Characterization and Expression Analysis of Genes from Megalobrama amblycephala Encoding Hemoglobins with Extracellular Microbicidal Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. RNA Isolation and cDNA Synthesis
2.4. Identification of M. amblycephala Hemoglobin Genes
2.5. Sequence and Phylogenetic Analysis
2.6. Quantitative Real-Time PCR Analysis
2.7. Antimicrobial Activities of Synthesized MaHbα and MaHbβ Peptides
2.8. Statistical Analyses
3. Results
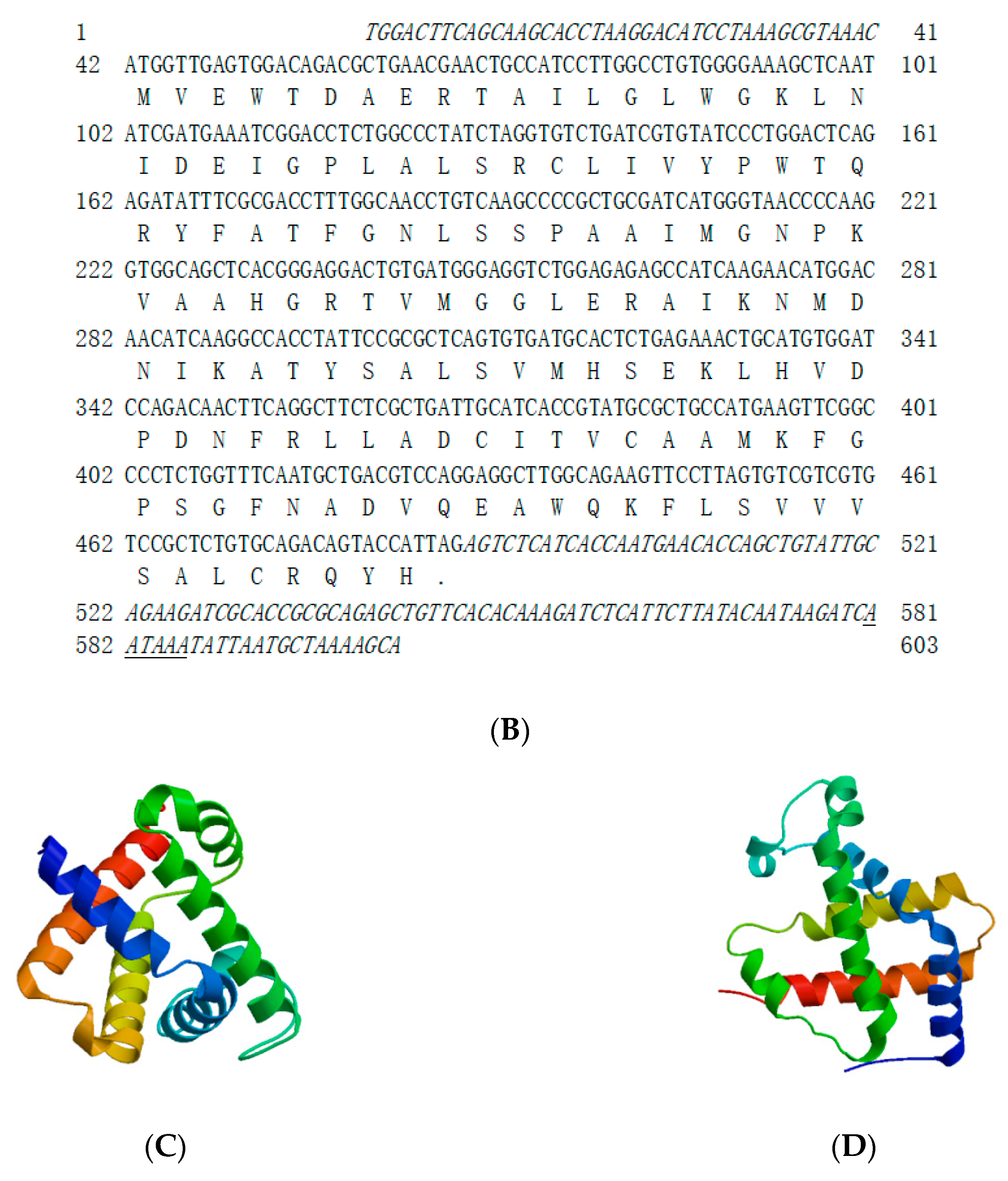
3.1. Identification and Characterization of MaHb Genes
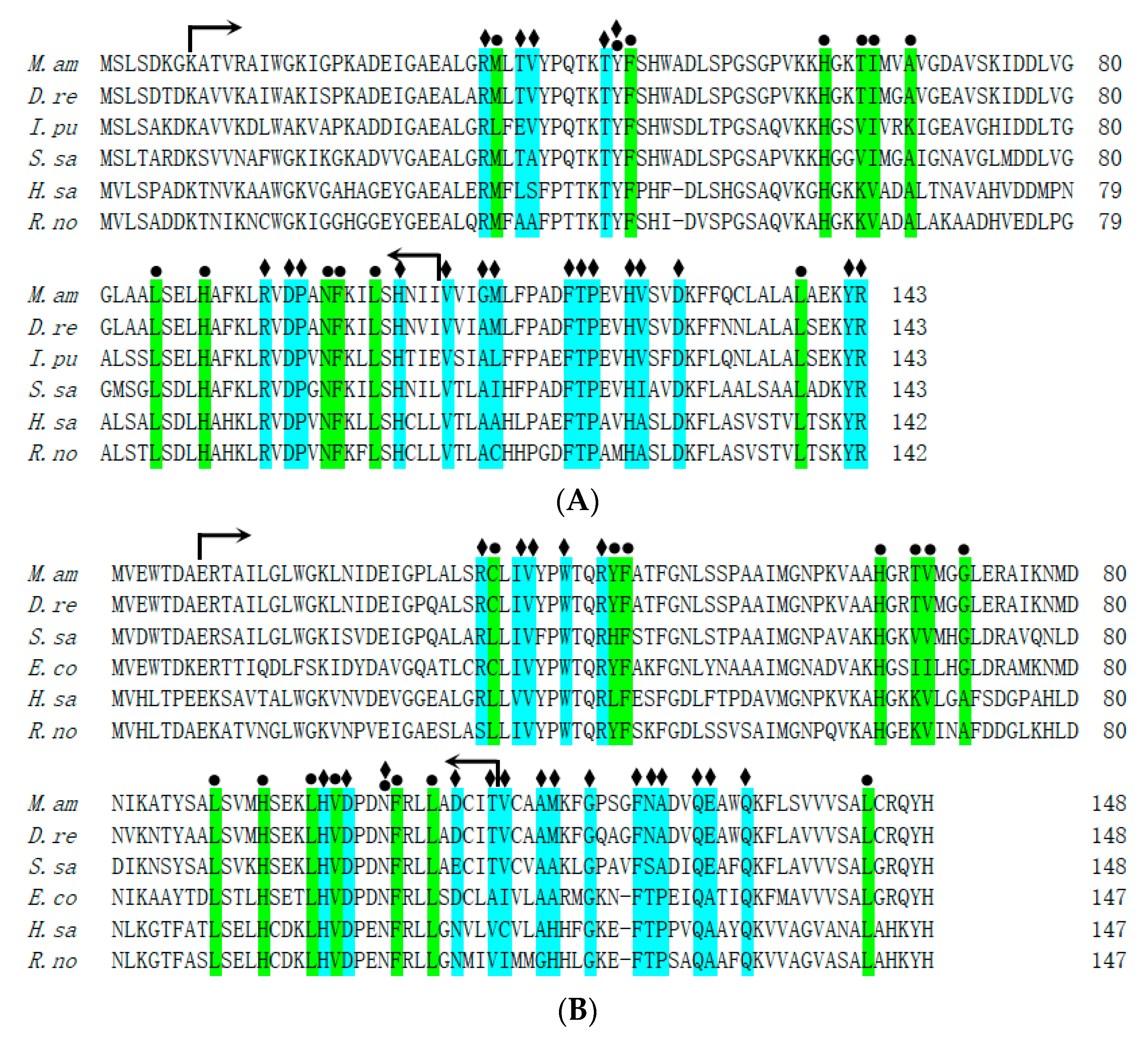
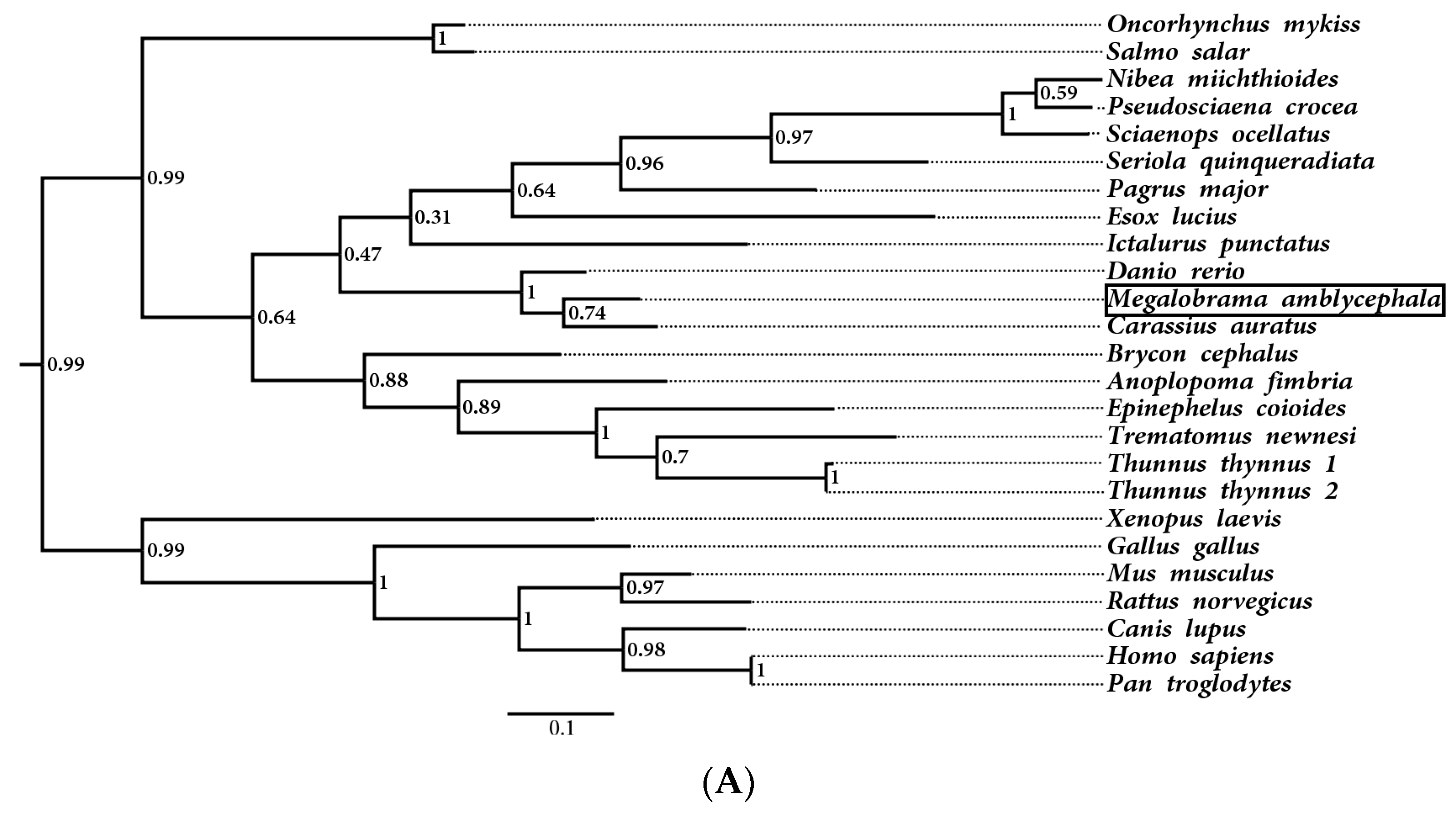
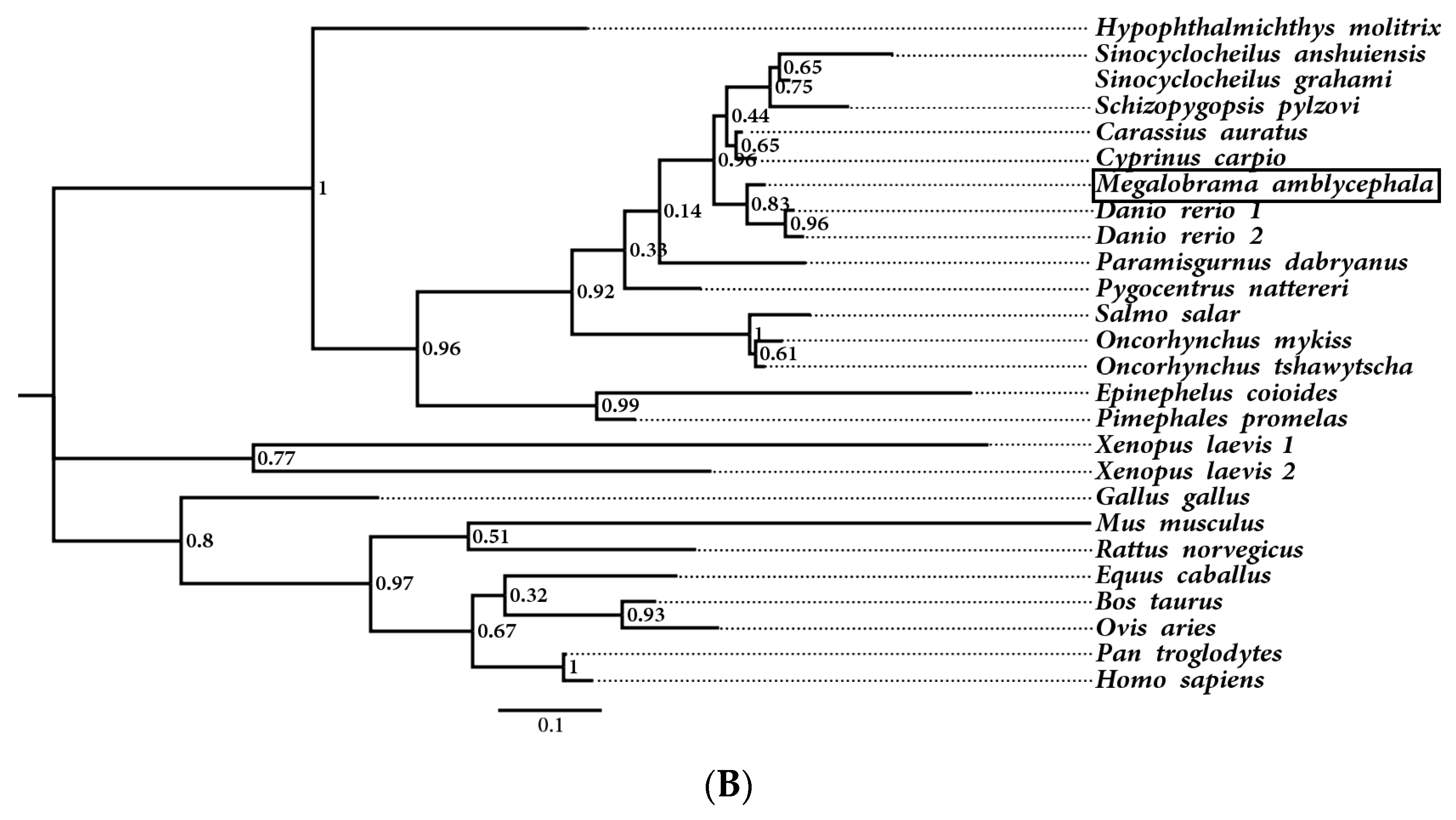
3.2. Multiple Sequence Alignment and Phylogenetic Analysis of MaHb Genes
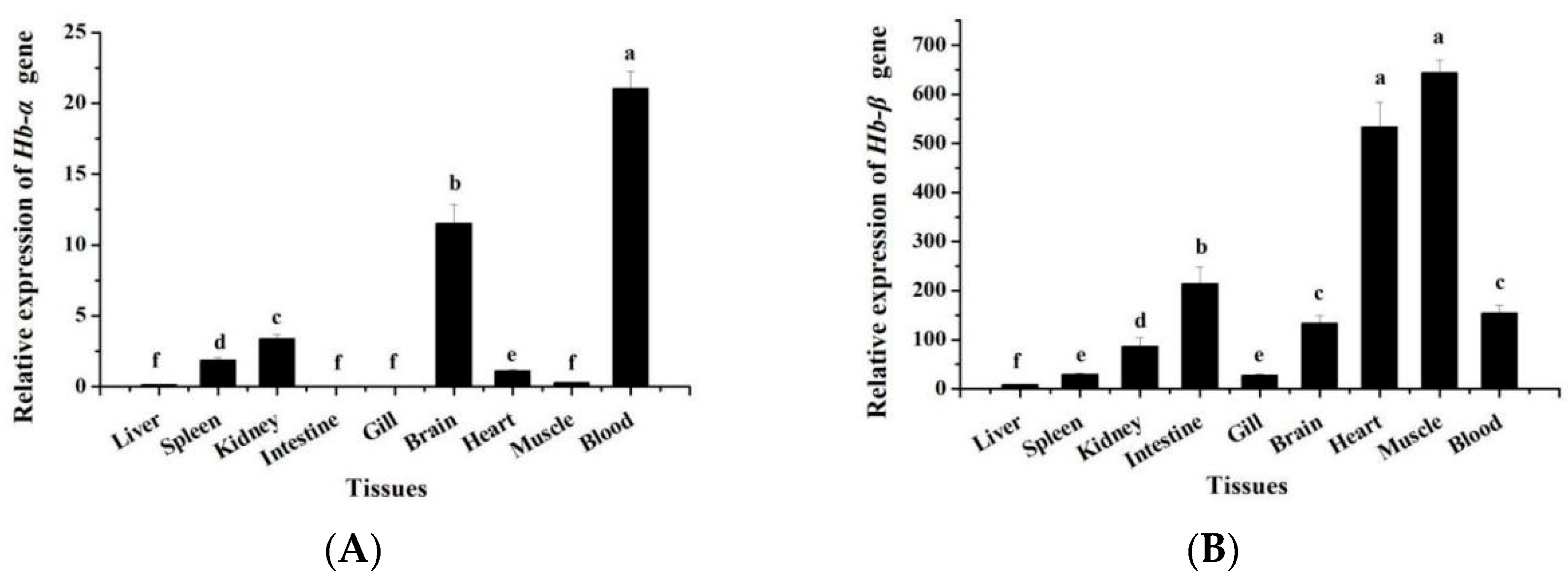
3.3. Expression Patterns of MaHbα and MaHbβ mRNA in Different Tissues and Various Developmental Stages
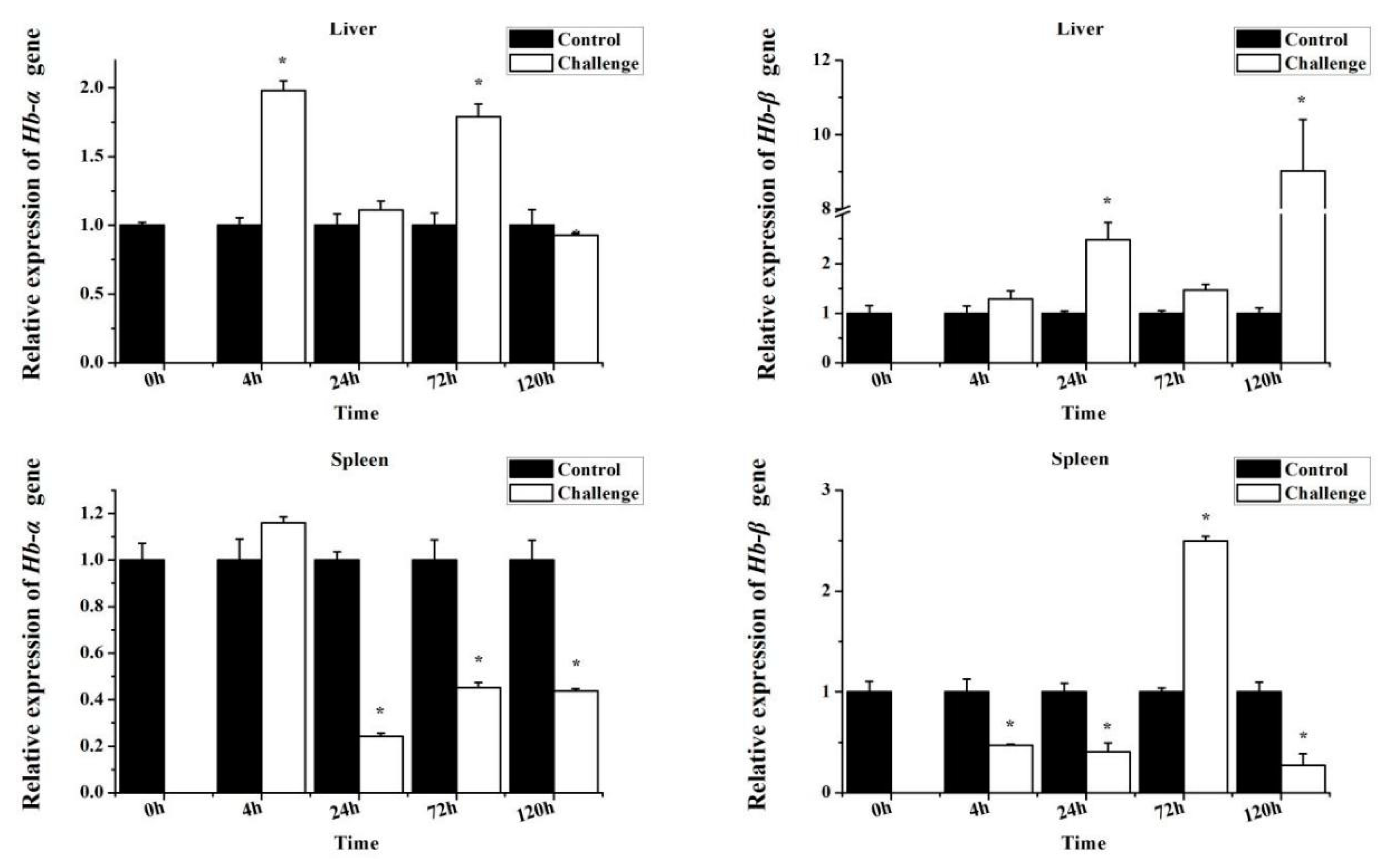
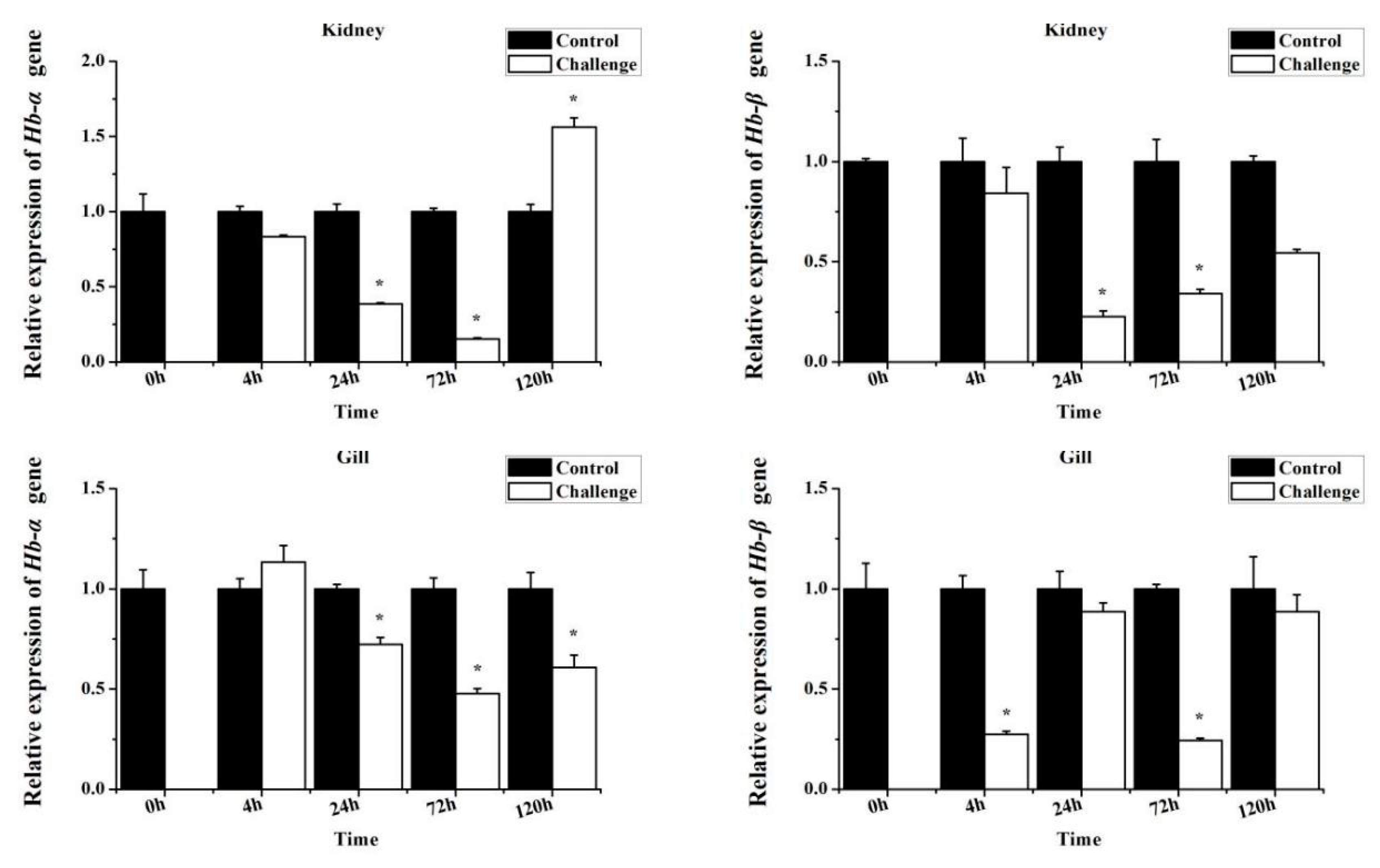
3.4. MaHbα and MaHbβ Expression in Response to Bacterial Infection
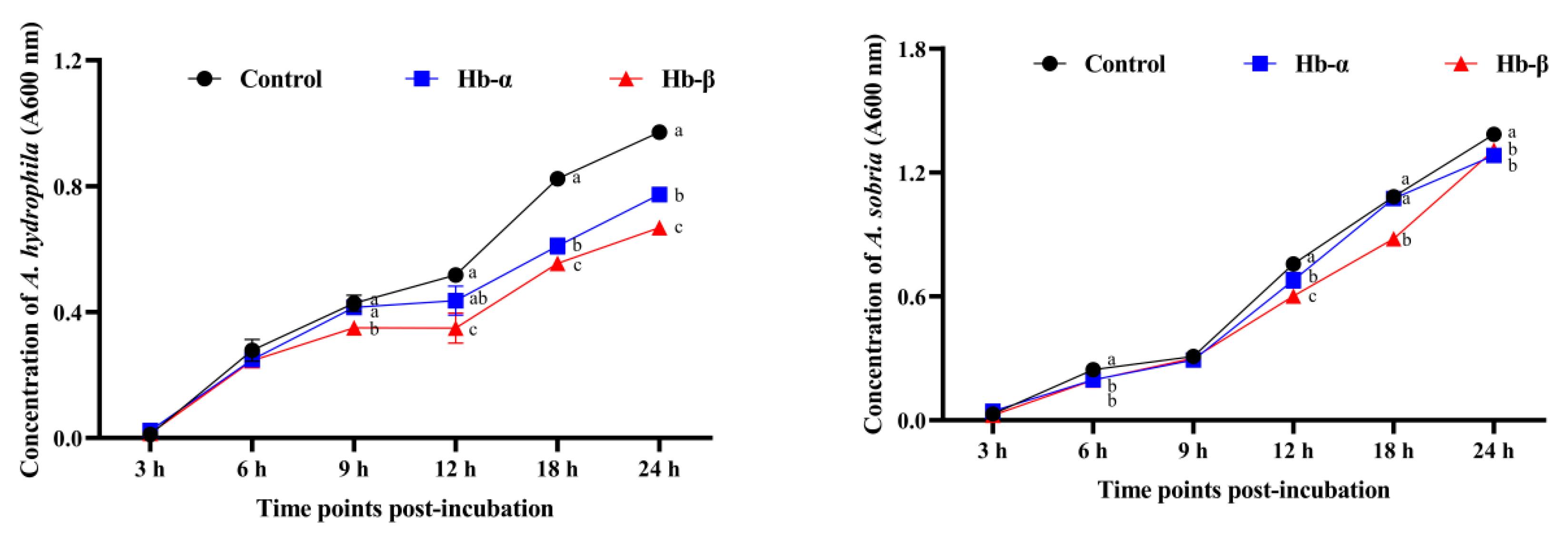
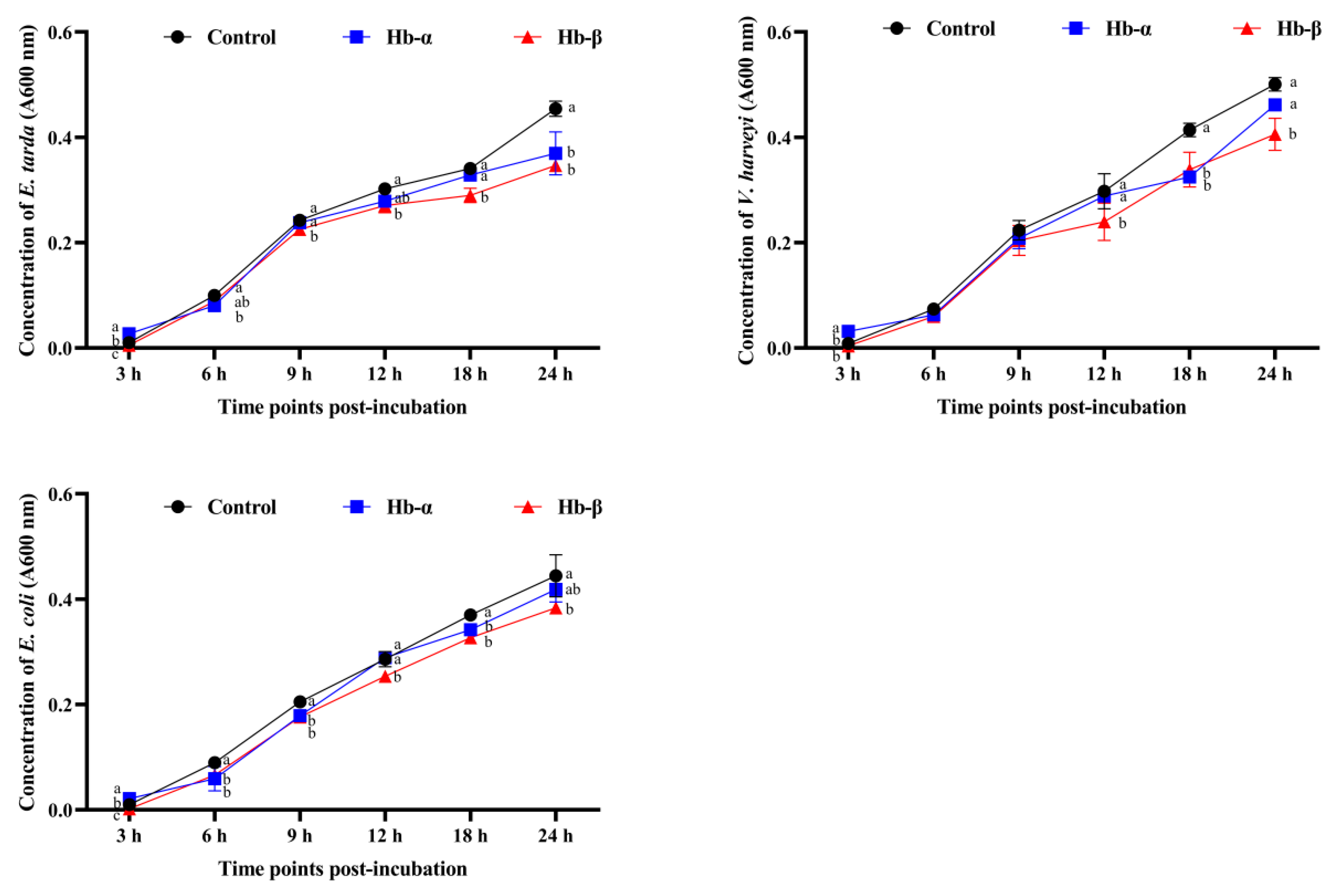
3.5. Antimicrobial Activities of Synthetic MaHbα and MaHbβ Peptides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Daoud, R.; Dubois, V.; Bors-Dodita, L.; Nedjar-Arroume, N.; Krier, F.; Chihib, N.-E.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. New antibacterial peptide derived from bovine hemoglobin. Peptides 2005, 26, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Baxmann, S.; Heine, C.; Breithaupt, N.; Ständker, L.; Forssmann, W.-G. Human hemoglobin-derived peptides exhibit antimicrobial activity: A class of host defense peptides. J. Chromatogr. B 2003, 791, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.A.; Jiang, H.; Tokiwa, Y.; Berova, N.; Nakanishi, K.; McCabe, D.; Zuckerman, W.; Xia, M.M.; Gabay, J.E. Broad-spectrum antimicrobial activity of hemoglobin. Bioorganic Med. Chem. 2001, 9, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Nedjar-Arroume, N.; Dubois-Delval, V.; Adje, E.Y.; Traisnel, J.; Krier, F.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. Bovine hemoglobin: An attractive source of antibacterial peptides. Peptides 2008, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Pinontoan, R.; Hosoya, H.; Muto, S. Monoamine-dependent production of reactive oxygen species catalyzed by pseudoperoxidase activity of human hemoglobin. Biosci. Biotechnol. Biochem. 2002, 66, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Jaenicke, E. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 2004, 28, 673–687. [Google Scholar] [CrossRef]
- Qin, Z.; Vijayaraman, S.B.; Lin, H.; Dai, Y.; Zhao, L.; Xie, J.; Lin, W.; Wu, Z.; Li, J.; Lin, L. Antibacterial activity of erythrocyte from grass carp (Ctenopharyngodon idella) is associated with phagocytosis and reactive oxygen species generation. Fish Shellfish. Immunol. 2019, 92, 331–340. [Google Scholar] [CrossRef]
- Wagner, A.; Deryckere, F.; McMorrow, T.; Gannon, F. Tail-to-tail orientation of the Atlantic salmon alpha- and beta-globin genes. J. Mol. Evol. 1994, 38, 28–35. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Takano, A.; Aoki, T.; Takashima, F. Rapid communication: Nucleotide sequence of rainbow trout alpha-globin I and IV cDNA. J. Anim. Sci. 1997, 75, 1426. [Google Scholar] [CrossRef][Green Version]
- Chan, F.-Y.; Robinson, J.; Brownlie, A.; Shivdasani, R.A.; Donovan, A.; Brugnara, C.; Kim, J.; Lau, B.-C.; Witkowska, H.E.; Zon, L.I. Characterization of adult alpha- and beta-globin genes in the zebrafish. Blood 1997, 89, 688–700. [Google Scholar] [CrossRef]
- Miyata, M.; Aoki, T. Head-to-head linkage of carp alpha- and beta-globin genes. Biochim. et Biophys. Acta 1997, 1354, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Sakai, M.; Miyata, M. Molecular cloning and sequence analysis of alpha- and beta-globin cDNAs from yellowtail, Seriola quinqueradiata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gillemans, N.; McMorrow, T.; Tewari, R.; Wai, A.W.K.; Burgtorf, C.; Drabek, D.; Ventress, N.; Langeveld, A.; Higgs, D.; Tan-Un, K.; et al. Functional and comparative analysis of globin loci in pufferfish and humans. Blood 2003, 101, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Yasumasu, S.; Iuchi, I. Characterization and expression of embryonic and adult globins of the teleost Oryzias latipes (medaka). J. Biochem. 2002, 132, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhao, J.; Jing, Z.; Zhang, Y.; Shi, Y.; Fan, T. Role of hemoglobin from blood clam Scapharca kagoshimensis beyond oxygen transport. Fish Shellfish. Immunol. 2015, 44, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, Y.; Jing, Z.; Fan, T. Molecular characteristics of hemoglobins in blood clam and their immune responses to bacterial infection. Int. J. Biol. Macromol. 2017, 99, 375–383. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, B.; Liu, Z.; Sun, X.; Zhou, L.; Yang, A.; Zhang, G. Molecular cloning, expression and biochemical characterization of hemoglobin gene from ark shell Scapharca broughtonii. Fish Shellfish. Immunol. 2018, 78, 60–68. [Google Scholar] [CrossRef]
- Cui, H.; Shen, X.; Zheng, Y.; Guo, P.; Gu, Z.; Gao, Y.; Zhao, X.; Cheng, H.; Xu, J.; Chen, X.; et al. Identification, expression patterns, evolutionary characteristics and recombinant protein activities analysis of CD209 gene from Megalobrama amblycephala. Fish Shellfish. Immunol. 2022, 126, 47–56. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.H.; Gao, Z.X.; Min, J.M.; Gu, Y.M.; Jian, J.B.; Jiang, X.W.; Cai, H.M.; Ebersberger, I.; Xu, M.; et al. The draft genome of blunt snout bream (Megalobrama amblycephala) reveals the development of intermuscular bone and adaptation to herbivorous diet. GigaScience 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Han, Z.; Zheng, Y.; Wang, X.; Zhang, M.; Li, H.; Xu, J.; Chen, X.; Ding, Z.; et al. Comparative effects of dietary soybean oil and fish oil on the growth performance, fatty acid composition and lipid metabolic signaling of grass carp, Ctenopharyngodon idella. Aquac. Rep. 2022, 22, 101002. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, H.; Zhang, M.; Li, H.; Wang, X.; Wang, Z.; Zhai, W.; Chen, X.; Cheng, H.; Xu, J.; et al. Molecular characterization, expression, evolutionary selection, and biological activity analysis of CD68 gene from Megalobrama amblycephala. Int. J. Mol. Sci. 2022, 23, 13133. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.J.; Litaker, R.W.; Noga, E.J. Antimicrobial peptides derived from hemoglobin are expressed in epithelium of channel catfish (Ictalurus punctatus, Rafinesque). Dev. Comp. Immunol. 2008, 32, 1301–1312. [Google Scholar] [CrossRef]
- Feng, J.; Liu, S.; Wang, X.; Wang, R.; Zhang, J.; Jiang, Y.; Li, C.; Kaltenboeck, L.; Li, J.; Liu, Z. Channel catfish hemoglobin genes: Identification, phylogenetic and syntenic analysis, and specific induction in response to heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 9, 11–22. [Google Scholar] [CrossRef]
- Vergara, A.; Vitagliano, L.; Merlino, A.; Sica, F.; Marino, K.; Verde, C.; di Prisco, G.; Mazzarella, L. An order-disorder transition plays a role in switching off the root effect in fish hemoglobins. J. Biol. Chem. 2010, 285, 32568–32575. [Google Scholar] [CrossRef]
- Maruyama, K.; Yasumasu, S.; Naruse, K.; Mitani, H.; Shima, A.; Iuchi, I. Genomic organization and developmental expression of globin genes in the teleost Oryzias latipes. Gene 2004, 335, 89–100. [Google Scholar] [CrossRef]
- Ganis, J.J.; Hsia, N.; Trompouki, E.; de Jong, J.L.; DiBiase, A.; Lambert, J.S.; Jia, Z.; Sabo, P.J.; Weaver, M.; Sandstrom, R.; et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev. Biol. 2012, 366, 185–194. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, X.; Ge, M.; Jin, X.; Wang, Z. Effects of Aeromonas hydrophila On haemoglobin of several kinds of fish. Hebei Fisheries. 2009, 11, 7–10. [Google Scholar] [CrossRef]
- Xiong, N.X.; Ou, J.; Fan, L.F.; Kuang, X.Y.; Fang, Z.X.; Luo, S.W.; Mao, Z.W.; Liu, S.J.; Wang, S.; Wen, M.; et al. Blood cell characterization and transcriptome analysis reveal distinct immune response and host resistance of different ploidy cyprinid fish following Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2021, 120, 547–559. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, J.; Gao, X.; Li, X.; Zhang, Y.; Liu, X.; Yang, H.; Bing, X.; Zhang, X. Histopathological analysis and the immune related gene expression profiles of mandarin fish (Siniperca chuatsi) infected with Aeromonas hydrophila. Fish Shellfish. Immunol. 2018, 83, 410–415. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.; Bonilla-Rodriguez, G. Fish hemoglobins. Braz. J. Med. Biol. Res. 2007, 40, 769–778. [Google Scholar] [CrossRef]
- Chiesa, M.E.; Rosenberg, C.E.; Fink, N.E.; Salibián, A. Serum protein profile and blood cell counts in adult toads Bufo arenarum (Amphibia: Anura: Bufonidae): Effects of sublethal lead acetate. Arch. Environ. Contam. Toxicol. 2006, 50, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wu, Q.; Song, S.; She, R.; Zhao, Y.; Yang, Y.; Zhang, M.; Du, F.; Soomro, M.H.; Shi, R. Antimicrobial activity and safety evaluation of peptides isolated from the hemoglobin of chickens. BMC Microbiol. 2016, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Tan, N.S.; Ho, B.; Ding, J.L. Respiratory protein–generated reactive oxygen species as an antimicrobial strategy. Nat. Immunol. 2007, 8, 1114–1122. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, Y.; Yang, L.; Yang, X.; Jiang, G.; Yu, M.; Cong, R. Identification and characterization of a hemocyanin-derived phenoloxidase from the crab Charybdis japonica. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 152, 144–149. [Google Scholar] [CrossRef]
- Catiau, L.; Traisnel, J.; Delval-Dubois, V.; Chihib, N.-E.; Guillochon, D.; Nedjar-Arroume, N. Minimal antimicrobial peptidic sequence from hemoglobin alpha-chain: KYR. Peptides 2010, 32, 633–638. [Google Scholar] [CrossRef]
- Catiau, L.; Traisnel, J.; Chihib, N.-E.; Le Flem, G.; Blanpain, A.; Melnyk, O.; Guillochon, D.; Nedjar-Arroume, N. RYH: A minimal peptidic sequence obtained from beta-chain hemoglobin exhibiting an antimicrobial activity. Peptides 2011, 32, 1463–1468. [Google Scholar] [CrossRef]
- Powers, J.-P.S.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]


| Primers | Primer Sequences (5′-3′) | Purpose |
|---|---|---|
| MaHbα-5′-1 | ATGCCCACTGGAGGTTTAGCGG | 5′-RACE |
| MaHbα-5′-2 | GCCGTGCTTCTTCACAGGACCAG | |
| MaHbα-3′-1 | CCCTCGGCAGAATGCTGACCGTCTACCCT | 3′-RACE |
| MaHbα-3′-2 | TCACACAACATCATAGTGGTCATTGGCAT | |
| MaHbα-F | CGGCAGAATGCTGACCGTC | ORF amplification |
| MaHbα-R | GCCCACTGGAGGTTTAGCG | |
| qMaHbα-F | ATGCTCTTCCCTGCTGACTTC | qRT-PCR |
| qMaHbα-R | GGATGCCCACTGGAGGTTTAG | |
| MaHbβ-5′-1 | AGCACAACTTTACCGTGAGCAGCAACC | 5′-RACE |
| MaHbβ-5′-2 | TTGCCAGGGCTTGAGGACCAACGACT | |
| MaHbβ-3′-1 | GCAGAAGTTCCTTAGTGTCGTCGTGTCC | 3′-RACE |
| MaHbβ-3′-2 | CAATGAACACCAGCTGTATTGCAGAAG | |
| MaHbβ-F | CATGGTTGAGTGGACAGACGC | ORF amplification |
| MaHbβ-R | GCGCGGTGCGATCTTCTGC | |
| qMaHbβ-F | GAAACCTCTACAACGCCGC | qRT-PCR |
| qMaHbβ-R | CTTTACCGTGAGCAGCAACC | |
| q18S rRNA-F | CGGAGGTTCGAAGACGATCA | qRT-PCR [18] |
| q18S rRNA-R | GGGTCGGCATCGTTTACG | |
| qβ-actin-F | GCTCTTACAGGAAACGGGTC | qRT-PCR [18] |
| qβ-actin-R | GCAGCAGCTCTGTAGGTCAT | |
| qEF1α-F | CTTCTCAGGCTGACTGTGC | qRT-PCR [18] |
| qEF1α-R | CCGCTAGCATTACCCTCC | |
| qGAPDH-F | TGCCGGCATCTCCCTCAA | qRT-PCR [18] |
| qGAPDH-R | TCAGCAACACGGTGGCTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhao, X.; Liu, Y.; Zheng, J.; Cui, H.; Wang, H.; Ding, H.; Liu, H.; Ding, Z. Characterization and Expression Analysis of Genes from Megalobrama amblycephala Encoding Hemoglobins with Extracellular Microbicidal Activity. Genes 2023, 14, 1972. https://doi.org/10.3390/genes14101972
Wang Q, Zhao X, Liu Y, Zheng J, Cui H, Wang H, Ding H, Liu H, Ding Z. Characterization and Expression Analysis of Genes from Megalobrama amblycephala Encoding Hemoglobins with Extracellular Microbicidal Activity. Genes. 2023; 14(10):1972. https://doi.org/10.3390/genes14101972
Chicago/Turabian StyleWang, Qijun, Xiaoheng Zhao, Yunlong Liu, Juan Zheng, Hujun Cui, Haotong Wang, Houxu Ding, Hong Liu, and Zhujin Ding. 2023. "Characterization and Expression Analysis of Genes from Megalobrama amblycephala Encoding Hemoglobins with Extracellular Microbicidal Activity" Genes 14, no. 10: 1972. https://doi.org/10.3390/genes14101972
APA StyleWang, Q., Zhao, X., Liu, Y., Zheng, J., Cui, H., Wang, H., Ding, H., Liu, H., & Ding, Z. (2023). Characterization and Expression Analysis of Genes from Megalobrama amblycephala Encoding Hemoglobins with Extracellular Microbicidal Activity. Genes, 14(10), 1972. https://doi.org/10.3390/genes14101972






