Tetracycline Resistance Genes in the Traditional Swedish Sour Herring surströmming as Revealed Using qPCR
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. qPCR Quantification of Tetracycline Resistance Genes
2.3. Statistical Analyses
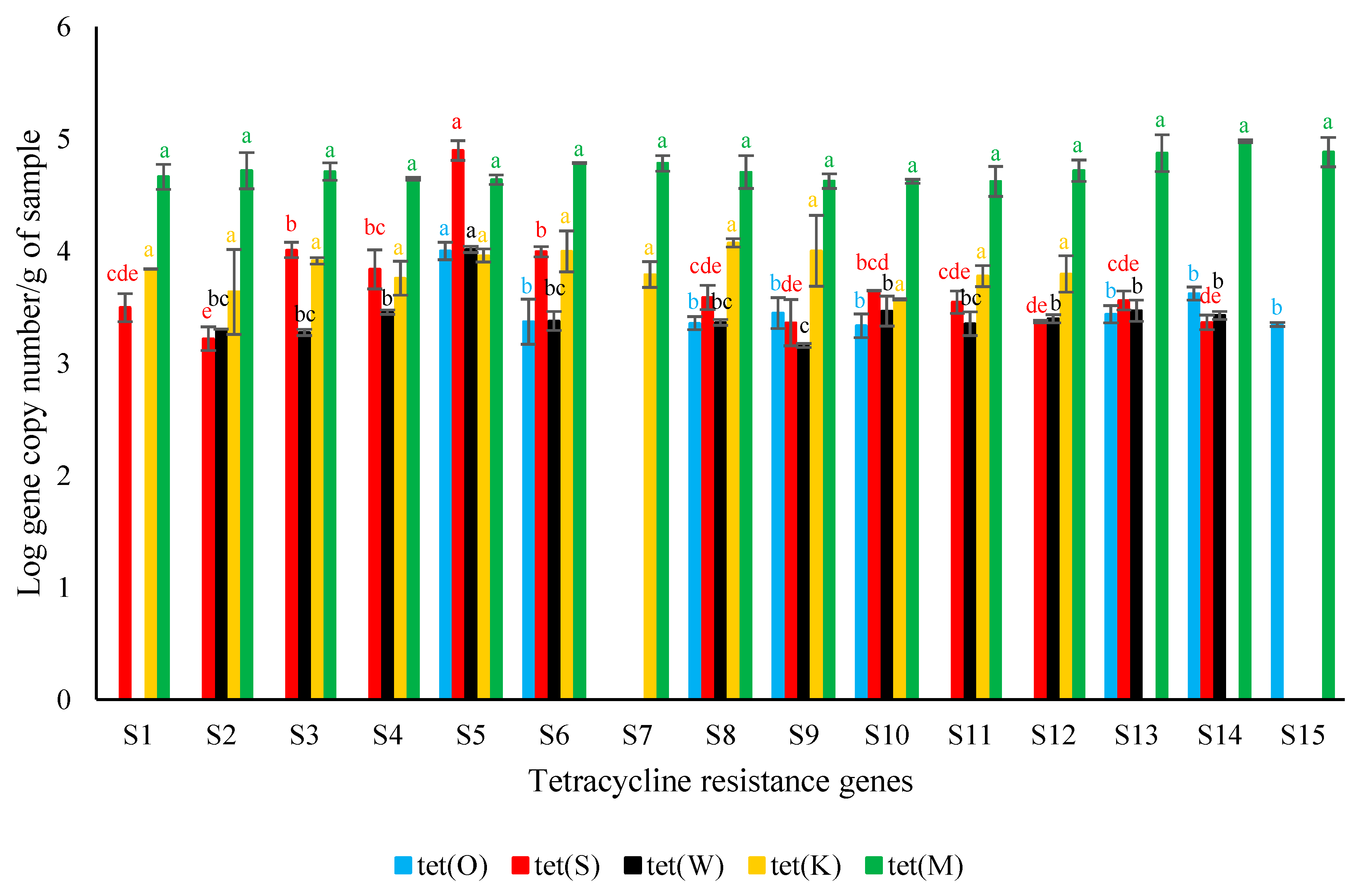
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Clementi, F. Ecology of lactic acid bacteria and coagulase negative cocci in fermented dry sausages manufactured in Italy and other Mediterranean countries: An overview. Int. Food Res. J. 2016, 2525243, 429–445. [Google Scholar]
- Akalin, A.S.; Gönç, S.; Akbaş, Y. Variation in organic acids content during ripening of pickled white cheese. J. Dairy Sci. 2002, 85, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef] [PubMed]
- Woegerbauer, M.; Bellanger, X.; Merlin, C. Cell-free DNA: An underestimated source of antibiotic resistance gene dissemination at the Interface between human activities and downstream environments in the context of wastewater reuse. Front. Microbiol. 2020, 11, 671. [Google Scholar] [CrossRef]
- OECD, ECDC, EFSA, and EMA (Organisation for Economic Co-operation and Development, European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicine Agency). Antimicrobial Resistance in the EU/EEA: A One Health Response 1–15. Available online: https://www.efsa.europa.eu/sites/default/files/topic/files/AMR-ECDC-Policy-Brief-2022.pdf (accessed on 1 December 2022).
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, 6490. [Google Scholar] [CrossRef]
- Dapkevicius, M.L.E.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current trends of enterococci in dairy products: A comprehensive review of their multiple roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Sornplang, P.; Leelavatcharamas, V.; Sukon, P.; Yowarach, S. Antibiotic resistance of lactic acid bacteria isolated from a fermented fish product, Pla-chom. Res. J. Microbiol. 2011, 6, 898–903. Available online: https://scialert.net/abstract/?doi=jm.2011.898.903 (accessed on 1 December 2022). [CrossRef]
- Yasir, M.; Al-Zahrani, I.A.; Bibi, F.; Abd El Ghany, M.; Azhar, E.I. New insights of bacterial communities in fermented vegetables from shotgun metagenomics and identification of antibiotic resistance genes and probiotic bacteria. Food Res. Int. 2022, 157, 111190. [Google Scholar] [CrossRef]
- Belleggia, L.; Aquilanti, L.; Ferrocino, I.; Milanović, V.; Garofalo, C.; Clementi, F.; Cocolin, L.; Mozzon, M.; Foligni, R.; Haouet, M.N.; et al. Discovering microbiota and volatile compounds of surströmming, the traditional Swedish sour herring. Food Microbiol. 2020, 91, 103503. [Google Scholar] [CrossRef]
- Skåra, T.; Axelsson, L.; Stefánsson, G.; Ekstrand, B.; Hagen, H. Fermented and ripened fish products in the northern European countries. J. Ethn. Foods 2015, 2, 18–24. [Google Scholar] [CrossRef]
- Belleggia, L.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Aquilanti, L.; Osimani, A. Prevalence of histidine decarboxylase genes of gram-positive bacteria in Surströmming as Revealed by qPCR. Indian J. Microbiol. 2021, 61, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Cardinali, F.; Aquilanti, L.; Maoloni, A.; Garofalo, C.; Zarantoniello, M.; Osimani, A. Quantitative assessment of transferable antibiotic resistance genes in zebrafish (Danio rerio) fed Hermetia illucens-based feed. Anim. Feed Sci. Technol. 2021, 277, 114978. [Google Scholar] [CrossRef]
- Florez, A.B.; Alegría, Á.; Rossi, F.; Delgado, S.; Felis, G.E.; Torriani, S.; Mayo, B. Molecular identification and quantification of tetracycline and erythromycin resistance genes in Spanish and Italian retail cheeses. Biomed. Res. Int. 2014, 2014, 746859. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Guo, X.; Yan, Z.; Wang, W.; Chen, B.; Ge, F.; Ye, B. A comprehensive analysis on spread and distribution characteristic of antibiotic resistance genes in livestock farms of southeastern China. PLoS ONE 2016, 11, e0156889. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Cardinali, F.; Aquilanti, L.; Maoloni, A.; Garofalo, C.; Zarantoniello, M.; Olivotto, I.; Riolo, P.; Ruschioni, S.; Isidoro, N.; et al. Quantification of antibiotic resistance genes in Siberian sturgeons (Acipenser baerii) fed Hermetia illucens-based diet. Aquaculture 2022, 560, 738485. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Thaker, M.; Spanogiannopoulos, P.; Wright, G.D. The tetracycline resistome. Cell. Mol. Life Sci. 2010, 67, 419–431. [Google Scholar] [CrossRef]
- Mei, H.; Arbeithuber, B.; Cremona, M.A.; DeGiorgio, M.; Nekrutenko, A. A high-resolution view of adaptive event dynamics in a plasmid. Genome Biol. Evol. 2019, 11, 3022–3034. [Google Scholar] [CrossRef]
- Biswas, K.; Sharma, P.; Joshi, S.R. Co-occurrence of antimicrobial resistance and virulence determinants in enterococci isolated from traditionally fermented fish products. J. Glob. Antimicrob. Resist. 2019, 17, 79–83. [Google Scholar] [CrossRef]
- Obayashi, Y.; Kadoya, A.; Kataoka, N.; Kanda, K.; Bak, S.M.; Iwata, H.; Suzuki, S. Tetracycline Resistance Gene Profiles in Red Seabream (Pagrus major) Intestine and Rearing Water After Oxytetracycline Administration. Front. Microbiol. 2020, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Le Neindre, K.; Dejoies, L.; Reissier, S.; Guérin, F.; Felden, B.; Cattoir, V. Small RNA-mediated regulation of the tet(M) resistance gene expression in Enterococcus faecium. Res. Microbiol. 2022, 173, 103941. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Nonaka, L.; Suzuki, S. Occurrence of tetracycline resistance genes tet(M) and tet(S) in bacteria from marine aquaculture sites. FEMS Microbiol. Lett. 2004, 237, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Vignaroli, C.; Luna, G.M.; Pasquaroli, S.; Biavasco, F. Antibiotic-resistant enterococci in seawater and sediments from a coastal fish farm. Microb. Drug Resist. 2012, 18, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Hedayatianfard, K.; Akhlaghi, M.; Sharifiyazdi, H. Detection of tetracycline resistance genes in bacteria isolated from fish farms using polymerase chain reaction. Vet. Res. Forum. 2014, 5, 269–275. [Google Scholar] [PubMed]
- Yamaguchi, A.; Shiina, Y.; Fujihira, E.; Sawai, T.; Noguchi, N.; Sasatsu, M. The tetracycline efflux protein encoded by the tet(K) gene from Staphylococcus aureus is a metal-tetracycline/H+ antiporter. FEBS Lett. 1995, 365, 193–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, Y.K.; Nho, S.W.; Shin, G.W.; Park, S.B.; Jang, H.B.; Cha, I.S.; Ha, M.A.; Kim, Y.R.; Dalvi, R.S.; Kang, B.J.; et al. Antibiotic susceptibility and resistance of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus). Vet. Microbiol. 2009, 136, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Nøhr-Meldgaard, K.; Struve, C.; Ingmer, H.; Agersø, Y. The Tetracycline Resistance Gene, tet(W) in Bifidobacterium animalis subsp. lactis Follows Phylogeny and Differs From tet(W) in Other Species. Front. Microbiol. 2021, 12, 658943. [Google Scholar] [CrossRef]
- Suzuki, S.; Kobayashi, T.; Suehiro, F.; Tuyen, B.C.; Tana, T.S. High occurrence rate of tetracycline (TC)-resistant bacteria and TC resistance genes relates to microbial diversity in sediment of Mekong River main waterway. Microb. Environ. 2008, 23, 149–152. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Shan, L.; Zhang, C.; Lei, Z.; Shang, Y. Isolation and Antibiotic Resistant Research of Tetragenococcus halophilus from Xuanwei Ham, A China High-Salt-Fermented Meat Products. Antibiotics 2019, 8, 151. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.H. Tetracycline resistance associated with commensal bacteria from representative ready-to-consume deli and restaurant foods. J. Food Prot. 2010, 73, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Helsens, N.; Calvez, S.; Prevost, H.; Bouju-Albert, A.; Maillet, A.; Rossero, A.; Hurtaud-Pessel, D.; Zagorec, M.; Magras, C. Antibiotic Resistance Genes and Bacterial Communities of Farmed Rainbow Trout Fillets (Oncorhynchus mykiss). Front. Microbiol. 2020, 11, 590902. [Google Scholar] [CrossRef] [PubMed]
- Moniz, K.; Walker, V.K.; Shah, V. Antibiotic resistance in mucosal bacteria from high Arctic migratory salmonids. Environ. Microbiol. Rep. 2022, 14, 385–390. [Google Scholar] [CrossRef]
- Juricova, H.; Matiasovicova, J.; Kubasova, T.; Cejkova, D.; Rychlik, I. The distribution of antibiotic resistance genes in chicken gut microbiota commensals. Sci. Rep. 2021, 11, 3290. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Gorlenko, Z.; Mindlin, S. Molecular structure and translocation of a multiple antibiotic resistance region of a Psychrobacter psychrophilus permafrost strain. FEMS Microbiol. Lett. 2009, 296, 190–197. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, T.H.; Jung, S.M.; Cho, S.H.; Jin, S.Y.; Park, N.H.; Kim, C.G.; Kim, J.S. Antibiotic resistance of bacteria isolated from the internal organs of edible snow crabs. PLoS ONE 2013, 21, e70887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zou, X.; Wu, S.; Wu, N.; Chen, X.; Zhou, W. Effects of Pyroligneous Acid on Diversity and Dynamics of Antibiotic Resistance Genes in Alfalfa Silage. Microbiol. Spectr. 2022, 10, e0155422. [Google Scholar] [CrossRef] [PubMed]
- Ciric, L.; Brouwer, M.S.M.; Mullany, P.; Roberts, A.P. Minocycline resistance in an oral Streptococcus infantis isolate is encoded by tet(S) on a novel small, low copy number plasmid. FEMS Microbiol. Lett. 2014, 353, 106–115. [Google Scholar] [CrossRef]
- Huang, C.Y.; Garcia, J.L.; Patel, B.K.; Cayol, J.L.; Baresi, L.; Mah, R.A. Salinivibrio costicola subsp. vallismortis subsp. nov., a halotolerant facultative anaerobe from Death Valley, and emended description of Salinivibrio costicola. Int. J. Syst. Evol. Microbiol. 2000, 2, 615–622. [Google Scholar] [CrossRef]
- Hsu, T.-T.D.; Lee, J. Global distribution and prevalence of arcobacter in food and water. Zoonoses Public Health 2015, 62, 579–589. [Google Scholar] [CrossRef]
- Sciortino, S.; Arculeo, P.; Alio, V.; Cardamone, C.; Nicastro, L.; Arculeo, M.; Alduina, R.; Costa, A. Occurrence and antimicrobial resistance of Arcobacter spp. recovered from aquatic environments. Antibiotics 2021, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Franco, D.; van Loosdrecht, M.C.M.; Abeel, T.; Weissbrodt, D.G. Free-floating extracellular DNA: Systematic profiling of mobile genetic elements and antibiotic resistance from wastewater. Water Res. 2021, 189, 116592. [Google Scholar] [CrossRef] [PubMed]
- Baltic Marine Environment Protection Commission, Expert Network on Hazardous Substances (EN-HZ 15-2021). Antimicrobial Resistance in the Baltic Sea—Monitoring and Indicator Assessments. Available online: https://portal.helcom.fi/meetings/EN-HZ%2015-2021-918/MeetingDocuments/EN-HZ%2015/13-1%20Antimicrobial%20resistance_initial%20draft.pdf (accessed on 1 December 2022).
- Gross, S.; Müller, A.; Seinige, D.; Wohlsein, P.; Oliveira, M.; Steinhagen, D.; Kehrenberg, C.; Siebert, U. Occurrence of antimicrobial-resistant Escherichia coli in marine mammals of the north and baltic seas: Sentinels for human health. Antibiotics 2022, 111, 1248. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Virta, M. The resistome of farmed fish feces contributes to the enrichment of antibiotic resistance genes in sediments below baltic sea fish farms. Front. Microbiol. 2017, 6, 2137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanović, V.; Maoloni, A.; Belleggia, L.; Cardinali, F.; Garofalo, C.; Cesaro, C.; Aquilanti, L.; Osimani, A. Tetracycline Resistance Genes in the Traditional Swedish Sour Herring surströmming as Revealed Using qPCR. Genes 2023, 14, 56. https://doi.org/10.3390/genes14010056
Milanović V, Maoloni A, Belleggia L, Cardinali F, Garofalo C, Cesaro C, Aquilanti L, Osimani A. Tetracycline Resistance Genes in the Traditional Swedish Sour Herring surströmming as Revealed Using qPCR. Genes. 2023; 14(1):56. https://doi.org/10.3390/genes14010056
Chicago/Turabian StyleMilanović, Vesna, Antonietta Maoloni, Luca Belleggia, Federica Cardinali, Cristiana Garofalo, Cristiana Cesaro, Lucia Aquilanti, and Andrea Osimani. 2023. "Tetracycline Resistance Genes in the Traditional Swedish Sour Herring surströmming as Revealed Using qPCR" Genes 14, no. 1: 56. https://doi.org/10.3390/genes14010056
APA StyleMilanović, V., Maoloni, A., Belleggia, L., Cardinali, F., Garofalo, C., Cesaro, C., Aquilanti, L., & Osimani, A. (2023). Tetracycline Resistance Genes in the Traditional Swedish Sour Herring surströmming as Revealed Using qPCR. Genes, 14(1), 56. https://doi.org/10.3390/genes14010056





