Molecular Mapping of Biofortification Traits in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP Based Linkage Map
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiments
2.2. Phenotyping for GFeC, GZnC, GPC, and TKW
2.3. Genotyping
2.4. Statistical Analysis and QTL Mapping
2.5. In Silico Analysis
3. Results
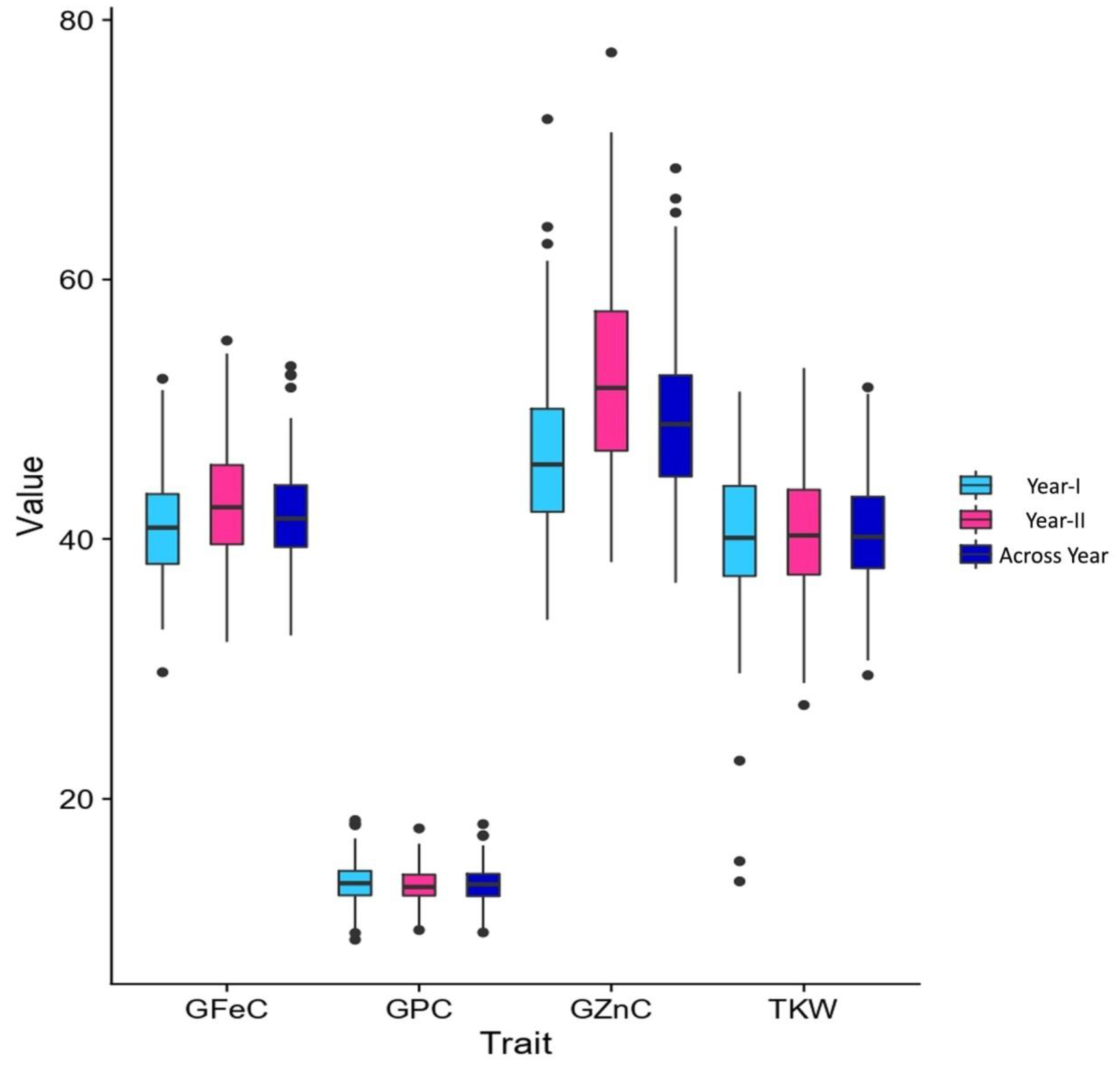
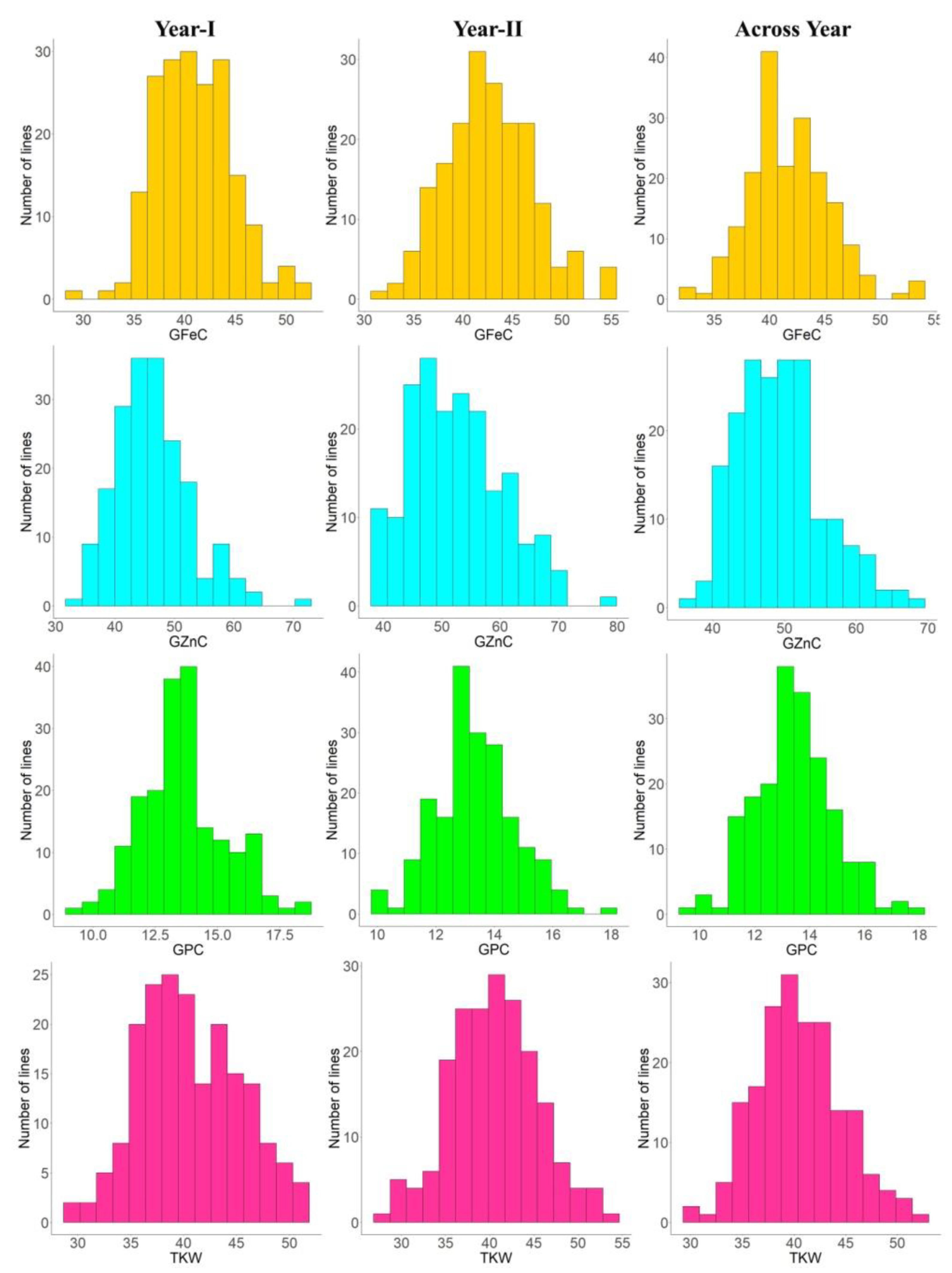
3.1. Variability and Correlations
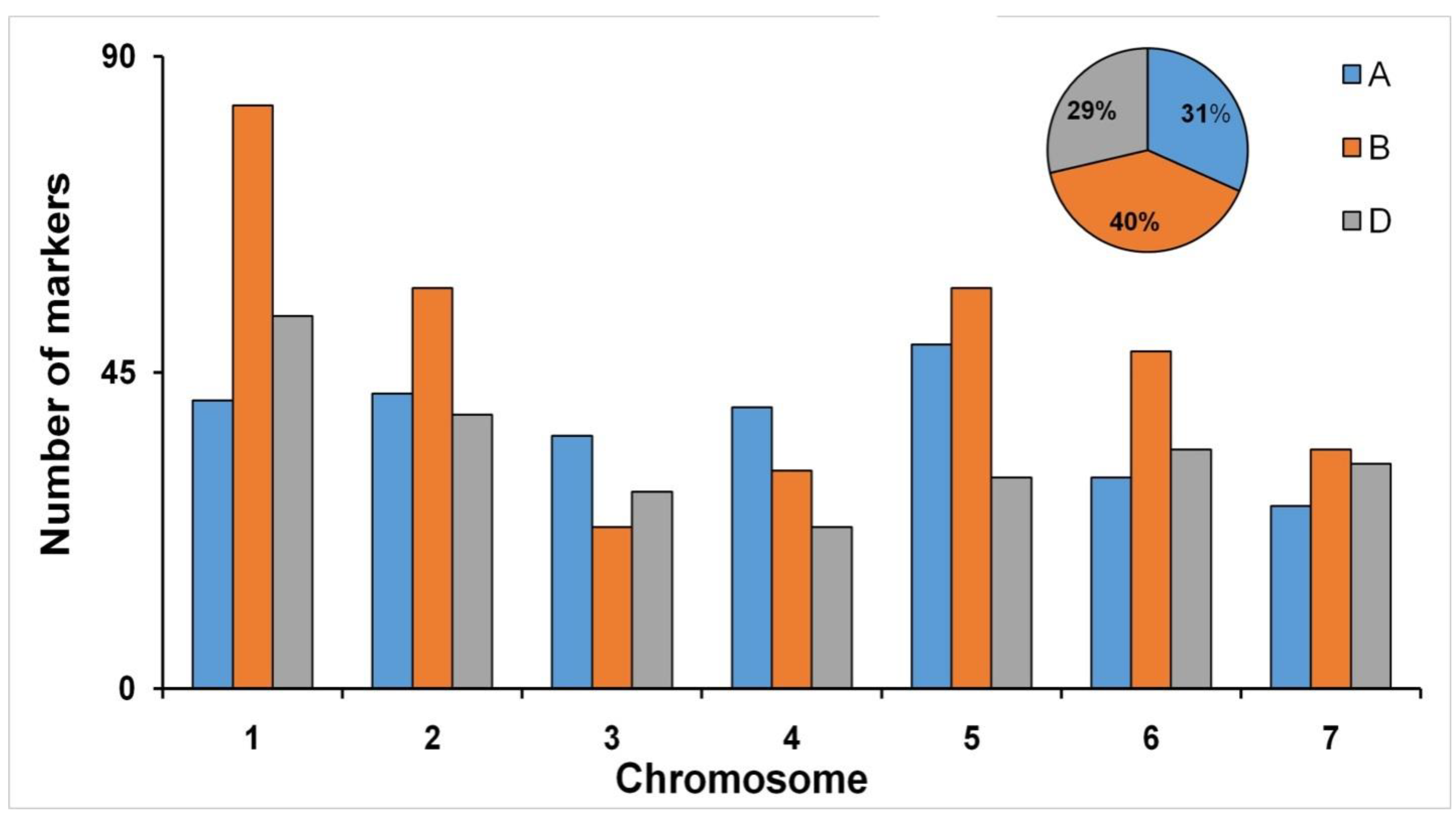
3.2. Genome-Wide Marker Distribution
3.3. Quantitative Trait Locus (QTL) Mapping
3.3.1. QTL Mapping for Grain Micronutrients
3.3.2. QTL Mapping for GPC and TKW
3.3.3. Stable and Co-Localised QTLs
3.4. Identification of Putative Candidate Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillespie, S.; Hodge, J.; Yosef, S.; Pandya-Lorch, R. Nourishing Millions: Stories of Change in Nutrition; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Palacios-Rojas, N.; Pixley, E.M.K.; Trethowan, R.; Pena, R.J. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J. Cereal. Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Anaemia Estimates, 2021 Edition. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 20 October 2022).
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative dataexternal icon. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Ackland, M.L.; Michalczyk, A.A. Zinc and infant nutrition. Arch. Biochem. Biophys. 2016, 611, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attribute able to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [Green Version]
- Kar, B.R.; Rao, S.L.; Chandramouli, B.A. Cognitive development in children with chronic protein energy malnutrition. Behav. Brain Funct. 2008, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triboi, E.; Triboi-Blondel, A.M. Productivity and grain or seed composition: A new approach to an old problem–invited paper. Eur. J. Agron. 2002, 16, 163–186. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, 105. [Google Scholar] [CrossRef] [Green Version]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to reduce zinc and iron deficits in food systems. Glob. Food Sec. 2017, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Pfeiffer, W.H.; Mcclafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. J. 2010, 87, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, A.; Jin, H.; Xiao, Y.; Zhang, Y.; Hao, Y.; Zhang, Y.; Hickey, L.T.; Morgounov, A.I.; Xia, X.; He, Z. Allelic effects and variations for key bread-making quali.ty genes in bread wheat using high-throughput molecular markers. J. Cereal Sci. 2019, 85, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2018, 8, 13526. [Google Scholar] [CrossRef] [Green Version]
- Çakmak, İ.; Torun, A.Y.F.E.R.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J.; Özkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Ozturk, L.; Yazici, A.M.; Budak, H.; Saranga, Y.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Plant Physiol. 2007, 129, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J.A. NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Calderini, D.F.; Ortiz-Monasterio, I. Are synthetic hexaploids a means of increasing grain element concentrations in wheat. Euphytica 2003, 134, 169–178. [Google Scholar] [CrossRef]
- Kumar, J.; Saripalli, G.; Gahlaut, V.; Goel, N.; Meher, P.K.; Mishra, K.K.; Mishra, P.C.; Sehgal, D.; Vikram, P.; Sansaloni, C.; et al. Genetics of Fe, Zn, b-carotene, GPC and yield traits in bread wheat (Triticum aestivum L.) using multi-locus and multi-traits GWAS. Euphytica 2018, 214, 219. [Google Scholar] [CrossRef]
- Hernández-Espinosa, N.; Mondal, S.; Autrique, E.; Gonzalez-Santoyo, H.; Crossa, J.; Huerta-Espino, J.; Singh, R.P.; Guzmán, C. Milling, processing and end-use quality traits of CIMMYT spring bread wheat germplasm under drought and heat stress. Field Crops Res. 2018, 215, 104–112. [Google Scholar] [CrossRef]
- Studnicki, M.; Wijata, M.; Sobczynski, G.; Samborski, S.; Gozdowski, D.; Rozbicki, J. Effect of genotype, environment and crop management on yield and quality traits in spring wheat. J. Cereal Sci. 2016, 72, 30–37. [Google Scholar] [CrossRef]
- Krishnappa, G.; Rathan, N.D.; Sehgal, D.; Ahlawat, A.K.; Singh, S.K.; Singh, S.K.; Shukla, R.B.; Jaiswal, J.P.; Solanki, I.S.; Singh, G.P.; et al. Identification of novel genomic regions for biofortification traits using an SNP marker-enriched linkage map in wheat (Triticum aestivum L.). Front. Nutr. 2021, 8, 669444. [Google Scholar] [CrossRef]
- Rathan, N.D.; Sehgal, D.; Thiyagarajan, K.; Singh, R.; Singh, A.-M.; Govindan, V. Identification of genetic loci and candidate genes related to grain zinc and iron concentration using a zinc enriched wheat ‘Zinc-Shakti’. Front. Genet. 2021, 12, 652653. [Google Scholar] [CrossRef]
- Goel, S.; Singh, K.; Singh, B.; Grewal, S.; Dwivedi, N.; Alqarawi, A.A.; Abd_Allah, E.F.; Ahmad, P.; Singh, N.K. Analysis of genetic control and QTL mapping of essential wheat grain quality traits in a recombinant inbred population. PLoS ONE 2019, 14, e0200669. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, B.; Singh, R.P.; Velu, G. QTL mapping for micronutrients concentration and yield component traits in a hexaploid wheat mapping population. J. Cereal Sci. 2019, 88, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, G.; Singh, A.M.; Chaudhary, S.; Ahlawat, A.K.; Singh, S.K.; Shukla, R.B.; Jaiswal, J.P.; Singh, G.P.; Solanki, I.S. Molecular mapping of the grain iron and zinc concentration, protein content and thousand kernel weight in wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0174972. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Tutus, Y.; Gomez-Becerra, H.F.; Hao, Y.; Demir, L.; Kara, R.; Crespo-Herrera, L.A.; Orhan, S.; Yazici, A.; Singh, R.P.; et al. QTL mapping for grain zinc and iron concentrations and zinc efficiency in a tetraploid and hexaploid wheat mapping populations. Plant Soil 2017, 411, 81–99. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, R.; Deng, Z.; Zhang, K.; Liu, B.; Tian, J. Conditional QTL mapping of three yield components in common wheat (Triticum aestivum L.). Crop J. 2016, 4, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Bai, S.; Li, J.; Hou, X.; Wang, X.; Li, H.; Zhang, B.; Chen, W.; Liu, D.; Liu, B.; et al. QTL positioning of thousand wheat grain weight in qaidam basin. J. Genet. 2014, 4, 239–244. [Google Scholar] [CrossRef]
- Mergoum, M.; Harilal, V.E.; Simsek, S.; Alamri, M.S.; Schatz, B.G.; Kianian, S.F.; Elias, E.; Kumar, A.; Bassi, F.M. Agronomic and quality QTL mapping in spring wheat. J. Plant Breed. Genet. 2013, 1, 19–33. [Google Scholar]
- Nezhad, K.Z.; Weber, W.E.; Roder, M.S.; Sharma, S.; Lohwasser, U.; Meyer, R.C.; Saal, B.; Borner, A. QTL analysis for thousand-grain weight under terminal drought stress in bread wheat (Triticum aestivum L.). Euphytica 2012, 186, 127–138. [Google Scholar] [CrossRef]
- Krishnappa, G.; Khan, H.; Krishna, H.; Kumar, S.; Mishra, C.N.; Parkash, O.; Devate, N.B.; Nepolean, T.; Rathan, N.D.; Mamrutha, H.M.; et al. Genetic dissection of grain iron and zinc, and thousand kernel weight in wheat (Triticum aestivum L.) using genome-wide association study. Sci. Rep. 2022, 12, 12444. [Google Scholar] [CrossRef] [PubMed]
- Rathan, N.D.; Krishna, H.; Ellur, R.K.; Sehgal, D.; Govindan, V.; Ahlawat, A.K.; Krishnappa, G.; Jaiswal, J.P.; Singh, J.B.; Sv, S.; et al. Genome-wide association study identifies loci and candidate genes for grain micronutrients and quality traits in wheat (Triticum aestivum L.). Sci. Rep. 2022, 12, 7037. [Google Scholar] [CrossRef]
- Gahlaut, V.; Jaiswal, V.; Balyan, H.S.; Joshi, A.K.; Gupta, P.K. Multi-locus GWAS for grain weight-related traits under rain-fed conditions in common wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 7531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, L.; Li, T.; Liu, Y.; Yan, Z.; Tang, G.; Zheng, Y.; Liu, D.; Wu, B. Genome-wide association study for grain micronutrient concentrations in wheat advanced lines derived from wild Emmer. Front. Plant Sci. 2021, 12, 651283. [Google Scholar] [CrossRef] [PubMed]
- Cu, S.T.; Guild, G.; Nicolson, A.; Velu, G.; Singh, R.; Stangoulis, J. Genetic dissection of zinc, iron, copper, manganese and phosphorus in wheat (Triticum aestivum L.) grain and rachis at two developmental stages. Plant Sci. 2020, 291, 110338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, X.; Zhao, G.; Qin, M.; Ibba, M.I.; Wang, Y.; Li, W.; Yang, P.; Wu, Z.; Lei, Z.; et al. Identification of novel genomic regions and superior alleles associated with Zn accumulation in wheat using a genome-wide association analysis method. Int. J. Mol. Sci. 2020, 21, 1928. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.P.; Brown-Guedira, G.; Kolb, F.L.; Van Sanford, D.A.; Tyagi, P.; Sneller, C.H.; Griffey, C.A. Genome-wide association studies for yield-related traits in soft red winter wheat grown in Virginia. PLoS ONE 2019, 14, e0208217. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Cheema, J.; Poland, J.; Uauy, C.; Chhuneja, P. Genome-wide association mapping of grain micronutrients concentration in Aegilops tauschii. Front. Plant Sci. 2019, 10, 54. [Google Scholar] [CrossRef]
- Bhatta, M.; Baenziger, P.S.; Waters, B.M.; Poudel, R.; Belamkar, V.; Poland, J.; Morgounov, A. Genome-wide association study reveals novel genomic regions associated with 10 grain minerals in synthetic hexaploid wheat. Int. J. Mol. Sci. 2018, 19, 3237. [Google Scholar] [CrossRef]
- Godoy, J.; Gizaw, S.; Chao, S.; Blake, N.; Carter, A.; Cuthbert, R.; Dubcovsky, J.; Hucl, P.; Kephart, K.; Pozniak, C.; et al. Genome-wide association study of agronomic traits in a spring-planted North American elite hard red spring wheat panel. Crop Sci. 2018, 58, 1838–1852. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, Y.; Bihamta, M.R.; Taleei, A.; Alipour, H.; Ingvarsson, P.K. Genome-wide association study of agronomic traits in bread wheat reveals novel putative alleles for future breeding programs. BMC Plant Biol. 2019, 19, 541. [Google Scholar] [CrossRef] [Green Version]
- Gajghate, R. Mapping of QTL for Drought Tolerance Related Traits in Bread Wheat (Triticum aestivum L. emThell) Using Recombinant Inbred Lines; ICAR-Indian Agricultural Research Institute: New Delhi, India, 2021. [Google Scholar]
- Murray, M.G.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Kosambi, D.D. The estimation of map distances from recombination values. Annal. Hum. Gene 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Horti. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of gene symbols for wheat. In Proceedings of the 12th International Wheat Genetics Symposium, Yokohama, Japan, 8–14 September 2013. [Google Scholar]
- Gollhofer, J.; Schlawicke, C.; Jungnick, N.; Schmidt, W.; Buckhout, T.J. Members of a small family of nodulin-like genes are regulated under iron deficiency in roots of Arabidopsis thaliana. Plant Physiol. Biochem. 2011, 49, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Mager, S.; Schonberger, B.; Ludewig, U. The transcriptome of zinc deficient maize roots and its relationship to DNA methylation loss. BMC Plant Biol. 2018, 18, 372. [Google Scholar] [CrossRef] [Green Version]
- Waters, B.M.; Uauy, C.; Dubcovsky, J.; Grusak, M.A. Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J. Exp. Bot. 2009, 60, 4263–4274. [Google Scholar] [CrossRef] [Green Version]
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A. kNACking on heaven’s door: How important are NAC transcription factors for leaf senescence and Fe/Zn remobilization to seeds? Front. Plant Sci. 2013, 4, 226. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Gao, S.; Liu, L.; Xu, F.; Che, R.; Wang, Y.; Tong, H.; Chu, C. Big Grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice. J. Integr. Plant Biol. 2019, 61, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Dionisio, G.; Uddin, M.N.; Vincze, E. Enrichment and identification of the most abundant Zinc binding proteins in developing barley grains by Zinc-IMAC capture and nano LC-MS/MS. Proteomes 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Wang, Q.; Li, Z.; Cheng, H.; Li, Z.; Liu, X.; Song, W.; Appels, R.; Zhao, H. Expression of TaCYP78A3, a gene encoding cytochrome P450 CYP78A3 protein in wheat (Triticum aestivum L.), affects seed size. Plant J. 2015, 83, 312–325. [Google Scholar] [CrossRef]
- Waqas, M.; Feng, S.; Amjad, H.; Letuma, P.; Zhan, W.; Li, Z.; Fang, C.; Arafat, Y.; Khan, M.U.; Tayyab, M.; et al. Protein phosphatase (pp2c9) induces protein expression differentially to mediate nitrogen utilization efficiency in rice under nitrogen-deficient condition. Int. J. Mol. Sci. 2018, 19, 2827. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, A.; Wang, M.; Zhu, Z.; Ouwerkerk, P.B. Functions of the CCCH type zinc finger protein OsGZF1 in regulation of the seed storage protein GluB-1 from rice. Plant Mol. Biol. 2014, 84, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Akhter, D.; Chengcong, Y.; Nath, U.K.; Eshag, J.; Xiaoli, J.; Chunhai, S. SRG1 encoding a kinesin-4 protein, is an important factor for determining grain shape in rice. Rice Sci. 2018, 25, 297–307. [Google Scholar] [CrossRef]
- Gopalareddy, K.; Singh, A.M.; Ahlawat, A.K.; Singh, G.P.; Jaiswal, J.P. Genotype-environment interaction for grain iron and zinc concentration in recombinant inbred lines of a bread wheat (Triticum aestivum L.) cross. Indian J. Gene Plant Breed 2015, 75, 307–313. [Google Scholar] [CrossRef]
- Krishnappa, G.; Ahlawat, A.K.; Shukla, R.B.; Singh, S.K.; Singh, S.K.; Singh, A.M.; Singh, G.P. Multi-environment analysis of grain quality traits in recombinant inbred lines of a biparental cross in bread wheat (Triticum aestivum L.). Cereal Res. Commun. 2019, 47, 334–344. [Google Scholar] [CrossRef]
- Devate, N.B.; Krishna, H.; Sunilkumar, V.P.; Manjunath, K.K.; Mishra, C.N.; Jain, N.; Singh, G.P.; Singh, P.K. Identification of genomic regions of wheat associated with grain Fe and Zn content under drought and heat stress using genome-wide association study. Front. Genet. 2022, 13, 1034947. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, G.; Guo, B.; Qu, C.; Zhang, M.; Kong, F.; Zhao, Y.; Li, S. QTL mapping for quality traits using a high-density genetic map of wheat. PLoS ONE 2020, 15, e0230601. [Google Scholar] [CrossRef] [PubMed]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum × wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef]
- Marcotuli, I.; Gadaleta, A.; Mangini, G.; Signorile, A.M.; Zacheo, S.A.; Blanco, A.; Simeone, R.; Colasuonno, P. Development of a High-Density SNP-Based Linkage Map and Detection of QTL for β-Glucans, Protein Content, Grain Yield per Spike and Heading Time in Durum Wheat. Int. J. Mol. Sci. 2017, 18, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.; Fan, X.; Chen, M.; Zhang, N.; Zhao, C.; Zhang, W.; Han, J.; Ji, J.; Zhao, X.; Yang, L.; et al. QTL detection for wheat kernel size and quality and the responses of these traits to low nitrogen stress. Theor. Appl. Genet. 2016, 129, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, K.; Zhao, Y.; Qian, Z.; Kong, F.; Guo, Y.; Wang, Y.; Li, S. Molecular genetic analysis of grain protein content and tour whiteness degree using RILs in common wheat. J. Genet. 2016, 95, 317–324. [Google Scholar] [CrossRef]
- Mahjourimajd, S.; Taylor, J.; Rengel, Z.; Khabaz-Saberi, H.; Kuchel, H.; Okamoto, M.; Langridge, P. The genetic control of grain protein content under variable nitrogen supply in an Australian wheat mapping population. PLoS ONE 2016, 11, e0159371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, D.; Gadaleta, A.; Mangini, G.; Colasuonno, P.; Marcotuli, I.; Giancaspro, A.; Giove, S.L.; Simeone, R.; Blanco, A. Candidate genes and genome-wide association study of grain protein content and protein deviation in durum wheat. Planta 2019, 249, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, X.; Zhou, Z.; Qin, M.; Wang, Y.; Li, W.; Yang, P.; Wu, Z.; Lei, Z. Genetic dissection of grain iron concentration in hexaploid wheat (Triticum aestivum L.) using a genome-wide association analysis method. PeerJ 2022, 10, e13625. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, L.; Wang, C.; Liu, Y.; Yan, Z.; Wang, Z.; Xiang, L.; Zhong, X.; Gong, F.; Zheng, Y.; et al. Genome-Wide Association Study Reveals Novel Genomic Regions Associated With High Grain Protein Content in Wheat Lines Derived From Wild Emmer Wheat. Front. Plant Sci. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Lou, H.; Wang, H.; Shi, J.; Liu, D.; Baogerile; Tao, J.; Miao, S.; Pei, Q.; Yu, L.; et al. Genome-Wide Association Study Reveals the Genetic Basis of Five Quality Traits in Chinese Wheat. Front. Plant Sci. 2022, 13, 835306. [Google Scholar] [CrossRef] [PubMed]


| Trait | Env. | HD2932 | SYN46 | RIL Population | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean + S.D | CV% | h2 (bs) | GCV | ECV | GA | ||||
| GFeC | Year-I | 35.10 | 45.05 | 29.75–52.35 | 40.96 ± 1.85 | 04.52 | 48.46 | 08.64 | 04.53 | 6.46 |
| Year-II | 32.40 | 46.25 | 32.10–55.30 | 42.61 ± 3.63 | 08.53 | 50.23 | 08.57 | 08.53 | 5.33 | |
| Across years | 33.75 | 45.65 | 32.60–53.32 | 41.79 ± 2.76 | 06.62 | 55.23 | 07.35 | 06.62 | 4.70 | |
| GZnC | Year-I | 46.95 | 56.30 | 33.80–72.35 | 46.30 ± 4.00 | 08.63 | 65.98 | 12.02 | 08.63 | 9.31 |
| Year-II | 43.21 | 58.45 | 38.25–77.50 | 52.55 ± 6.67 | 12.69 | 47.96 | 12.18 | 12.69 | 9.13 | |
| Across years | 45.08 | 57.37 | 36.65–68.57 | 49.42 ± 5.32 | 10.76 | 44.57 | 09.65 | 10.76 | 6.56 | |
| GPC | Year-I | 11.61 | 13.35 | 09.16–18.38 | 13.50 ± 0.59 | 04.32 | 88.26 | 11.84 | 04.32 | 3.11 |
| Year-II | 10.16 | 13.60 | 09.90–17.73 | 13.33 ± 0.86 | 06.48 | 65.52 | 08.93 | 06.48 | 1.98 | |
| Across years | 10.88 | 13.47 | 09.71–18.05 | 13.45 ± 0.75 | 05.60 | 75.00 | 07.35 | 06.62 | 4.70 | |
| TKW | Year-I | 34.93 | 46.15 | 25.20–51.35 | 40.36 ± 6.55 | 16.22 | 51.26 | 15.97 | 16.22 | 2.35 |
| Year-II | 35.43 | 46.27 | 27.24–53.17 | 40.48 ± 3.40 | 08.39 | 61.18 | 10.54 | 08.39 | 6.87 | |
| Across years | 35.18 | 46.21 | 26.22–52.26 | 40.54 ± 3.39 | 08.36 | 49.85 | 08.34 | 08.36 | 4.91 | |
| Trait | QTL Name | Env. | Position | Flanking Markers | LOD | PVE (%) | Add | Confidence Interval |
|---|---|---|---|---|---|---|---|---|
| GFeC | QGfec.iari_5B | Year I | 680 | AX-94797162–Xgwm159 | 3.9 | 09.0 | 1.42 | 670.5–698.5 |
| Across Years | 681 | AX-94797162–Xgwm159 | 2.7 | 06.7 | 1.10 | 670.5–686.5 | ||
| QGfec.iari_6B | Across Years | 299 | AX-94520583–AX-94387975 | 2.9 | 05.2 | −0.97 | 292.5–305.5 | |
| GZnC | QGznc.iari_7A | Year I | 349 | AX-94575185–AX-94708164 | 2.8 | 06.6 | 1.98 | 338.5–363.5 |
| GPC | QGpc.iari_1B | Year I | 72 | Xwmc406–Xgwm124 | 2.6 | 04.9 | 0.50 | 60.5–84.5 |
| Across Years | 72 | Xwmc406–Xgwm124 | 2.6 | 04.9 | 0.50 | 60.5–84.5 | ||
| QGpc.iari_4A | Year I | 389 | AX-94409394–Xwmc698 | 2.7 | 10.0 | 0.72 | 371.5–409.5 | |
| Across Years | 389 | AX-94409394–Xwmc698 | 2.7 | 10.0 | 0.72 | 371.5–409.5 | ||
| QGpc.iari_4B | Year I | 0 | Xgwm149–AX-94559916 | 3.0 | 03.7 | 0.44 | 0–21.5 | |
| Year II | 0 | Xgwm149–AX-94559916 | 5.0 | 07.4 | 0.42 | 0–14.5 | ||
| Across Years | 0 | Xgwm149–AX-94559916 | 3.0 | 03.7 | 0.44 | 0–21.5 | ||
| QGpc.iari_5D | Year II | 141 | Xcfd29–AX-94687667 | 2.7 | 10.7 | −0.50 | 128.5–159.5 | |
| QGpc.iari_6B | Year II | 298 | AX-94996310–AX-94520583 | 3.9 | 05.6 | −0.37 | 293.5–302.5 | |
| TKW | QTkw.iari_4B | Year I | 0 | Xgwm149–AX-94559916 | 3.7 | 10.5 | 1.77 | 0–11.5 |
| Year II | 0 | Xgwm149–AX-94559916 | 5.6 | 13.4 | 1.75 | 0–12.5 |
| Trait | QTL Name | Marker Interval | TraesID | Putative Candidate Genes | Functions |
|---|---|---|---|---|---|
| GFeC | QGfec.iari_6B | AX-94520583-AX-94387975 | TraesCS6B02G127300 | L-aspartate oxidase | – |
| TraesCS6B02G086000 | F-box domain | – | |||
| GZnC | QGznc.iari_7A | AX-94575185–AX-94708164 | TraesCS7A02G041000 | P-loop containing nucleoside triphosphate hydrolase | Zinc ion binding |
| TraesCS7A02G000900 | Protein kinase domain | – | |||
| TraesCS7A02G000800 | Nodulin-like protein | Iron homeostasis in arabidopsis [51], Zinc transportation in Maize [52] | |||
| TraesCS7A02G000300 | NAC domain | Zn, Fe and Protein remobilization to the developing grain [19]. Translocation of iron, zinc, and nitrogen from vegetative tissues to grain [53], Zn and Fe remobilization to seeds in Rice [54] | |||
| GPC | QGpc.iari_1B | Xwmc406–Xgwm124 | TraesCS1B02G413500 | Purine permease | Regulates grain size via modulating cytokinin transport in rice [55] |
| QGpc.iari_4A | AX-94409394–Xwmc698 | TraesCS4A02G019000 | Zinc-binding ribosomal protein | Binding of barley grain proteins [56] | |
| TraesCS4A02G019400 | Cytochrome P450 | Regulates grain size by affecting the extent of integument cell proliferation [57] | |||
| TraesCS4A02G341600 | Protein phosphatase 2A | Increased nitrogen use efficiency in Rice [58] | |||
| TraesCS4A02G341500 | GDSL lipase/esterase | – | |||
| QGpc.iari_6B | AX-94996310–AX-94520583 | TraesCS6B02G167200 | Zinc finger, CCCH-type | Regulation of GluB-1 promoter and controls the accumulation of glutelins protein during grain development in Rice [59] | |
| TKW & GPC | QGpc.iari_4B | Xgwm149-AX-94559916 | TraesCS4B02G269800 | Kinesin motor domain | Grain shape in rice [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadon, V.; Sharma, S.; Krishna, H.; Krishnappa, G.; Gajghate, R.; Devate, N.B.; Panda, K.K.; Jain, N.; Singh, P.K.; Singh, G.P. Molecular Mapping of Biofortification Traits in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP Based Linkage Map. Genes 2023, 14, 221. https://doi.org/10.3390/genes14010221
Jadon V, Sharma S, Krishna H, Krishnappa G, Gajghate R, Devate NB, Panda KK, Jain N, Singh PK, Singh GP. Molecular Mapping of Biofortification Traits in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP Based Linkage Map. Genes. 2023; 14(1):221. https://doi.org/10.3390/genes14010221
Chicago/Turabian StyleJadon, Vasudha, Shashi Sharma, Hari Krishna, Gopalareddy Krishnappa, Rahul Gajghate, Narayana Bhat Devate, Kusuma Kumari Panda, Neelu Jain, Pradeep Kumar Singh, and Gyanendra Pratap Singh. 2023. "Molecular Mapping of Biofortification Traits in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP Based Linkage Map" Genes 14, no. 1: 221. https://doi.org/10.3390/genes14010221
APA StyleJadon, V., Sharma, S., Krishna, H., Krishnappa, G., Gajghate, R., Devate, N. B., Panda, K. K., Jain, N., Singh, P. K., & Singh, G. P. (2023). Molecular Mapping of Biofortification Traits in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP Based Linkage Map. Genes, 14(1), 221. https://doi.org/10.3390/genes14010221






