A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report
2.2. Laboratory Investigations
2.3. Molecular Investigations
3. Results
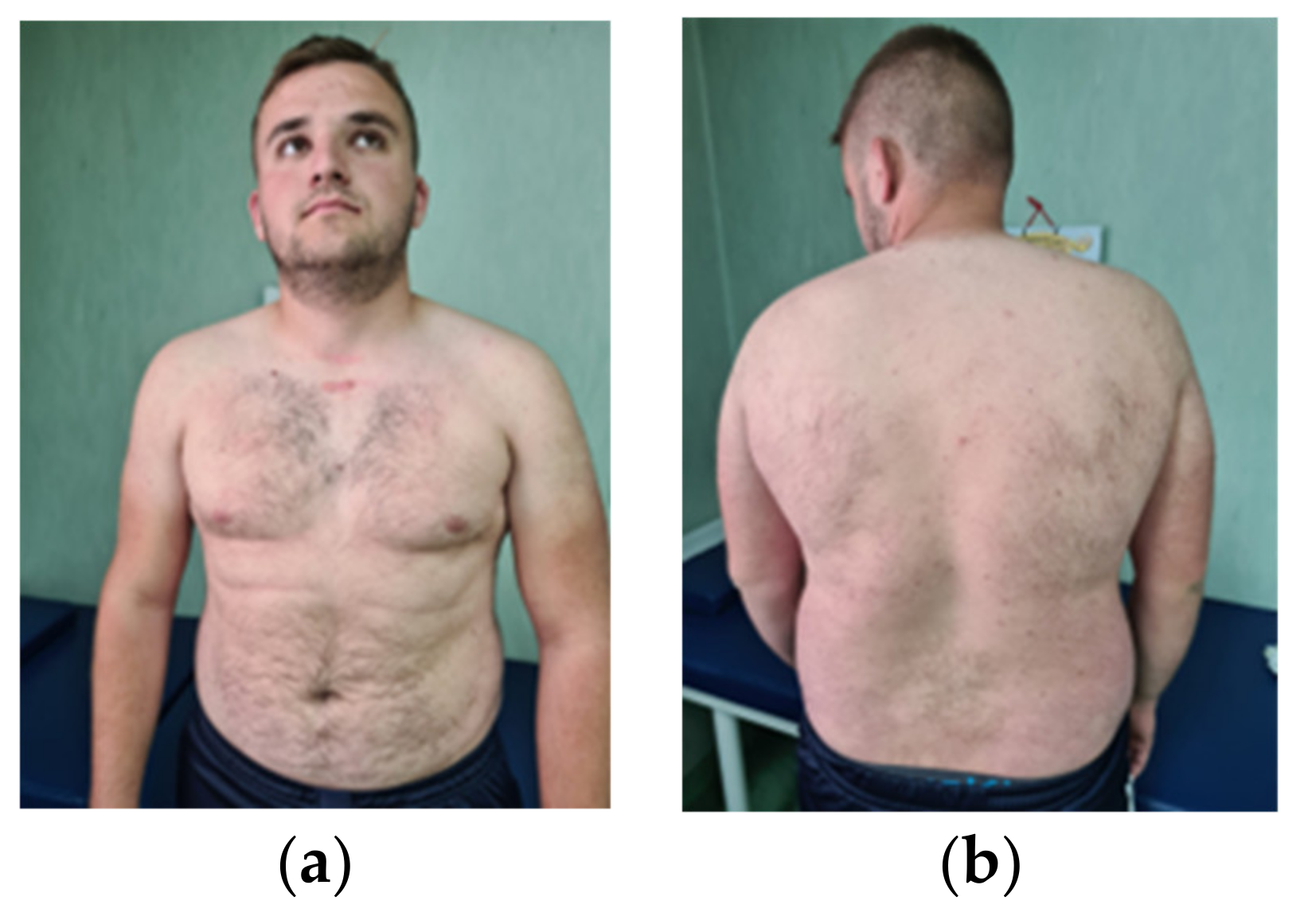
3.1. Clinical Evaluation of the Patient
3.2. Laboratory Investigations
3.3. Interdisciplinary Consultations
3.4. Molecular Investigations
4. Discussion
4.1. Clinical Aspects
4.1.1. Craniofacial Dysmorphism
4.1.2. Thyroid Involvement
4.1.3. Lymphoid Involvement
4.1.4. Gastrointestinal Manifestations
4.1.5. Skin Changes
4.1.6. Cancer Risk
4.1.7. Neurodevelopmental Anomalies
4.2. Genetics
The PTEN Gene
4.3. Genotype–Phenotype Correlation
4.4. Treatment
4.5. Screening Methods and Genetic Counseling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballester Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Lloyd, K.M., II; Dennis, M. Cowden’s disease. A possible new symptom complex with multiple system involvement. Ann. Intern. Med. 1963, 58, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef]
- Nelen, M.R.; Padberg, G.W.; Peeters, E.A.; Lin, A.Y.; van den Helm, B.; Frants, R.R.; Coulon, V.; Goldstein, A.M.; van Reen, M.M.; Easton, D.F.; et al. Localization of the Gene for Cowden Disease to Chromosome 10q22-23. Nat. Genet. 1996, 13, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, Y.; Chen, W.; Merlino, G.; Yu, Y. PTEN Dual Lipid- and Protein-Phosphatase Function in Tumor Progression. Cancers 2022, 14, 3666. [Google Scholar] [CrossRef]
- Hobert, J.; Eng, C. PTEN hamartoma tumor syndrome: An overview. Genet. Med. 2009, 11, 687–694. [Google Scholar] [CrossRef]
- Porto, A.C.S.; Roider, E.; Ruzicka, T. Cowden Syndrome: Report of a Case and Brief Review of Literature. An. Bras. Dermatol. 2013, 88 (Suppl. 1), 52–55. [Google Scholar] [CrossRef]
- Yehia, L.; Eng, C. PTEN Hamartoma Tumor Syndrome. 29 November 2001 [Updated 11 February 2021]. In GeneReviews®®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1488/ (accessed on 20 July 2023).
- Shaco-Levy, R.; Jasperson, K.W.; Martin, K.; Samadder, N.J.; Burt, R.W.; Ying, J.; Bronner, M.P. Gastrointestinal Polyposis in Cowden Syndrome. J. Clin. Gastroenterol. 2017, 51, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R. Hamartoma Tumor Syndrome: A Clinical Overview. Cancers 2019, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- The National Comprehensive Cancer Network (NCCN). The NCCN Guidelines Genetic/Familial High-Risk Assessment: Breast and Ovarian. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar]
- Eng, C. Will the real Cowden syndrome please stand up: Revised diagnostic criteria. J. Med. Genet. 2000, 37, 828–830. [Google Scholar] [CrossRef]
- Hereditary Gastrointestinal Cancer Panel. Available online: https://blueprintgenetics.com/tests/panels/hereditary-cancer/hereditary-gastrointestinal-cancer-panel/ (accessed on 30 July 2023).
- Macken, W.L.; Tischkowitz, M.; Lachlan, K.L. PTEN Hamartoma Tumor Syndrome in Childhood: A Review of the Clinical Literature. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 591–610. [Google Scholar] [CrossRef]
- Fombonne, E.; Roge, B.; Claverie, J.; Courty, S.; Fremolle, J. Microcephaly and macrocephaly in autism. J. Autism Dev. Disord. 1999, 29, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Bongiovanni, M.; Tille, J.-C.; Kozakewich, H.; Nosé, V. Thyroid Pathology in PTEN -Hamartoma Tumor Syndrome: Characteristic Findings of a Distinct Entity. In Thyroid New; Mary Ann Liebert, Inc.: Rochelle, NY, USA, 2011; Volume 21, pp. 135–144. [Google Scholar]
- Plamper, M.; Schreiner, F.; Gohlke, B.; Kionke, J.; Korsch, E.; Kirkpatrick, J.; Born, M.; Aretz, S.; Woelfle, J. Thyroid Disease in Children and Adolescents with PTEN Hamartoma Tumor Syndrome (PHTS). Eur. J. Pediatr. 2018, 177, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Smpokou, P.; Fox, V.L.; Tan, W.-H. PTEN Hamartoma Tumour Syndrome: Early Tumour Development in Children. Arch. Dis. Child. 2015, 100, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Kiss, E.; Beinkampen, S.; Adler, B.; Frazier, T.; Prior, T.; Erdman, S.; Eng, C.; Herman, G. A Retrospective Chart Review of the Features of PTEN Hamartoma Tumour Syndrome in Children. J. Med. Genet. 2017, 54, 471–478. [Google Scholar] [CrossRef]
- Chen, H.H.; Handel, N.; Ngeow, J.; Muller, J.; Huhn, M.; Yang, H.-T.; Heindl, M.; Berbers, R.-M.; Hegazy, A.N.; Kionke, J.; et al. Immune dysregulation in patients with PTEN hamartoma tumor syndrome: FOXP3 regulatory T cells analysis. J. Allergy Clin. Immunol. 2017, 139, 607–620.e15. [Google Scholar] [CrossRef]
- Hady-Cohen, R.; Maharshak, I.; Michelson, M.; Yosovich, K.; Lev, D.; Constantini, S.; Leiba, H.; Lerman-Sagie, T.; Blumkin, L. Familial Intracranial Hypertension in 2 Brothers with PTEN Mutation: Expansion of the Phenotypic Spectrum. J. Child Neurol. 2019, 34, 506–510. [Google Scholar] [CrossRef]
- Omoyinmi, E.; Standing, A.; Keylock, A.; Price-Kuehne, F.; Melo Gomes, S.; Rowczenio, D.; Nanthapisal, S.; Cullup, T.; Nyanhete, R.; Ashton, E.; et al. Clinical Impact of a Targeted Next-Generation Sequencing Gene Panel for Autoinflammation and Vasculitis. PLoS ONE 2017, 12, e0181874. [Google Scholar] [CrossRef]
- Webber, L.P.; Martins, M.D.; Carrard, V.C.; Trevizani Martins, M.A.; Munerato, M.C. Cowden Syndrome—A Case Report Emphasizing the Role of the Dental Surgeon in Diagnosis: Cowden Syndrome Diagnosed for Dental Surgeon. Spec. Care Dent. 2015, 35, 51–54. [Google Scholar] [CrossRef]
- Sharma, M.R.; Petty, E.M.; Lesperance, M.M. Airway Obstruction Caused by PTEN Hamartoma (Bannayan-Tiley-Ruvalcaba) Syndrome. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Latiff, A.; Warouw Atmawidjaja, Z.; Raja Lope, R.; Syed Omar, R.; Syed Zakaria, S.; Ar, S. Letter to the Editor Bannayan Riley Ruvalcaba Syndrome. Ann. Acad. Med. 2010, 39, 578–579. [Google Scholar]
- Omote, K.; Kawamata, T.; Imaizumi, H.; Namiki, A. Case of Cowden’s Disease That Caused Airway Obstruction during Induction of Anesthesia. Anesthesiology 1999, 91, 1537–1540. [Google Scholar] [CrossRef]
- Heald, B.; Mester, J.; Rybicki, L.; Orloff, M.S.; Burke, C.A.; Eng, C. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology 2010, 139, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Kethman, W.; Rao, A.; Devereaux, K.; Ouellet, E.; Kin, C. Say What? Bannayan-Riley-Ruvalcaba Syndrome Presenting with Gastrointestinal Bleeding Due to Hamartoma-Induced Intussusception. Dig. Dis. Sci. 2017, 62, 2293–2297. [Google Scholar] [CrossRef]
- Ballester, V.; Rashtak, S.; Boardman, L. Clinical and Molecular Features of Young-Onset Colorectal Cancer. World J. Gastroenterol. 2016, 22, 1736–1744. [Google Scholar] [CrossRef]
- Cone, M.M. Hamartomatous Polyps and Associated Syndromes. Clin. Colon Rectal Surg. 2016, 29, 330–335. [Google Scholar] [CrossRef]
- Stanich, P.P.; Pilarski, R.; Rock, J.; Frankel, W.L.; El-Dika, S.; Meyer, M.M. Colonic Manifestations of PTEN Hamartoma Tumor Syndrome: Case Series and Systematic Review. World J. Gastroenterol. 2014, 20, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Ngeow, J. The Skin in Cowden Syndrome. Front. Med. 2021, 8, 658842. [Google Scholar] [CrossRef] [PubMed]
- Salem, O.S.; Steck, W.D. Cowden’s Disease (Multiple Hamartoma and Neoplasia Syndrome): A Case Report and Review of the English Literature. J. Am. Acad. Dermatol. 1983, 8, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, C.; Sánchez, S.; Castillo, M.; Moleón, L.; Moreno, D.; López, S. Multiple Oral Fibropapillomatosis as an Initial Manifestation of Cowden Syndrome. Case Report. Med Oral Patol Oral Cir Bucal. Cancers 2006, 11. [Google Scholar]
- Marshall, M.; Otero, D.; Niklander, S.; Martínez-Flores, R. Cowden’s Syndrome Diagnosed through Oral Lesions: A Case Report. J. Clin. Exp. Dent. 2021, 13, e1162–e1166. [Google Scholar] [CrossRef] [PubMed]
- Nosé, V. Genodermatosis Affecting the Skin and Mucosa of the Head and Neck: Clinicopathologic, Genetic, and Molecular Aspect--PTEN-Hamartoma Tumor Syndrome/Cowden Syndrome. Head Neck Pathol. 2016, 10, 131–138. [Google Scholar] [CrossRef]
- Miguelote, S.; Silva, R.; Fougo, J.L.; Barbosa, L.E.; Araújo Teixeira, J.P. Cowden Syndrome Is a Risk Factor for Multiple Neoplasm: A Case Report. World J. Surg. Oncol. 2020, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, L.A.J.; Hoogerbrugge, N.; Schuurs-Hoeijmakers, J.H.M.; Vos, J.R. A review on age-related cancer risks in PTEN hamartoma tumor syndrome. Clin Genet. 2021, 99, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.; Alfonso, A.; Hughes, E.; Kucera, M.; Mabey, B.; Singh, N.; Eng, C. Cancer Risk Associated With PTEN Pathogenic Variants Identified Using Multigene Hereditary Cancer Panel Testing. JCO Precis. Oncol. 2023, 7, e2200415. [Google Scholar] [CrossRef] [PubMed]
- Prvanović, M.; Nedeljković, M.; Tanić, N.; Tomić, T.; Terzić, T.; Milovanović, Z.; Maksimović, Z.; Tanić, N. Role of PTEN, PI3K, and mTOR in Triple-Negative Breast Cancer. Life 2021, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Fackenthal, J.D.; Marsh, D.J.; Richardson, A.L.; Cummings, S.A.; Eng, C.; Robinson, B.G.; Olopade, O.I. Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J. Med. Genet. 2001, 38, 159–164. [Google Scholar] [CrossRef][Green Version]
- Zahedi Abghari, F.; Moradi, Y.; Akouchekian, M. PTEN gene mutations in patients with macrocephaly and classic autism: A systematic review. Med. J. Islam. Repub. Iran. 2019, 33, 10. [Google Scholar] [CrossRef]
- Cummings, K.; Watkins, A.; Jones, C.; Dias, R.; Welham, A. Behavioural and psychological features of PTEN mutations: A systematic review of the literature and meta-analysis of the prevalence of autism spectrum disorder characteristics. J. Neurodev. Disord. 2022, 14, 1. [Google Scholar] [CrossRef]
- Bigler, E.D.; Abildskov, T.J.; Petrie, J.A.; Johnson, M.; Lange, N.; Chipman, J.; Lu, J.; McMahon, W.; Lainhart, J.E. Volumetric and voxel-based morphometry findings in autism subjects with and without macrocephaly. Dev. Neuropsychol. 2010, 35, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Embacher, R.; Tilot, A.K.; Koenig, K.; Mester, J.; Eng, C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol. Psychiatry 2015, 20, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Balci, T.B.; Davila, J.; Lewis, D.; Boafo, A.; Sell, E.; Richer, J.; Nikkel, S.M.; Armour, C.M.; Tomiak, E.; Lines, M.A.; et al. A broad spectrum of neuropsychiatric phenotypes associated with white matter disease in PTEN hamartoma tumor syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 101–109. [Google Scholar] [CrossRef]
- McMahon, M.E.; Murray, D.; MacNally, S.; O’Brien, D.F. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma) in the setting of Cowden syndrome: A case report and literature review on COLD syndrome. Br. J. Neurosurg. 2022. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It is all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a Putative Protein Tyrosine Phosphatase Gene Mutated in Human Brain, Breast, and Prostate Cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef]
- Pollack, I.F.; Hamilton, R.L.; James, C.D.; Finkelstein, S.D.; Burnham, J.; Yates, A.J.; Holmes, E.J.; Zhou, T.; Finlay, J.L.; Children’s Oncology Group. The rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: Results from the Children’s Cancer Group 945 cohort. J. Neurosurg. 2006, 105, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Bubien, V.; Bonnet, F.; Brouste, V.; Hoppe, S.; Barouk-Simonet, E.; David, A.; Edery, P.; Bottani, A.; Layet, V.; Caron, O.; et al. Highcumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J. Med. Genet. 2013, 50, 255–263. [Google Scholar] [CrossRef]
- Gimm, O.; Perren, A.; Weng, L.P.; Marsh, D.J.; Yeh, J.J.; Ziebold, U.; Gil, E.; Hinze, R.; Delbridge, L.; Lees, J.A.; et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 2000, 156, 1693–1700. [Google Scholar] [CrossRef]
- Cristofano, D.; Pesce, A.; Cordon-Cardo, B.; Pandolfi, C. Pten is Essential for Embryonic Development and Tumour Suppression. Nat. Genet. 1998, 19, 348–355. [Google Scholar] [CrossRef]
- Weng, L.P.; Smith, W.M.; Dahia, P.L.; Ziebold, U.; Gil, E.; Lees, J.A.; Eng, C. PTEN Suppresses Breast Cancer Cell Growth by Phosphatase Activity-Dependent G1 Arrest Followed by Cell Death. Cancer Res. 1999, 59, 5808–5814. [Google Scholar] [PubMed]
- Jurca, C.M.; Kozma, K.; Petchesi, C.D.; Zaha, D.C.; Magyar, I.; Munteanu, M.; Faur, L.; Jurca, A.; Bembea, D.; Severin, E.; et al. Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/MTOR Signaling Pathways-Case Report and Literature Review. Genes 2023, 14, 433. [Google Scholar] [CrossRef]
- Tamura, M.; Gu, J.; Matsumoto, K.; Aota, S.; Parsons, R.; Yamada, K.M. Inhibition of Cell Migration, Spreading, and Focal Adhesions by Tumor Suppressor PTEN. Science 1998, 280, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Biomembranele, Unitate în Diversitate. Available online: https://www.mircea-leabu.ro/wp-content/uploads/2015/02/Semnalizare-celular%C4%83-text.pdf (accessed on 4 September 2023).
- Lee, Y.-R.; Chen, M.; Pandolfi, P.P. The Functions and Regulation of the PTEN Tumour Suppressor: New Modes and Prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Lachlan, K.L.; Lucassen, A.M.; Bunyan, D.; Temple, I.K. Cowden Syndrome and Bannayan Riley Ruvalcaba Syndrome Represent One Condition with Variable Expression and Age-Related Penetrance: Results of a Clinical Study of PTEN Mutation Carriers. J. Med. Genet. 2007, 44, 579–585. [Google Scholar] [CrossRef]
- Ngeow, J.; Mester, J.; Rybicki, L.A.; Ni, Y.; Milas, M.; Eng, C. Incidence and Clinical Characteristics of Thyroid Cancer in Prospective Series of Individuals with Cowden and Cowden-like Syndrome Characterized by Germline PTEN, SDH, or KLLN Alterations. J. Clin. Endocrinol. Metab. 2011, 96, E2063–E2071. [Google Scholar] [CrossRef]
- Mighell, T.L.; Evans-Dutson, S.; O’Roak, B.J. A Saturation Mutagenesis Approach to Understanding PTEN Lipid Phosphatase Activity and Genotype-Phenotype Relationships. Am. J. Hum. Genet. 2018, 102, 943–955. [Google Scholar] [CrossRef]
- Tan, M.-H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef]
- Nelen, M.R.; Kremer, H.; Konings, I.B.M.; Schoute, F.; van Essen, A.J.; Koch, R.; Woods, C.G.; Fryns, J.-P.; Hamel, B.; Hoefsloot, L.H.; et al. Novel PTEN Mutations in Patients with Cowden Disease: Absence of Clear Genotype–Phenotype Correlations. Eur. J. Hum. Genet. 1999, 7, 267–273. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; PHTS Guideline Development Group; European Reference Network GENTURIS. Cancer Surveillance Guideline for Individuals with PTEN hamartoma tumor syndrome. Eur. J. Hum. Genet. 2020, 28, 1387–1393. [Google Scholar] [CrossRef]
- Karn, R.; Emerson, I.A. Molecular dynamic study on PTEN frameshift mutations in breast cancer provide c2 domain as a potential biomarker. J. Biomol. Struct. Dyn. 2022, 40, 3132–3143. [Google Scholar] [CrossRef]
- Mukamal, L.V.; Ferreira, A.F.; Jacques, C.D.M.C.; Amorim, C.A.F.L.; Pineiro-Maceira, J.; Ramos-e-Silva, M. Cowden Syndrome: Review and Report of a Case of Late Diagnosis: Cowden Syndrome. Int. J. Dermatol. 2012, 51, 1494–1499. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kingston, B.; Bailleux, C.; Delaloge, S.; Schiavon, G.; Scott, V.; Lacroix-Triki, M.; Carr, T.H.; Kozarewa, I.; Gevensleben, H.; Kemp, Z.; et al. Exceptional Response to AKT Inhibition in Patients With Breast Cancer and Germline PTEN Mutations. JCO Precis. Oncol. 2019, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W.; American College of Gastroenterology. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Clinical Practice Guidelines. Available online: https://genturis.eu/l=eng/Guidelines-and-pathways/Clinical-practice-guidelines.html (accessed on 6 September 2023).



| Pathognomonic lesions: Mucocutaneous lesions: Facial trichilemmomas. Acral keratoses Papillomatous lesions Mucosal lesions | ||
| Major criteria | Minor criteria | Observations |
| Breast cancer Endometrial cancer Thyroid cancer (mainly follicular thyroid carcinoma) Gastrointestinal hamartomas Lhermitte–Duclos disease, adult Macrocephaly | Other thyroid lesions. Mental retardation (IQ ≤ 75). Gastrointestinal hamartomas. Fibrocystic disease of the breast. Lipomas and fibromas. Genitourinary tumors or malformations. | |
| For a person with a negative family history of CS, the following criteria must be taken into account: | ||
| ||
| In the case of a person affected by CS, the diagnostic criteria for his relatives are: | ||
| ||
| Description | Recommendations | ||
|---|---|---|---|
| ENT (Ear-Nose-Throat) | 2006 3 ½ years | Tonsillar polyp, approx. 0.5/0.5 cm, surgically removed. | Pathological anatomy: tonsillar papilloma. |
| 2007 4 years | Tonsillar polyp significantly increased in size, approx. 4/5 cm, associating respiratory distress and oral breathing. | Tonsillectomy. | |
| Surgery | 2012 9 years | Left plantar hemangioma. | Plantar ultrasound: Slightly hypoechoic structure of approx. 6.5/3.1 cm in the continuity of the subcutaneous cellular tissue. Doppler examination: Well-represented vasculature with high velocities and extremely variable impedance indices. The appearance suggests plantar hemangioma. Surgical excision of the hemangioma. |
| 2018 15 years | Left thyroid lobe nodules. | Left thyroid lobectomy. Pathological examination confirms a follicular adenoma. | |
| 2020 17 years | Contusion wound with partial section of right Achilles tendon. | ||
| Endocrinology | 2015 12 years | Left multinodular goiter. | Thyroid ultrasound: Right lobe without changes; left lobe: 3 nodular lesions of approx. 3.5/2.7/2 cm, 2.5/2/1.5 cm, and 2/1.5/1.5 cm, respectively. |
| 2018 15 years | Right thyroid lobe nodule (cyst). | Ultrasound of the right thyroid lobe: Average volume, echogenic, homogeneous structure. Inferior, a hyperechoic restricted lesion with minimal proliferation on the anterior wall, non-vascularized cc 0.5 cm (cyst?); vascularization of the thyroid parenchyma of normal appearance. | |
| 2020 17 years | Right thyroid nodule. | ||
| Gastroenterology | 2023 April 20 years | Colonic polyposis under observation. | Lower digestive endoscopy: Internal hemorrhoids gr II. Suspected inflammatory bowel disease; a colonoscopy was recommended. Colonoscopy: At the level of the ileum, colon, and rectum, multiple polyps (hundreds of sessile polyps); sigma: pedunculated polyp with dysplastic appearance—excision; anal canal: polypoid poly-lobed formation of approx. 2.5 cm—biopsy (Figure 3a–d). Pathological anatomy: Hyperplastic polyps at the level of the rectum; juvenile hamartomatous polyps at the level of the anus (Figure 4a,b). |
| Dermatological | 2023, May, 20 years | Facial acne and papular lesion on the forehead with the appearance of trichilemmoma. | A biopsy of the papillomatous lesion was recommended. |
| System | Screening and Prophylaxis |
|---|---|
| Thyroid |
|
| Kidneys |
|
| Colon |
|
| Breast |
|
| Dermatological |
|
| Growth |
|
| Genetic counseling |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurca, C.M.; Frățilă, O.; Iliaș, T.; Jurca, A.; Cătana, A.; Moisa, C.; Jurca, A.D. A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature. Genes 2023, 14, 1909. https://doi.org/10.3390/genes14101909
Jurca CM, Frățilă O, Iliaș T, Jurca A, Cătana A, Moisa C, Jurca AD. A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature. Genes. 2023; 14(10):1909. https://doi.org/10.3390/genes14101909
Chicago/Turabian StyleJurca, Claudia Maria, Ovidiu Frățilă, Tiberia Iliaș, Aurora Jurca, Andreea Cătana, Corina Moisa, and Alexandru Daniel Jurca. 2023. "A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature" Genes 14, no. 10: 1909. https://doi.org/10.3390/genes14101909
APA StyleJurca, C. M., Frățilă, O., Iliaș, T., Jurca, A., Cătana, A., Moisa, C., & Jurca, A. D. (2023). A New Frameshift Mutation of PTEN Gene Associated with Cowden Syndrome—Case Report and Brief Review of the Literature. Genes, 14(10), 1909. https://doi.org/10.3390/genes14101909






