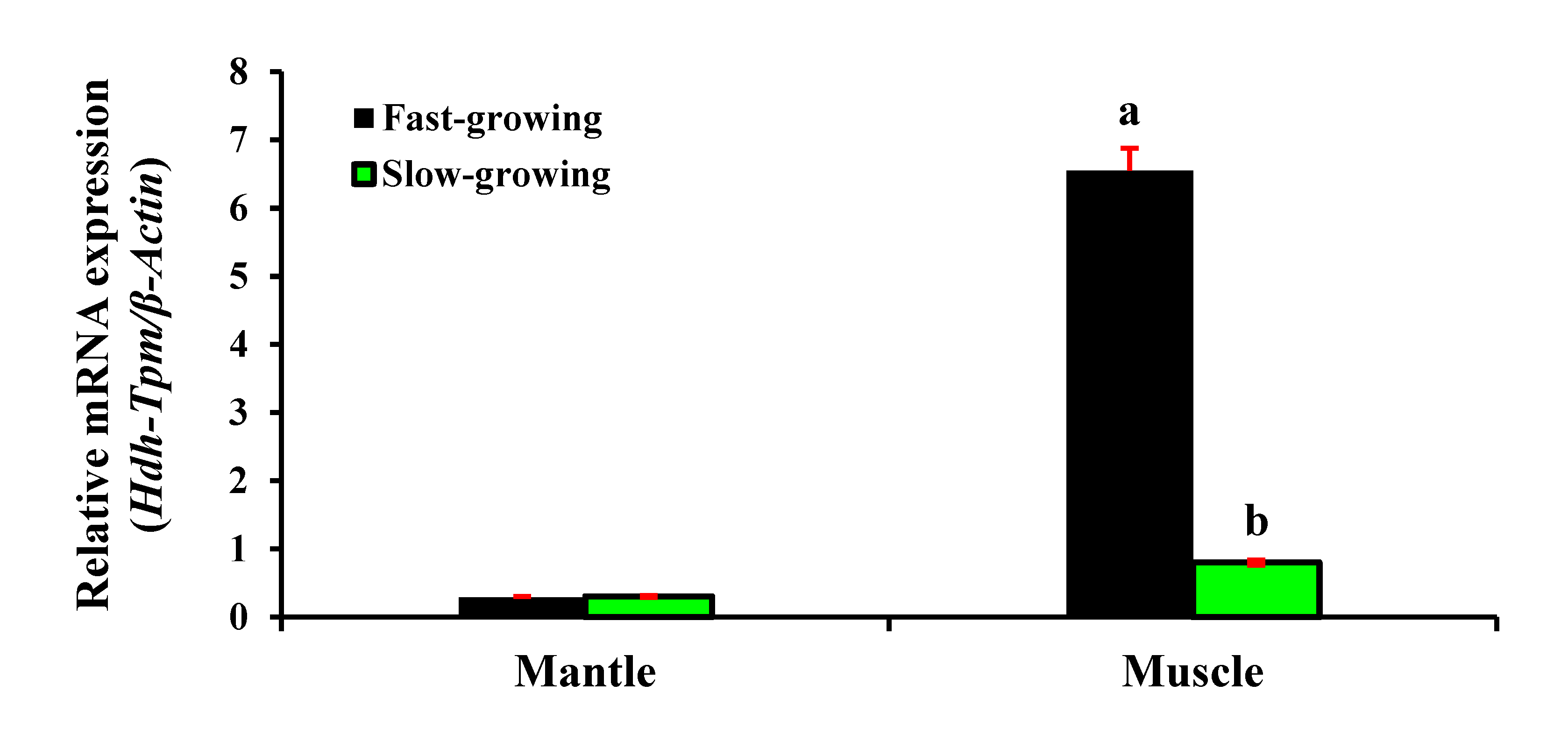
3.7. Expression of Hdh-TPM in Different Growth Types
3.8. Expression of Hdh-TPM at Different Heat Stress Conditions
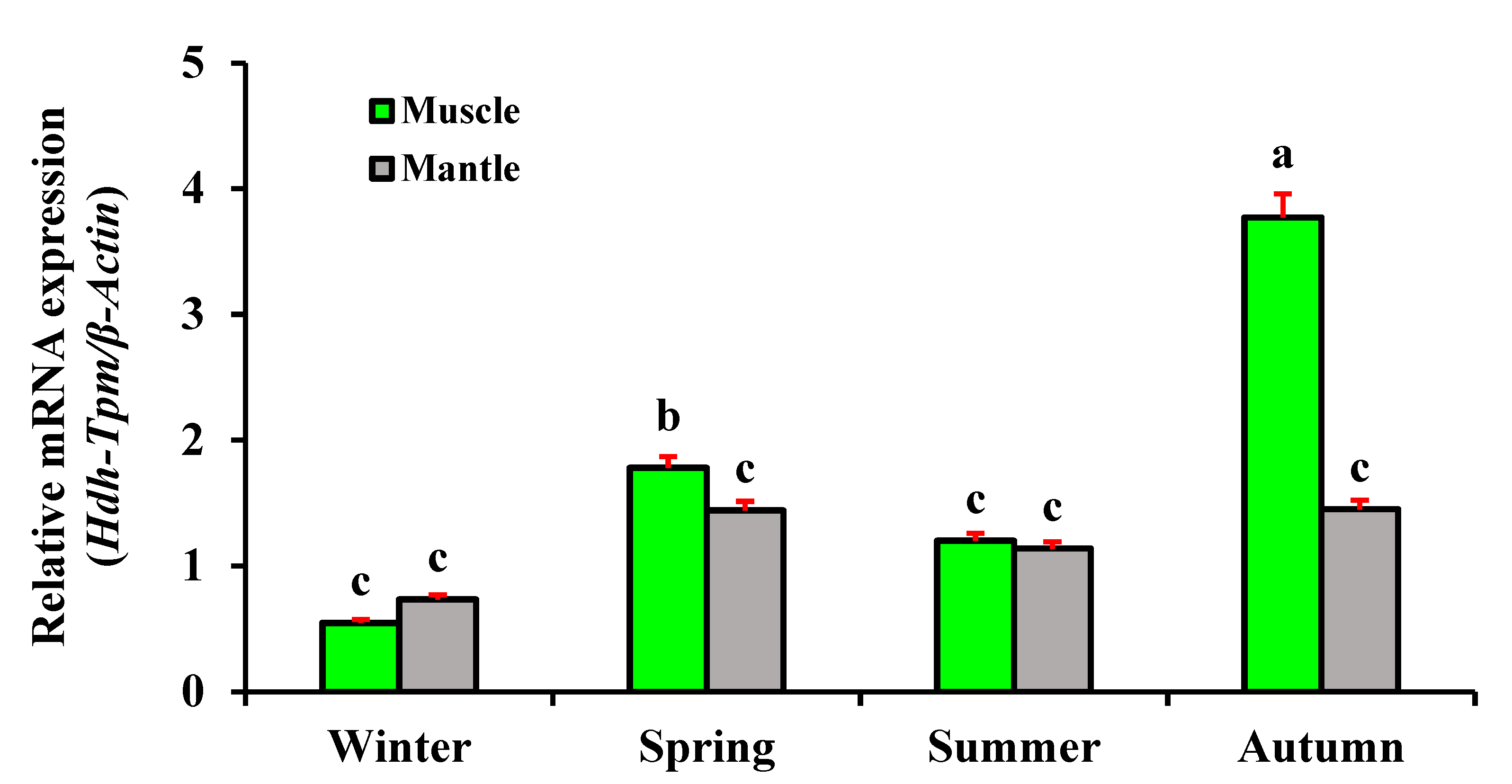
3.9. Expression of Hdh-TPM in Different Seasons
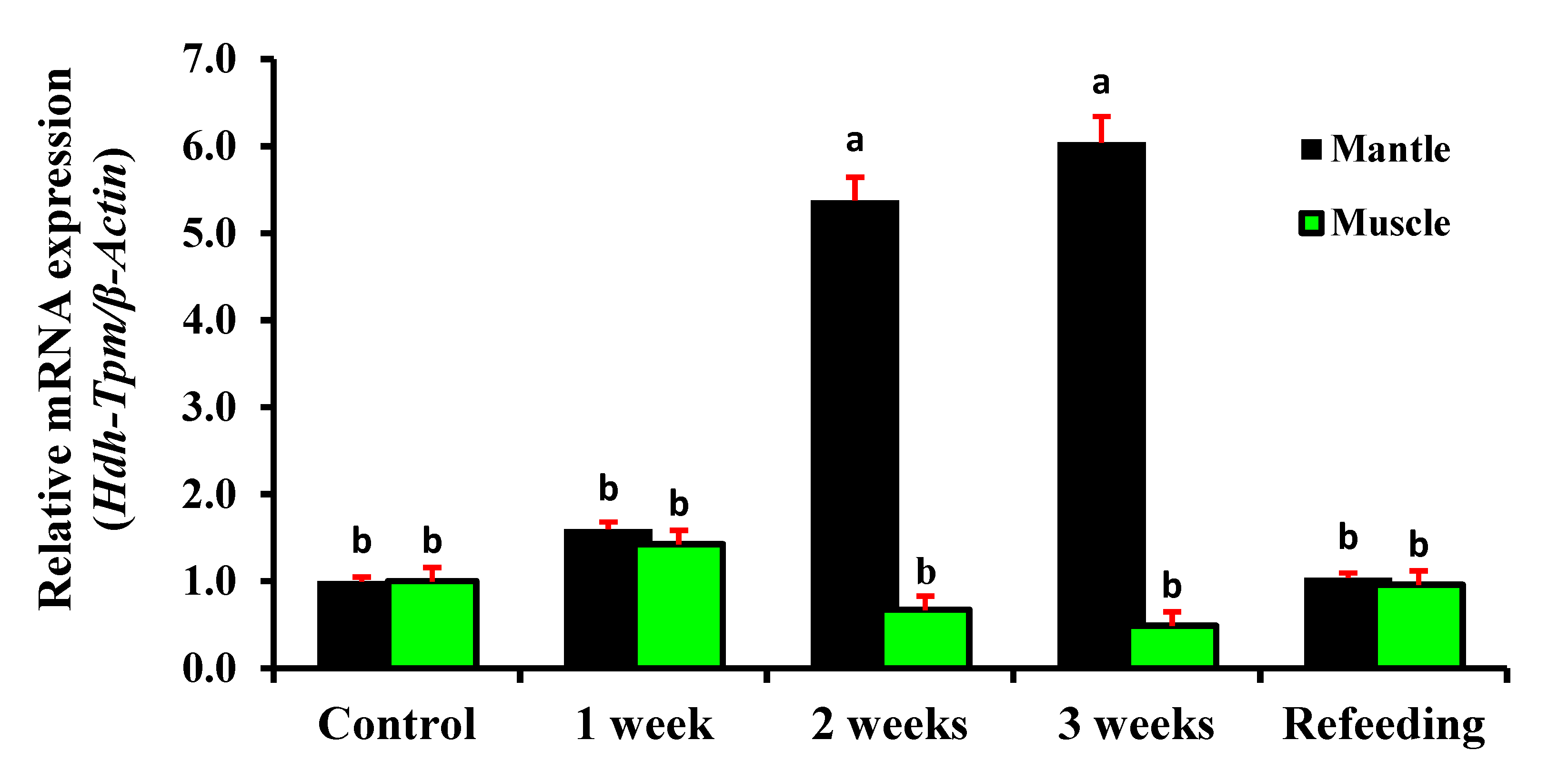
3.10. Expression of Hdh-TPM in Starvation Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalyva, A.; Schmidtmann, A.; Geeves, M.A. In vitro formation and characterization of the skeletal muscle α·β Tropomyosin heterodimers. Biochemistry 2012, 51, 6388–6399. [Google Scholar] [CrossRef] [Green Version]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrmann, E.; Muller, M.; Penczek, P.A.; Mannherz, H.G.; Manstein, D.J.; Raunser, S. Structure of the rigor actin-Tropomyosin-myosin complex. Cell 2012, 150, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, B.; Winkelmann, D.A.; White, H.D.; Hitchcock-DeGregori, S.E. Regulation of actin-myosin interaction by conserved periodic sites of Tropomyosin. Proc. Natl. Acad. Sci. USA 2012, 109, 18425–18430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loong, C.K.; Badr, M.A.; Chase, P.B. Tropomyosin flexural rigidity and single Ca2+ regulatory unit dynamics: Implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy. Front. Physiol. 2012, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Pavadai, E.; Lehman, W.; Rynkiewicz, M.J. Protein-Protein Docking Reveals Dynamic interactions of Tropomyosin on actin filaments. Biophys. J. 2020, 119, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lehman, W.; Rynkiewicz, M.J.; Moore, J.R. A new twist on tropomyosin binding to actin filaments: Perspectives on thin filament function, assembly and biomechanics. J. Muscle Res. Cell Motil. 2020, 41, 23–38. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic adaptations to eccentric training: A systematic review. Sports Med. 2017, 47, 17–41. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Li, X.Y.; Lin, Y.S.; Zhang, H.W. Phylogenetic analysis and expression patterns of Tropomyosin in amphioxus. Dongwuxue Yanjiu 2012, 33, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, K.; Ono, S. Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol. Biol. Cell. 2004, 15, 2782–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.Y.; Lee, Y.-J.; Song, W.-Y.; Kim, T.-I.; Lee, W.-C.; Kim, T.Y.; Kang, C.-K. Physiological responses of the abalone Haliotis discus hannai to daily and seasonal temperature variations. Sci. Rep. 2019, 9, 8019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, G.A.; Hittleman, K.J.; Faulkner, J.A.; Beyer, R.E. Tissue temperature and whole-animal oxygen consumption after exercise. Am. J. Physiol. 1971, 221, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G. Fatigue in working muscles. J. Appl. Physiol. 2009, 106, 358–359. [Google Scholar] [CrossRef] [Green Version]
- Locke, M.; Celotti, C. The effect of heat stress on skeletal muscle contractile properties. Cell Stress Chaperones 2014, 19, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Saunderson, E.A.; Spiers, H.; Mifsud, K.R.; Gutierrez-Mecinas, M.; Trollope, A.F.; Shaikh, A.; Mill, J.; Reul, J.M. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2016, 113, 4830–4835. [Google Scholar] [CrossRef] [Green Version]
- Britz, P.J.; Hecht, T.; Mangold, S. Effect of temperature on growth, feed consumption and nutritional indices of Haliotis midae fed a formulated diet. Aquaculture 1997, 152, 191–203. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Hossen, S.; Cho, Y.; Lee, W.K.; Kho, K.H. Hdh-Tektin-4 Regulates Motility of Fresh and Cryopreserved Sperm in Pacific Abalone, Haliotis discus hannai. Front. Cell Dev. Biol. 2022, 10, 870743. [Google Scholar] [CrossRef]
- Hsu, T.H.; Gwo, J.C. Genetic diversity and stock identification of small abalone (Haliotis diversicolor) in Taiwan and Japan. PLoS ONE 2017, 12, e0179818. [Google Scholar] [CrossRef]
- Young, M.A.; Treml, E.A.; Beher, J.; Fredle, M.; Gorfine, H.; Miller, A.D.; Swearer, S.E.; Ierodiaconou, D. Using species distribution models to assess the long-term impacts of changing oceanographic conditions on abalone density in southeast Australia. Ecography 2020, 43, 1052–1064. [Google Scholar] [CrossRef]
- Kyeong, D.; Kim, J.; Shin, Y.; Subramaniyam, S.; Kang, B.-C.; Shin, E.-H.; Park, E.H.; Noh, E.S.; Kim, Y.-O.; Park, J.Y.; et al. Expression of Heat Shock Proteins in Thermally Challenged Pacific Abalone Haliotis discus hannai. Genes 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, S.; Sukhan, Z.P.; Cho, Y.; Kho, K.H. Effects of Cryopreservation on Gene Expression and Post Thaw Sperm Quality of Pacific Abalone, Haliotis discus hannai. Front. Mar. Sci. 2021, 8, 652390. [Google Scholar] [CrossRef]
- Park, C.-J.; Kim, S.Y. Abalone aquaculture in Korea. J. Shellfish Res. 2013, 32, 17–19. [Google Scholar] [CrossRef]
- Xu, F.; Gao, T.; Liu, X. Metabolomics Adaptation of Juvenile Pacific Abalone Haliotis discus hannai to Heat Stress. Sci. Rep. 2020, 10, 6353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharker, M.R.; Kim, S.C.; Hossen, S.; Sumi, K.R.; Choi, S.K.; Choi, K.S.; Kho, K.H. Carbonic Anhydrase in Pacific Abalone Haliotis discus hannai: Characterization, Expression, and Role in Biomineralization. Front. Mol. Biosci. 2021, 8, 655115. [Google Scholar] [CrossRef]
- Huang, J.; Luo, X.; Huang, M.; Liu, G.; You, W.; Ke, C. Identification and characteristics of muscle growth-related microRNA in the Pacific abalone, Haliotis discus hannai. BMC Genom. 2018, 19, 915. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Hanif, M.A.; Hossen, S.; Cho, Y.; Sukhan, Z.P.; Choi, C.Y.; Kho, K.H. Characterization and Expression Analysis of Mollusk-like Growth Factor: A Secreted Protein Involved in Pacific Abalone Embryonic and Larval Development. Biology 2022, 11, 1445. [Google Scholar] [CrossRef]
- Meiring, J.C.M.; Bryce, N.S.; Wang, Y.; Taft, M.H.; Manstein, D.J.; Lau, S.L.; Steer, J.; Hardeman, E.C.; Gunning, P.W. Co-polymers of Actin and Tropomyosin Account for a Major Fraction of the Human Actin Cytoskeleton. Curr. Biol. 2018, 28, 2331–2337. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Manstein, D.J.; Meiring, J.C.M.; Hardeman, E.C.; Gunning, P.W. Actin–Tropomyosin distribution in non-muscle cells. J. Muscle Res. Cell Motil. 2020, 41, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillberg, L.; Zhao-Rathje, L.S.; Nyakern-Meazza, M.; Helfand, B.; Goldman, R.D.; Schutt, C.E.; Lindberg, U. Tropomyosins are present in lamellipodia of motile cells. Eur. J. Cell Biol. 2006, 85, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A. The Sliding-Filament Theory of Muscle Contraction; Springer: Cham, Switzerland, 2018; p. 426. [Google Scholar] [CrossRef]
- England, J.; Granados-Riveron, J.; Polo-Parada, L.; Kuriakose, D.; Moore, C.; Brook, J.D.; Rutland, C.S.; Setchfield, K.; Gell, C.; Ghosh, T.K.; et al. Tropomyosin 1: Multiple roles in the developing heart and in the formation of congenital heart defects. J. Mol. Cell. Cardiol. 2017, 106, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Routh, A.L.; Kuyumcu-Martinez, M.N. Nanopore sequencing reveals full-length Tropomyosin 1 isoforms and their regulation by RNA-binding proteins during rat heart development. J. Cell. Mol. Med. 2021, 25, 8352–8362. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Schevzov, G.; Hotulainen, P.; Naumanen, P.; Martin, C.; Gunning, P.W.; Lappalainen, P. A molecular pathway for myosin II recruitment to stress fibers. Curr. Biol. 2011, 21, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Gateva, G.; Kremneva, E.; Reindl, T.; Kotila, T.; Kogan, K.; Gressin, L.; Gunning, P.W.; Manstein, D.J.; Michelot, A.; Lappalainen, P. Tropomyosin isoforms specify functionally distinct actin filament populations in vitro. Curr. Biol. 2017, 27, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Creed, S.J.; Desouza, M.; Bamburg, J.R.; Gunning, P.; Stehn, J. Tropomyosin isoform 3 promotes the formation of filopodia by regulating the recruitment of actin-binding proteins to actin filaments. Exper. Cell. Res. 2011, 317, 249–261. [Google Scholar] [CrossRef]
- Gimona, M.; Kazzaz, J.A.; Helfman, D.M. Forced expression of tropomyosin 2 or 3 in v-ki-ras-transformed fibroblasts results in distinct phenotypic effects. Proc. Natl. Acad. Sci. USA 1996, 93, 9618–9623. [Google Scholar] [CrossRef] [Green Version]
- Gooding, C.; Smith, C.W. Tropomyosin exons as models for alternative splicing. Adv. Exp. Med. Biol. 2008, 644, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Dalby-Payne, J.R.; O’Loughlin, E.V.; Gunning, P. Polarization of specific tropomyosin isoforms in gastrointestinal epithelial cells and their impact on cftr at the apical surface. Mol. Biol. Cell. 2003, 14, 4365–4375. [Google Scholar] [CrossRef] [PubMed]
- Brayford, S.; Bryce, N.S.; Schevzov, G.; Haynes, E.M.; Bear, J.E.; Hardeman, E.C.; Gunning, P.W. Tropomyosin promotes lamellipodial persistence by collaborating with arp2/3 at the leading edge. Curr. Biol. 2016, 26, 1312–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepaniak, K.; Bukala, A.; da Silva Neto, A.M.; Ludwiczak, J.; Dunin-Horkawicz, S. A library of coiled-coil domains: From regular bundles to peculiar twists. Bioinformatics 2020, 36, 5368–5376. [Google Scholar] [CrossRef] [PubMed]
- Djinovic-Carugo, K.; Gautel, M.; Ylanne, J.; Young, P. The spectrin repeat: A structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002, 513, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Ishimoda-Takagi, T. Localization of tropomyosin in sea urchin eggs. Exp. Cell Res. 1979, 119, 423–428. [Google Scholar] [CrossRef]
- Di, G.; Kong, X.; Miao, X.; Zhang, Y.; Huang, M.; Gu, Y.; You, W.; Zhang, J.; Ke, C. Proteomic analysis of trochophore and veliger larvae development in the small abalone Haliotis diversicolor. BMC Genom. 2017, 18, 809. [Google Scholar] [CrossRef] [Green Version]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Nofrizal; Ramdhani, F.; Arimoto, T. Temperature effect on the maximum swimming speed of jack mackerel Trachurus japonicus through muscle contraction monitoring. Anim. Behav. Biometeorol. 2020, 8, 160–167. [Google Scholar] [CrossRef]
- Yanase, K.; Eayrs, S.; Arimoto, T. Influence of water temperature and fish length on the maximum swimming speed of sand flathead, Platychephalus bassensis: Implication for trawl selectivity. Fish. Res. 2007, 84, 180–188. [Google Scholar] [CrossRef]
- Kwon, K.; Yoo, B.-K.; Ko, Y.; Choi, J.-Y.; Kwon, O.-Y.; Kim, S.-W. Effect of starvation on expression of troponin complex genes and ER stress associated genes in skeletal muscles. Biomed Res. 2018, 29, 2368–2372. [Google Scholar] [CrossRef] [Green Version]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]




| Primer Name | Nucleotide Sequences | Purpose |
|---|---|---|
| Oligo dT (OdT) | GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT | cDNA synthesis |
| Oligo dT adapter | GGCCACGCGTCGACTAGTAC | |
| TPM Fw | CAAACTACAGCCATGGATGC | Fragment PCR |
| TPM Rv | GAGATGCCTCTTGTTCACTG | Fragment PCR |
| TPM 5′ | GATTACGCCAAGCTTGTGACAGAGATGGAACAAGAGGTGAGTG | 5′ RACE PCR |
| TPM 3′ | GATTACGCCAAGCTT GAGAACCGGGCAACTGAGGCTGAAAG | 3′ RACE PCR |
| TPM-ORF-Fw | CAAACTACAGCCATGGATGC | Full length PCR |
| TPM-ORF-Rv | GCCATATGGATTTTGTAGGC | Full length PCR |
| TPM-qRT-Fw | CAGTGAACAAGAGGCATCTC | qPCR |
| TPM-qRT-Rv | CGTCACTAATGGCCTTGTAC | qPCR |
| Hdh-β-Actin-Fw | CCGTGAAAAGATGACCCAGA | qRT-PCR |
| Hdh-β-Actin-Rv | TACGACCGGAAGCGTACAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, M.A.; Hossen, S.; Lee, W.K.; Kho, K.H. Molecular Characterization of Tropomyosin and Its Potential Involvement in Muscle Contraction in Pacific Abalone. Genes 2023, 14, 2. https://doi.org/10.3390/genes14010002
Hanif MA, Hossen S, Lee WK, Kho KH. Molecular Characterization of Tropomyosin and Its Potential Involvement in Muscle Contraction in Pacific Abalone. Genes. 2023; 14(1):2. https://doi.org/10.3390/genes14010002
Chicago/Turabian StyleHanif, Md Abu, Shaharior Hossen, Won Kyo Lee, and Kang Hee Kho. 2023. "Molecular Characterization of Tropomyosin and Its Potential Involvement in Muscle Contraction in Pacific Abalone" Genes 14, no. 1: 2. https://doi.org/10.3390/genes14010002
APA StyleHanif, M. A., Hossen, S., Lee, W. K., & Kho, K. H. (2023). Molecular Characterization of Tropomyosin and Its Potential Involvement in Muscle Contraction in Pacific Abalone. Genes, 14(1), 2. https://doi.org/10.3390/genes14010002






