The Role of Pancreatic Fatty Acid Synthesis in Islet Morphology and Function after Caloric Restriction or Roux-En-Y Gastric Bypass Surgery in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatments
2.3. RYGB Procedures
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.5. Measurement of Plasma Insulin
2.6. Histological Analysis
2.7. Immunohistochemistry
2.8. Determination of Triglycerides in the Pancreas
2.9. Western Blot Analysis
2.10. RNA Extraction, Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
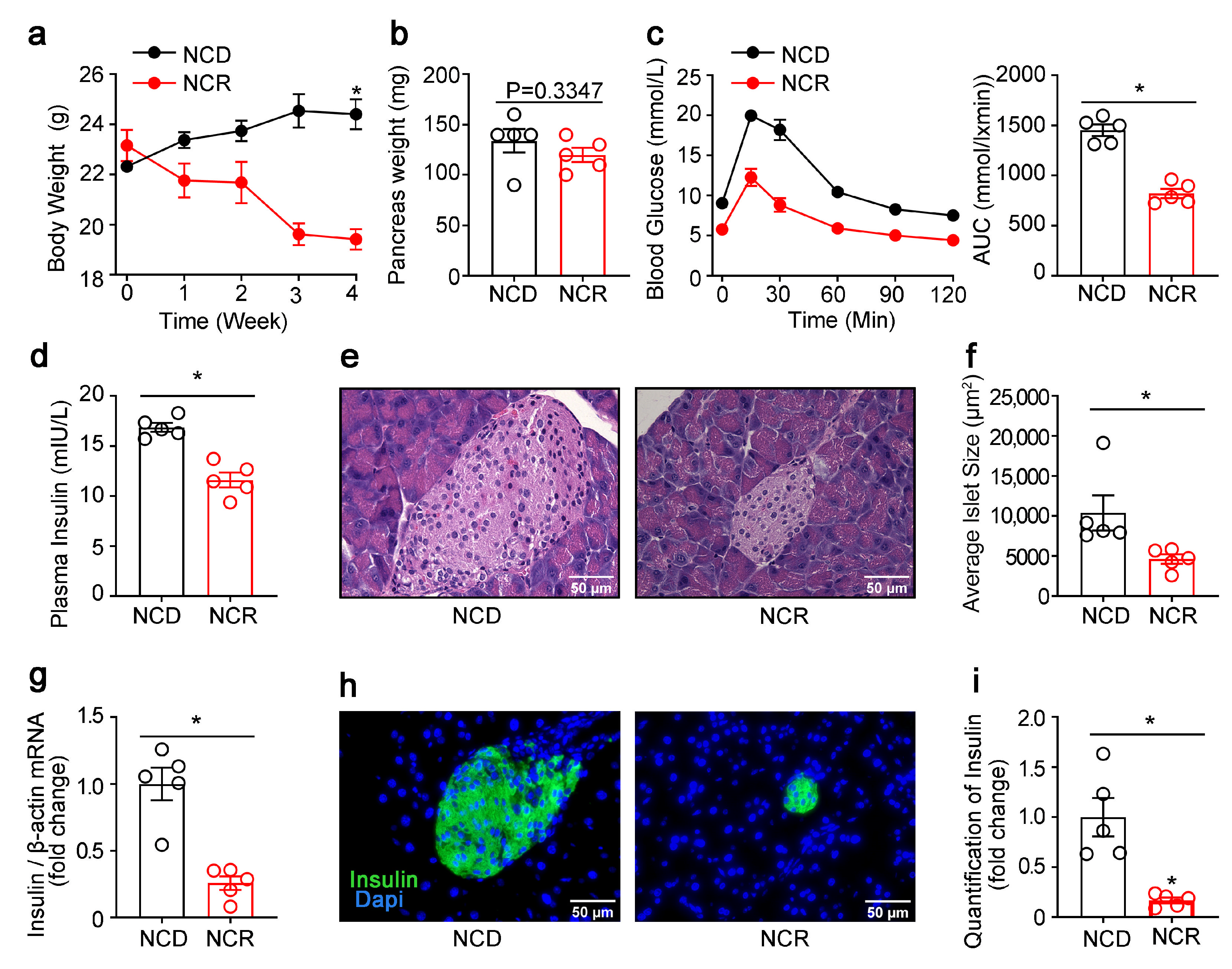
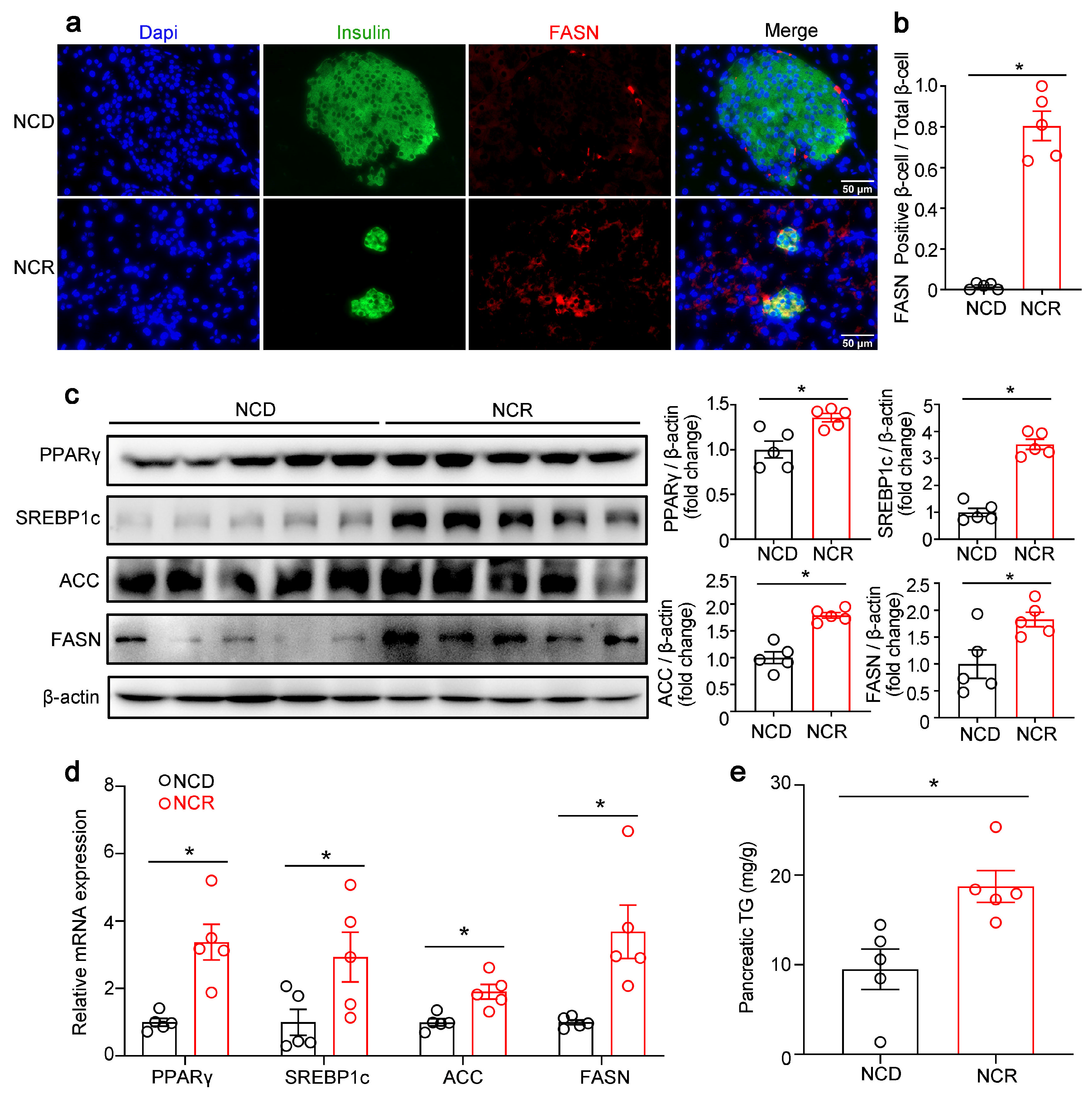
3.1. Effects of CR on the Morphology of Islets and Pancreatic Lipogenic Gene Expression in Normal Mice
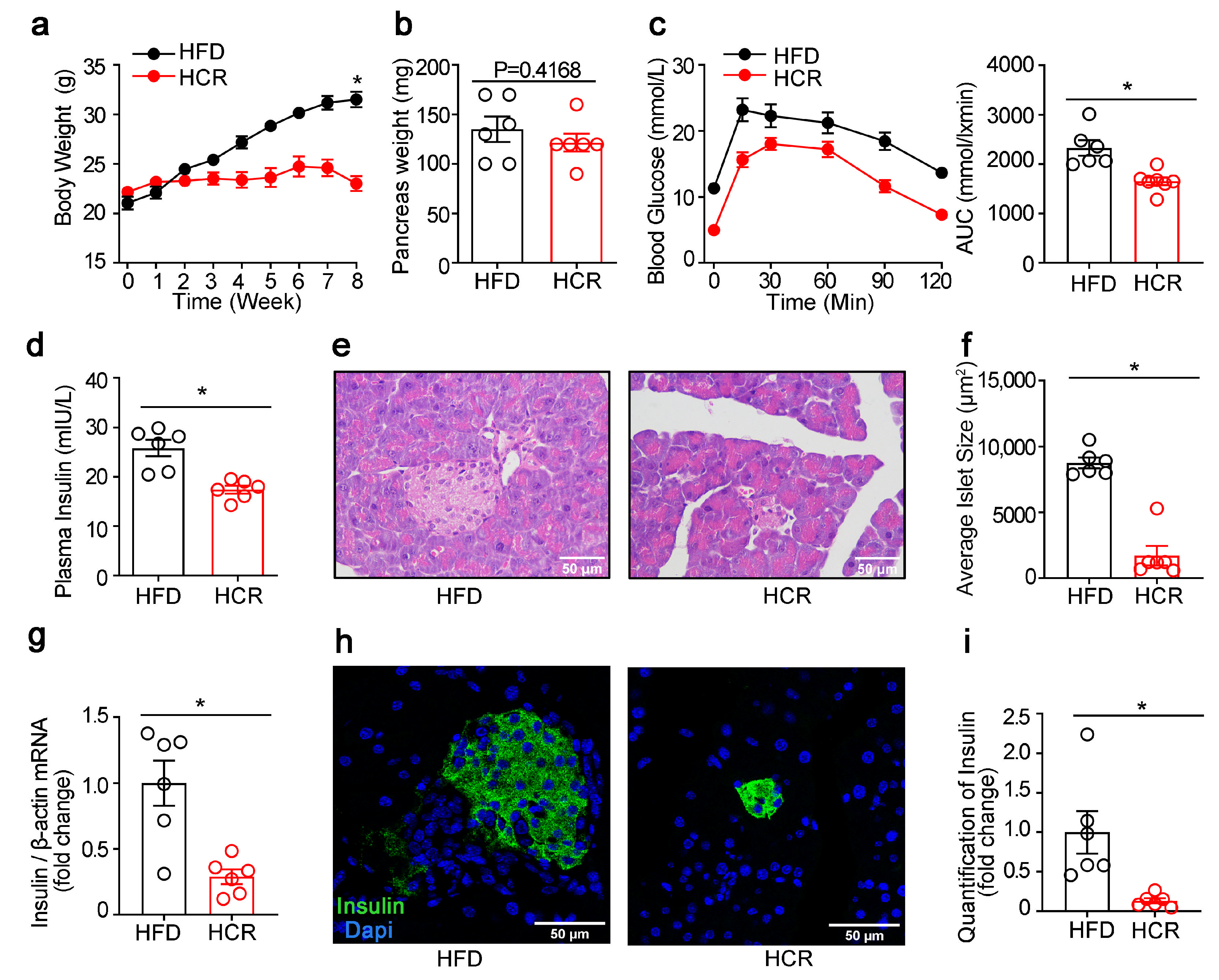
3.2. Effects of CR on the Function and Morphology of Pancreatic Islets as well as Lipogenic Gene Expression in Diabetic Mice
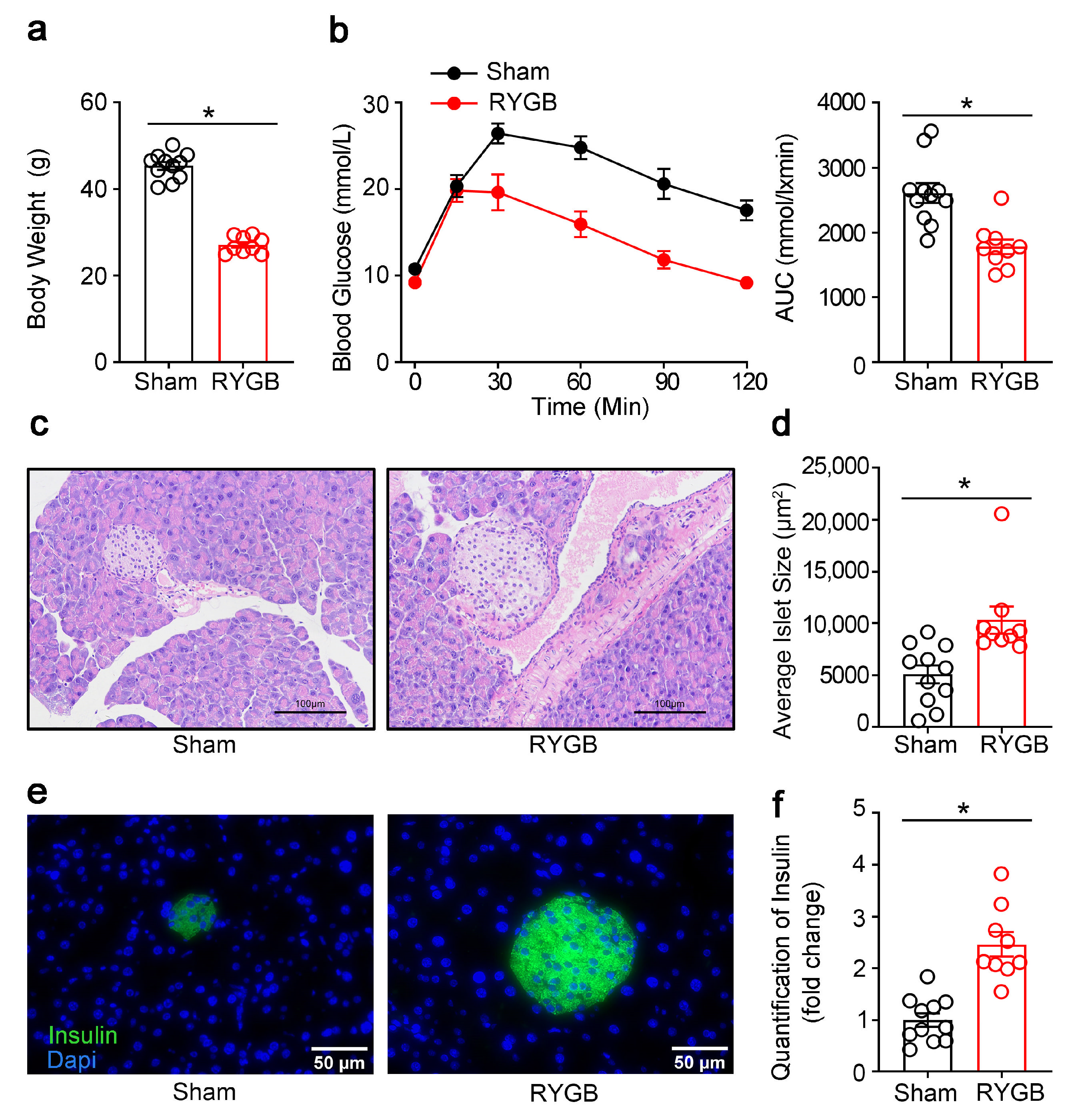
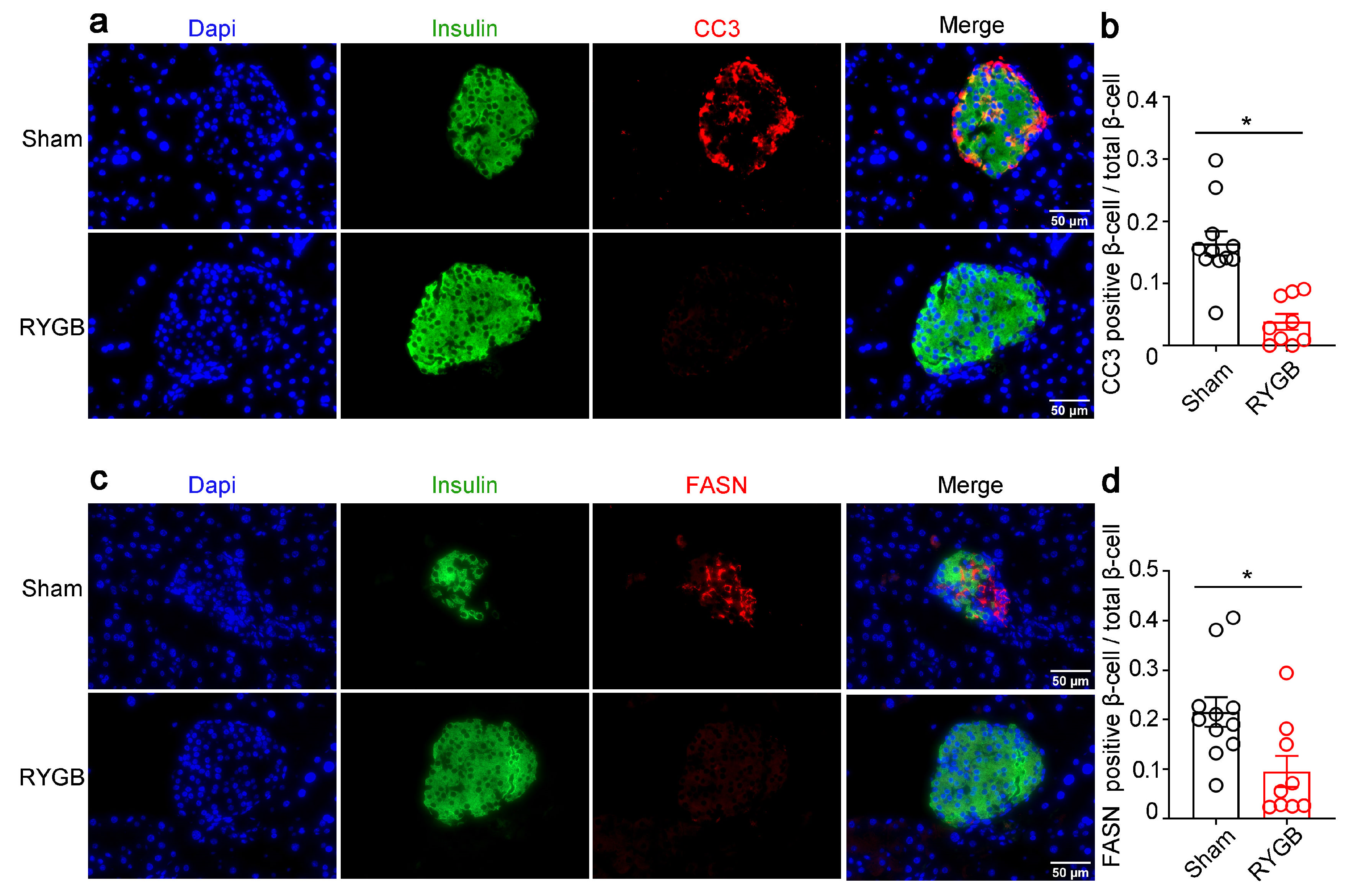
3.3. Effects of RYGB on the Islet Morphology in Fatty Diet-Induced Diabetic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CR | Caloric restriction |
| RYGB | Roux-en-Y gastric bypass |
| SREBP1c | Sterol regulatory element-binding protein-1c |
| Fas | Death receptor Fas/CD95 |
| FASN | Fatty acid synthase |
| ACC | Acetyl-CoA carboxylase |
| FFA | Free fatty acid |
| TG | Triglyceride |
| CC3 | Cleaved caspase-3 |
| GSIS | Glucose-stimulated insulin secretion |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Tschen, S.I.; Dhawan, S.; Gurlo, T.; Bhushan, A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009, 58, 1312–1320. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Given, B.D.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Investig. 1988, 81, 442–448. [Google Scholar] [CrossRef]
- Costes, S.; Langen, R.; Gurlo, T.; Matveyenko, A.V.; Butler, P.C. β-Cell failure in type 2 diabetes: A case of asking too much of too few? Diabetes 2013, 62, 327–335. [Google Scholar] [CrossRef]
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Shetty, S.; Orci, L.; Unger, R.H.; Scherer, P.E. Diabetes and apoptosis: Lipotoxicity. Apoptosis 2009, 14, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Haataja, L.; Gurlo, T.; Huang, C.J.; Butler, P.C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 2008, 29, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Kanda, Y.; Hashiramoto, M.; Shimoda, M.; Hamamoto, S.; Tawaramoto, K.; Kimura, T.; Hirukawa, H.; Nakashima, K.; Kaku, K. Dietary restriction preserves the mass and function of pancreatic β cells via cell kinetic regulation and suppression of oxidative/ER stress in diabetic mice. J. Nutr. Biochem. 2015, 26, 219–226. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Cline, G.W.; Wang, Y.; Rabin-Court, A.; Song, J.D.; Zhang, D.; Zhang, X.M.; Nozaki, Y.; Dufour, S.; et al. Mechanisms by which a Very-Low-Calorie Diet Reverses Hyperglycemia in a Rat Model of Type 2 Diabetes. Cell Metab. 2018, 27, 210–217.e3. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, W.; Wu, J.; Gong, L.; Li, Q.; Xiao, X.; Zhang, J.; Wang, Z. Increased beta-Cell Mass in Obese Rats after Gastric Bypass: A Potential Mechanism for Improving Glycemic Control. Med. Sci. Monit. 2017, 23, 2151–2158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosinski, J.D.; Aminian, A.; Axelrod, C.L.; Batayyah, E.; Romero-Talamas, H.; Daigle, C.; Mulya, A.; Scelsi, A.; Schauer, P.R.; Brethauer, S.A.; et al. Roux-en-Y gastric bypass restores islet function and morphology independent of body weight in ZDF rats. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E392–E398. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Spégel, P.; Ekelund, M.; Garcia Vaz, E.; Pierzynowski, S.; Gomez, M.F.; Mulder, H.; Hedenbro, J.; Groop, L.; Wierup, N. Gastric bypass improves β-cell function and increases β-cell mass in a porcine model. Diabetes 2014, 63, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Mark, V.; Ligon, C.; Dutia, R.; Nair, N.; Shah, A.; Laferrère, B. Role of the Gut in the Temporal Changes of β-Cell Function After Gastric Bypass in Individuals With and Without Diabetes Remission. Diabetes Care 2022, 45, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Chester, M.W.; Daniels, M.B.; McGarry, J.D.; Stein, D.T. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 1998, 47, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.T.; Esser, V.; Stevenson, B.E.; Lane, K.E.; Whiteside, J.H.; Daniels, M.B.; Chen, S.; McGarry, J.D. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Investig. 1996, 97, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Diraison, F.; Parton, L.; Ferré, P.; Foufelle, F.; Briscoe, C.P.; Leclerc, I.; Rutter, G.A. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: Modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Biochem. J. 2004, 378, 769–778. [Google Scholar] [CrossRef]
- Andreolas, C.; da Silva Xavier, G.; Diraison, F.; Zhao, C.; Varadi, A.; Lopez-Casillas, F.; Ferré, P.; Foufelle, F.; Rutter, G.A. Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Diabetes 2002, 51, 2536–2545. [Google Scholar] [CrossRef]
- Dubois, M.; Kerr-Conte, J.; Gmyr, V.; Bouckenooghe, T.; Muharram, G.; D’Herbomez, M.; Martin-Ponthieu, A.; Vantyghem, M.C.; Vandewalle, B.; Pattou, F. Non-esterified fatty acids are deleterious for human pancreatic islet function at physiological glucose concentration. Diabetologia 2004, 47, 463–469. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.P.; Grill, V.E. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J. Clin. Investig. 1994, 93, 870–876. [Google Scholar] [CrossRef]
- Nolan, C.J.; Leahy, J.L.; Delghingaro-Augusto, V.; Moibi, J.; Soni, K.; Peyot, M.L.; Fortier, M.; Guay, C.; Lamontagne, J.; Barbeau, A.; et al. Beta cell compensation for insulin resistance in Zucker fatty rats: Increased lipolysis and fatty acid signalling. Diabetologia 2006, 49, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Li, Z.; Peng, M.; Huang, Z.; Qin, T.; Chen, L.; Li, H.; Zhang, H.; Zhang, W.; Xu, G. Takeda G Protein-Coupled Receptor 5-Mechanistic Target of Rapamycin Complex 1 Signaling Contributes to the Increment of Glucagon-Like Peptide-1 Production after Roux-en-Y Gastric Bypass. EBioMedicine 2018, 32, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Rahier, J.; Guiot, Y.; Goebbels, R.; Sempoux, C.; Henquin, J.-C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, R.Y.; Song, J.; Guan, Y.F.; Xu, T.Y.; Du, H.; Viollet, B.; Miao, C.Y. Loss of AMP-activated protein kinase-α2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes 2012, 61, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Li, F.; Lin, Z.; Zhang, M.; Yang, P.; Bu, L.; Sheng, H.; Li, H.; Qu, S. Reversibility of β-Cell-Specific Transcript Factors Expression by Long-Term Caloric Restriction in db/db Mouse. J. Diabetes Res. 2016, 2016, 6035046. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, D.; Zhao, Y.; Tao, H.; Zhou, Y. Moderate calorie restriction to achieve normal weight reverses β-cell dysfunction in diet-induced obese mice: Involvement of autophagy. Nutr. Metab. 2015, 12, 34. [Google Scholar] [CrossRef]
- Loweth, A.C.; Williams, G.T.; James, R.F.; Scarpello, J.H.; Morgan, N.G. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1beta and Fas ligation. Diabetes 1998, 47, 727–732. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yu, H.; Cooper, G.J. Fas-associated death receptor signaling evoked by human amylin in islet beta-cells. Diabetes 2008, 57, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Wang, Y.; Geng, D.; Liu, J. Roux-en-Y gastric bypass-induced improvement of glucose tolerance and insulin resistance in type 2 diabetic rats are mediated by glucagon-like peptide-1. Obes. Surg. 2011, 21, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.O.; Mulya, A.; Huang, H.; Dan, O.; Schauer, P.R.; Dinischiotu, A.; Brethauer, S.A.; Kirwan, J.P. Effect of Roux-en-Y Gastric Bypass on the NLRP3 Inflammasome in Pancreatic Islets from Zucker Diabetic Fatty Rats. Obes. Surg. 2016, 26, 3076–3081. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 2008, 51, 1781–1789. [Google Scholar] [CrossRef]
- Lee, Y.; Lingvay, I.; Szczepaniak, L.S.; Ravazzola, M.; Orci, L.; Unger, R.H. Pancreatic steatosis: Harbinger of type 2 diabetes in obese rodents. Int. J. Obes. 2010, 34, 396–400. [Google Scholar] [CrossRef]
- Warnotte, C.; Gilon, P.; Nenquin, M.; Henquin, J.C. Mechanisms of the stimulation of insulin release by saturated fatty acids: A study of palmitate effects in mouse beta-cells. Diabetes 1994, 43, 703–711. [Google Scholar] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Small, P.K.; Woodcock, S.A.; Pucci, A.; Aribisala, B.; Al-Mrabeh, A.; Daly, A.K.; Batterham, R.L.; Taylor, R. Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care 2016, 39, 158–165. [Google Scholar] [CrossRef] [PubMed]



| Upstream Primer (5′-3′) | Downstream Primer (5′-3′) | Accession Number(s) | |
|---|---|---|---|
| PPARγ | TCAGCTCTGTGGACCTCTCC | ACCCTTGCATCCTTCACAAG | XM_017321456.3 |
| SREBP1c | GGAGCCATGGATTGCACATT | GGAAGTCACTGTCTTGGTTGTTGA | XM_006532716.4 |
| FASN | TGGGTTCTAGCCAGCAGAGT | ACCACCAGAGACCGTTATGC | NM_007988.3 |
| ACC | TGGTCGTGACTGCTCTGTGC | GTAGCCGAGGGTTCAGTTCC | XM_006531956.3 |
| insulin | CCTGGTGGAGGCTCTCTACCT | CAGAGGGGTAGGCTGGGTAGT | NM_001185084.2 |
| β-actin | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC | NM_007393.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, H.; Liu, Y.; Zhang, M.; Qiu, Z.; Li, Y.; Zhang, Z.; Li, Y.; Xu, G. The Role of Pancreatic Fatty Acid Synthesis in Islet Morphology and Function after Caloric Restriction or Roux-En-Y Gastric Bypass Surgery in Mice. Genes 2023, 14, 5. https://doi.org/10.3390/genes14010005
Mo H, Liu Y, Zhang M, Qiu Z, Li Y, Zhang Z, Li Y, Xu G. The Role of Pancreatic Fatty Acid Synthesis in Islet Morphology and Function after Caloric Restriction or Roux-En-Y Gastric Bypass Surgery in Mice. Genes. 2023; 14(1):5. https://doi.org/10.3390/genes14010005
Chicago/Turabian StyleMo, Haocong, Yang Liu, Mengyuan Zhang, Zirui Qiu, Yilin Li, Zhejiao Zhang, Yanting Li, and Geyang Xu. 2023. "The Role of Pancreatic Fatty Acid Synthesis in Islet Morphology and Function after Caloric Restriction or Roux-En-Y Gastric Bypass Surgery in Mice" Genes 14, no. 1: 5. https://doi.org/10.3390/genes14010005
APA StyleMo, H., Liu, Y., Zhang, M., Qiu, Z., Li, Y., Zhang, Z., Li, Y., & Xu, G. (2023). The Role of Pancreatic Fatty Acid Synthesis in Islet Morphology and Function after Caloric Restriction or Roux-En-Y Gastric Bypass Surgery in Mice. Genes, 14(1), 5. https://doi.org/10.3390/genes14010005





