Abstract
Niemann–Pick type C (NPC, ORPHA: 646) is a neuro-visceral, psychiatric disease caused predominantly by pathogenic variants in the NPC1 gene or seldom in NPC2. The rarity of the disease, and its wide range of clinical phenotypes and ages of onset, turn the diagnosis into a significant challenge. Other than the detailed clinical history, the typical diagnostic work-up for NPC includes the quantification of pathognomonic metabolites. However, the molecular basis diagnosis is still of utmost importance to fully characterize the disorder. Here, the authors provide an overview of splicing variants in the NPC1 and NPC2 genes and propose a new workflow for NPC diagnosis. Splicing variants cover a significant part of the disease-causing variants in NPC. The authors used cDNA analysis to study the impact of such variants, including the collection of data to classify them as leaky or non-leaky pathogenic variants. However, the presence of naturally occurring spliced transcripts can misdiagnose or mask a pathogenic variant and make the analysis even more difficult. Analysis of the NPC1 cDNA in NPC patients in parallel with controls is vital to assess and detect alternatively spliced forms. Moreover, nonsense-mediated mRNA decay (NMD) analysis plays an essential role in evaluating the naturally occurring transcripts during cDNA analysis and distinguishing them from other pathogenic variants’ associated transcripts.
1. Introduction
Lysosomal storage disorders (LSDs) are a group of about 70 inherited diseases, most of which are quite rare and present with vast clinical heterogeneity, ranging from severe, early-onset diseases to milder forms, of later onset. This remarkable variability may be observed not only between different diseases from the same group but also—and most importantly—amongst patients suffering from the same exact disease. Overall, this clinical heterogeneity has a direct impact on their diagnosis. Over the past several years, the number of available treatments for patients with LSDs has rapidly increased, namely, enzyme replacement and substrate reduction therapies, the use of molecular chaperones, gene therapy, and bone marrow transplant, among others [1]. Nevertheless, molecular diagnosis is the ultimate and essential step to provide access to therapy. The identification of biallelic nonsense and frameshift variants, as well as missense variants in conserved regions, provides a straightforward direct gene target analysis. Nevertheless, next-generation sequencing (NGS)-targeted panels for LSD-associated genes or other NGS methodologies provide a quick way to identify the molecular defect underlying diseases with such clinical variability [2]. However, in specific cases, the molecular diagnosis timeline can be even longer, when such pathogenic variants affect splicing and mRNA processing. These situations represent an additional challenge, with the identification and effect prediction of abnormal transcripts. In addition, some naturally spliced forms can raise another layer of difficulty and even mimic the molecular defect and its impact on splicing. The IDS, GNPTAB, and NPC1 genes—whose pathogenic variants underly Mucopolysaccharidosis type II, Mucolipidosis types II or III, and Niemann–Pick type C, respectively—are some examples of LSD-associated genes with naturally occurring spliced forms already reported in the databases (https://www.uniprot.org/uniprotkb/P22304/entry#sequences (accessed on 28 September 2023)); (https://www.uniprot.org/uniprotkb/Q3T906/entry#sequences (accessed on 28 September 2023)); and (https://www.uniprot.org/uniprotkb/O15118/entry#sequences (accessed on 28 September 2023)).
Niemann–Pick type C (NPC, ORPHA: 646) in particular is a devastating neurodegenerative LSD, caused by loss-of-function variants in either the NPC1 gene (in approximately 95% of cases) [3] or the NPC2 gene (in 5% of cases). Analysis of next-generation sequencing (NGS) data sets indicates that the incidence rate of NPC for the classical clinical manifestations is ~1:90,000 but suggests that, for the late-onset phenotype or variant forms, the frequency might be higher [4].
Overall, the wide range of clinical phenotypes and the different ages of onset it may present with, together with the rarity of the disease and the fact it may be caused by mutations in two different genes, make its diagnosis a significant challenge. At a clinical level, NPC’s infantile forms present varying degrees of neurologic involvement and frequently present visceral manifestations, such as splenomegaly, hepatomegaly, neonatal jaundice, and hyperbilirubinemia [5,6]. Adolescent- or adult-onset NPC, on the other hand, presents with varying combinations of progressive neurologic deficits, e.g., ataxia, dystonia and/or dementia, vertical supranuclear gaze palsy (VSGP), or major psychiatric illness, including schizophrenia, depression, and psychosis, among others [6].
That is why a definitive NPC diagnosis must rely on additional laboratorial analyses. The classical method of establishing a NPC diagnosis relies on the filipin staining of cultured fibroblasts from skin biopsies [7]. This is a microscopy-based test that takes advantage of the fact that filipin specifically binds to unesterified cholesterol, allowing the evaluation of cholesterol accumulation in the perinuclear vesicular compartments [8]. This rationale is consistent with the current assumption that the impaired egress of cholesterol from the late endosome/lysosome (LE/L) is a key element of NPC pathogenesis. Nevertheless, even the most severely affected patients may fail to be diagnosed through this method [9,10]. In fact, patients with proven NPC disease may present with variable filipin patterns, from typical “classical” or “intermediate” to “atypical” or “variant” ones, which fail to be classified as a NPC by filipin staining alone. Recent advances in the field are actively contributing to an increase in the detection of NPC patients. Among those advances is the development of rapid and reliable biomarkers, including oxysterols [11,12,13], lysosphingomyelin derivatives [14,15], and bile acids [16,17], even though none of them are specific to NPC [18]. However, N-palmitoyl-O-phosphocholineserine, (PPCS, previously known as lysosphingomyelin-509) has been shown to be elevated in the plasma and dried blood spots of NPC patients [19,20]. But, these novel biomarkers are not the sole contributors to the increased recognition of this disorder and its more expedited diagnosis. The increased availability of NGS has also contributed to the update of the overall NPC diagnostic algorithm while actively contributing to an increase in the number of positive molecular NPC diagnoses. Currently, there are a number of fully described diagnostic workflows for NPC [18], which may slightly vary between different labs depending on the tests each of them has available. However, NPC1 and NPC2 molecular analysis is mandatory in all of them and usually represents the ultimate step towards diagnosis [21]. Indeed, a rapid molecular diagnosis of a potential NPC patient is essential, not just for swift access to available therapies (currently miglustat is the only one approved within the European Union) but also to slow the progression of the disease and ultimately because it is the sole method of offering prenatal diagnosis to affected families [22].
2. A Brief Overview of the Diagnostic Workflow for Niemann-Pick Type C
2.1. The Picture in Black and White: Standard Workflows and Straightforward Diagnoses
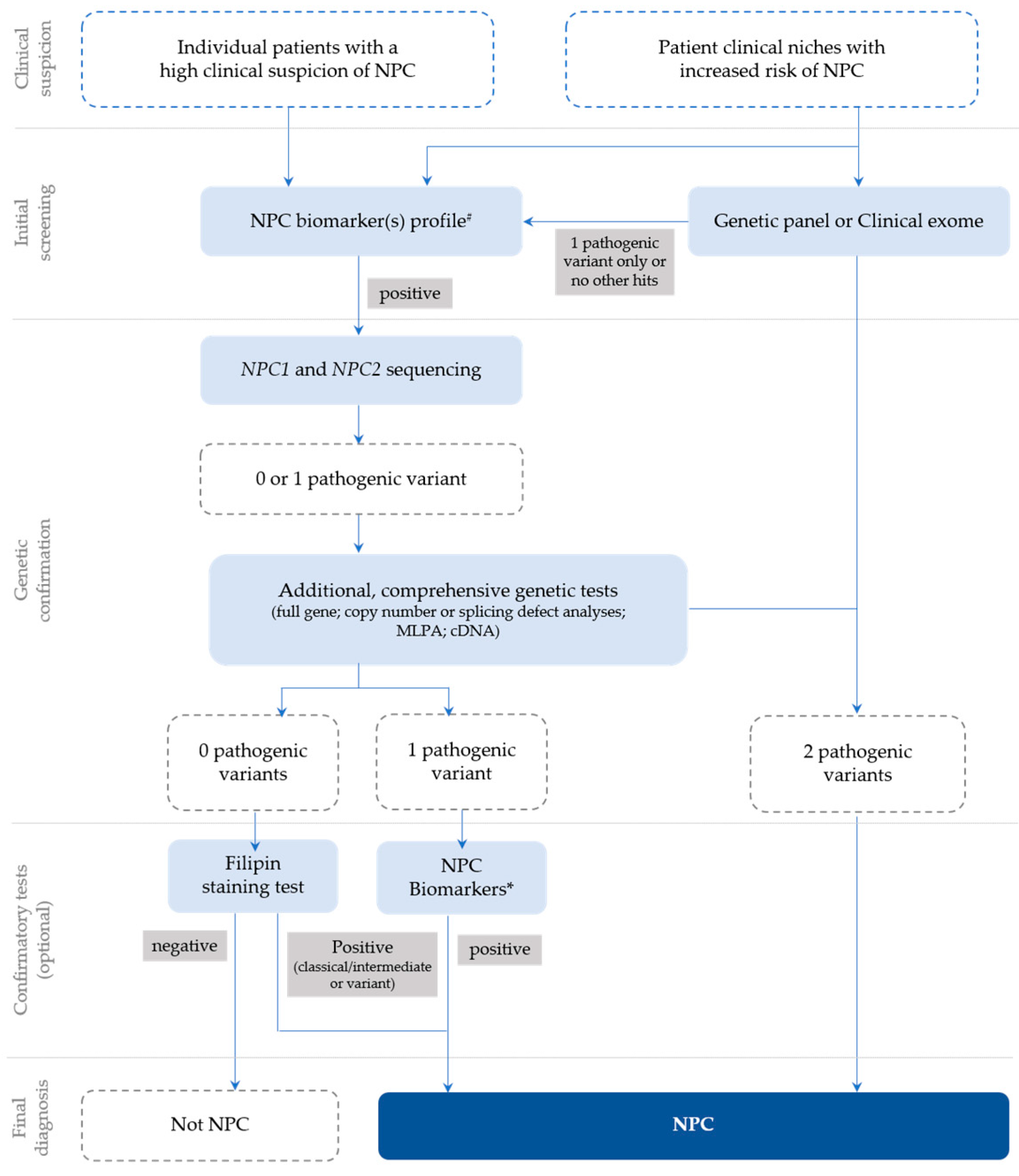
In general, following a suspicious timeline of clinical manifestation and/or a biomarker profile consistent with NPC, the next step is the NPC1 and NPC2 sequencing of the index case (Figure 1) and subsequent segregation studies of the parents [18]. The NPC1 gene (MIM# 607623) comprises 25 exons and over 600 disease-causing variants have been reported to date [23], most of which encode missense alleles. For the NPC2 gene (MIM# 601015), thirty-four disease-causing variants have been described and four of them are splicing (https://my.qiagendigitalinsights.com/, HGMD Professional 2023.3, accessed on 17 October 2023). Among the most common NPC1 pathogenic variants are p.Ile1061Thr, found in 20% of patients of Western European descent [24], and p.Pro1007Ala, associated with milder forms of the disease [25,26]. In Portugal, however, the most frequent disease-causing variant is the missense p.Ala1035Val, which accounts for 15–20% of the affected cases (unpublished data transmitted by Quelhas D and Ribeiro I); it was recently reported as the most common in patients from Latin America [27].
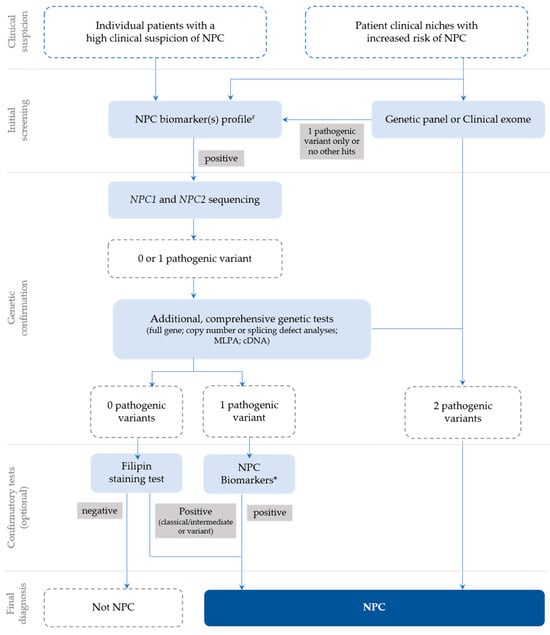
Figure 1.
Recommendations for the detection and diagnosis of NPC, based on Patterson et al. [18] with slight updates to accommodate the most recent technologies, which are now commonly used for diagnostic purposes (e.g., clinical exome), as well as the current nomenclature. # Negative biomarkers may be suggestive that the diagnosis is not NPC; * Biomarker(s) profiling (if not initially conducted) or extended biomarker(s) profiling (in addition to those already conducted).
However, the highly polymorphic nature of NPC1 can muddle diagnostic conclusions and turn the interpretation of novel variants of unknown significance (VUSs) into a challenge. In addition, cDNA sequencing is necessary to address mRNA processing in the presence of silent variants, or other VUSs, including missense variants near the (exonic or intronic) splicing regions.
More specifically, the cDNA analysis of exonic variants may help confirm the pathogenic effect of variants predicted to affect splice sites [28]. Several splice-site pathogenic variants have been identified in NPC and in many other LSDs [29]. In some instances, these variants do not allow the generation of functional mRNAs [30]. However, they are leaky and frequently produce a small percentage of correctly spliced and translated transcripts, leading to attenuated phenotypic expression of the disease [31].
Whenever conventional gDNA analysis leads to a single variant identification, the genetic study focuses on detecting the second damaging variant. For this reason, complementary studies, such as multiplex ligation-dependent probe amplification (MLPA) in gDNA to cover intragenic deletions or duplications or cDNA sequencing, may also be required for proper diagnosis of NPC [18,22]. As straightforward as this approach may sound, reaching a conclusive molecular diagnosis of NPC may, in some cases, be harder than it seems.
2.2. The Grayscale Image
Among the confounding factors that can either hinder or delay a definitive diagnosis of NPC is the presence of genetic variants affecting the normal NPC1 and NPC2 splicing patterns.
Several pathogenic variants affecting both NPC1 and NPC2 mRNA splicing, occurring in intronic and exonic regions, have already been described [32]. Although quite rare, three pathogenic intronic variants have been described in the NPC2 gene [12,28,33]. One additional variant affecting splicing was found in both healthy controls and patients [4].
Interestingly, missense variants, such as the c.1553G>A (p.Arg518Gln), were proven to have an additional impact on the splicing mechanism in the NPC1 gene, as long as they occur in the coding exons’ splicing regulatory sequences [28,34,35,36].
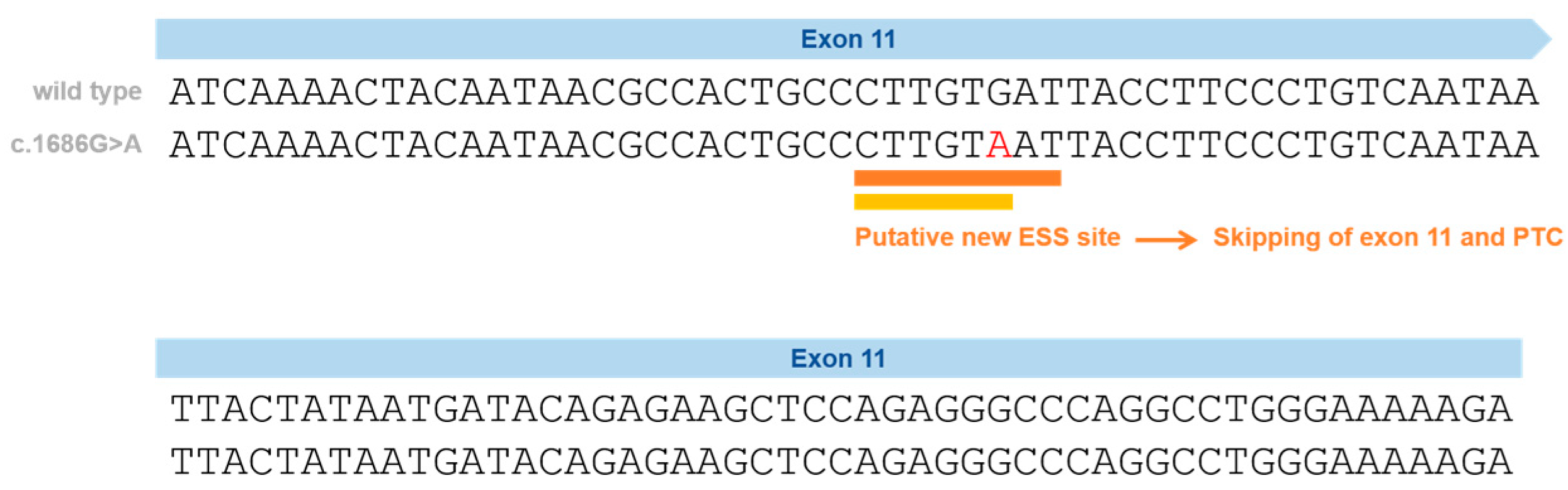
Following a combined approach (gDNA and cDNA studies), we have previously proven the impact of a silent variant in the NPC1 gene that leads to exon skipping—p.Val562= (Figure 2) [37]. This variant is located in Exon 11 and was initially reported in Spanish NPC patients and classified, at that time, as a VUS or polymorphism, after a genomic DNA study [38].
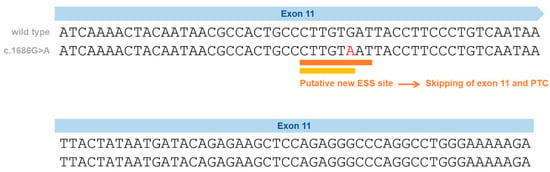
Figure 2.
Schematic representation of the silent variants in the NPC1 exonic region affecting splicing and the effect on splicing based on in silico predictions (Human Splicing Finder—HSF and EX-SKIP tools and Maxent). P.Val562= localization on Exon 11 (red) and the effect on splicing based on in silico predictions. EX-SKIP compares the Exonic Splicing Enhancer (ESE)/Exonic Splicing Silencer (ESS) profile of a wild type (WT) and a mutated allele to determine if a specific exonic variant increases the chance of exon skipping. It calculates the total number of ESSs, ESEs, and their ratio. The p.Val562= mutant is associated with a change in the ESE/ESS ratio, which is compatible with a higher chance of exon skipping than in the WT allele. In addition, the HSF (a tool to predict the effects of pathogenic variants on splicing signals or to identify splicing motifs in any human sequence) predicts that the p.Val562= mutant leads to the creation of an ESS site. It involves the cDNA sequences CTTGTAAT (orange) [39] and CTTGTA (yellow) [40], which might be associated with a potential alteration of splicing. In the case of the silent variant p.Val562=, functional cDNA analysis was performed [19], confirming the bioinformatic prediction of Exon 11 skippings.
In our previous study, cDNA analysis of the affected patient and his mother (heterozygous carrier) made it possible to identify a transcript with the skipping of Exon 11. This caused a shift in the reading frame and the emergence of a premature termination codon [37], leading to its reclassification as a disease-causing variant. Importantly, the p.Val562= variant was found not only in three independent Portuguese families but also in previously reported Spanish [38] and French patients [41] (Table 1). In a French cohort, this variant was reported in heterozygosity with the p.Ile1061Thr in two siblings [41,42]; however, its functional consequence was not ascertained. Despite the sequencing of five overlapping NPC1 cDNA fragments in the two siblings carrying the p.Val562=, the pathogenic effect of the variant was considered unknown (Supplementary Table S1, patients 25 and 25 from Nadjar et al. [41]). The most likely explanation is the degradation of the aberrant transcript by NMD. There is no information about the frequency of this variant in gnomAD. Looking to other repositories, this variant is only reported in a database from Tubingen University (NPC-db2; https://medgen.medizin.uni-tuebingen.de/NPC-db2/search.php (accessed on 28 September 2023))—it was found in one patient but not in the controls. No information was provided regarding the homo- or heterozygosity of that patient; however, both in the literature and in our cohort, only heterozygous patients were identified.

Table 1.
Patients identified as compound heterozygous for the splicing variant c.1686G>A in the NPC1 gene.
Another example of a NPC-causing variant associated with complex mRNA processing is the c.190+5G>A variant. This particular variant is located not in NPC1, the most obvious candidate to harbor a disease-causing mutation, but in Intron 2 of the NPC2 gene. Again, this variant seems to be associated with a milder clinical course since both reported patients—two siblings homozygous for this variant—presented with a juvenile onset of the neurological disease and prolonged survival. A more detailed study showed that this splice variant generated multiple abnormal mRNAs [43]. However, in fibroblasts, a very small proportion of the correctly spliced transcript was also observed. Although this was not sufficient in producing enough NPC2 protein for Western Blot detection, the presence of low levels of functional protein presumably accounts for the milder clinical course. The question of whether different tissues could display variable levels of abnormally/normally spliced RNA transcribed from the c.190+5G>A variant can also be raised.
Ideally, however, these variants should be easily detected whenever an adequate cDNA analysis of any of the involved genes is performed. Still, that is not always the case, as we will demonstrate with a few practical examples.
2.2.1. Splice Site Prediction Software
In light of a high NPC-suspicion index (a high suspicion score is assigned to patients who present with either two of seven key symptoms or VSGP alone) [44], the synonymous and nonsynonymous variants in either NPC1 or NPC2 should be studied. The predicted impact of potential splice site variants can be analyzed with the splicing prediction module of Alamut Visual software v.2.11 (Interactive Biosoftware, Rouen, France), which integrates data from three methods: Splice Site Prediction by Neural Network (NNSplice), MaxEntScan, and Human Splicing Finder (HSF) [39]. In the past few years, the SpliceAI algorithm has demonstrated the highest sensitivity and specificity when compared with other tools [45] and is nowadays the most widely recommended [46]. However, cDNA analysis is useful to study the effect of the novel variants on splicing, as well as to improve the current knowledge of the underlying molecular mechanisms and, essentially, to analyze if the variants are leaky. Other than cDNA analysis, other procedures can help to assess if a variant is leaky, namely, cloning procedures; allele-specific RNA expression quantification using PCR-based methods, among others; and procedures depending on the studied cases.
2.2.2. Naturally Occurring Spliced Forms of mRNA May Mask Disease-Associated Transcripts
While analyzing cDNA samples obtained from the skin fibroblasts of NPC patients and controls, we observed the presence of an additional amplification product comprising Exons 9, 12, and 13 and missing Exons 10 and 11. This was detected both with and without cycloheximide (CHX) treatment while the pathogenic transcript due to p.Val562= was responsive to CHX. CHX is a potent NMD inhibitor that can be used to prevent the degradation of PTC-containing transcripts. In the case of disease-causing variants that affect splicing, the visualization of the aberrant transcripts can be tricky and consequently overlooked. When patients’ cells are available, the treatment of cell cultures with CHX can significantly increase the signal of the aberrant transcripts that have PTCs and are, thus, degraded by NMD but do not have any effect on the naturally occurring transcripts (a detailed protocol is described in Encarnação et al., 2020 [37].
This observation prompted us to further analyze the transcript, searching for its presence in other tissue samples. After a number of independent assessments with different primer sets and PCR conditions, it was possible to observe the presence of a smaller NPC1 transcript, amplified in both controls and NPC patients, missing NPC1 Exons 10 and 11. This alternative transcript may be detected after RT-PCR electrophoresis as a lower molecular weight band. This may be confusing when analyzing actual aberrant transcripts resulting from real pathogenic variants causing the constitutive splicing errors of the NPC1 gene. This transcript lacking Exons 10 and 11 has already been reported in the Ensembl genome database (Transcript ID ENST00000591051.1).
Interestingly, this alternative skipping produces an in-frame transcript and the overall NPC1 reading frame remains unaltered, despite missing 204 nucleotides, corresponding to 64 coding triplets.
In order to verify the expression levels of this transcript in other cells/tissues, RNA was extracted from blood samples. cDNA was then synthesized using the same amount of RNA as that used for fibroblasts samples and both transcripts were detected. Nevertheless, their expression levels in blood seemed significantly lower than those of fibroblasts. That pattern is in full agreement with previous reports on mRNA expression in normal human tissues.
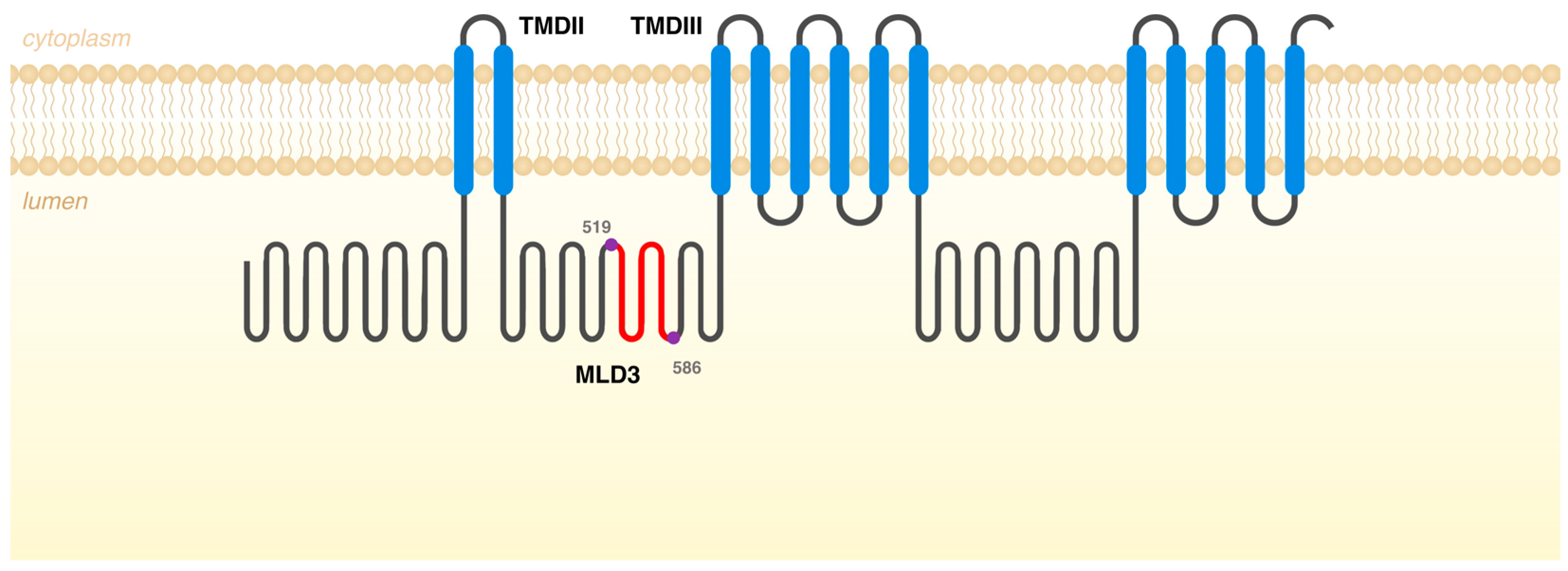
The presence of this naturally occurring NPC1 alternatively spliced mRNA is actually in accordance with the in silico estimates on splice junction strengths predicted by the MaxEntScan. This tool is based on the approach for modeling the sequences of short sequence motifs, such as those involved in RNA splicing. It simultaneously scores non-adjacent as well as adjacent dependencies between positions [5]. Interestingly, when using the tool to evaluate these regions in NPC1 Exons 9, 10, 11, and 12, we observed that the lowest 3′ss score was predicted for Exon 10 while the weakest 5′ss was predicted for Exon 11. This is in accordance with our data and can justify the existence of a naturally occurring NPC1 transcript, which does not encompass Exons 10 and 11. If translated, one such differently spliced form would give rise to a protein, which would not include amino acids 519 to 586 (68 amino acids in total). That segment is located in Middle Luminal Domain 3 (MLD3), between transmembrane domains (TMDs) II and III of the NPC1 protein (Figure 3). MLD3 mediates the transfer of NPC2-bound cholesterol to the sterol binding domain located at the N-terminal domain of NPC1 and also the Ebola virus binding [47,48]. There are 20 disease-causing missense variants in this region (HGMD Professional 2023.3 in October 2023) but this is not the most conserved region of the NPC1 protein. Interestingly, however, the predicted size of that protein product would be 1210 aa but none of the transcripts listed in Ensembl match that prediction. Still, when a more detailed analysis of those transcripts is performed, namely, by Clustal omega multiple sequence alignment, it becomes evident that there is one NPC1 transcript that does lack Exons 10 and 11 (ID ENST00000591051.1). Remarkably, however, that is not the only difference between this naturally occurring transcript and the wild type one. There is a significant difference between the transcription initiation of both transcripts, with the ENST00000591051.1 transcript Exon 1 corresponding to a sequence that partially overlaps Exon 6 of the wild-type transcript, thus comprising only 18 coding exons, in opposition to the wild-type one, which spans 25 exons.
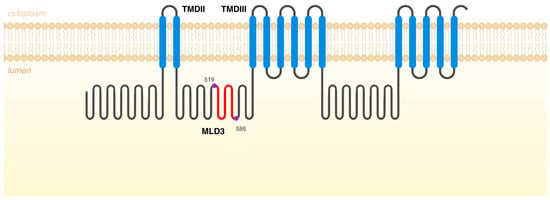
Figure 3.
Schematic representation of NPC1 protein with the different domains. In red are the missing amino acids, if the differently spliced form would give rise to a protein. That segment is located in Middle Luminal Domain 3 (MLD3), between transmembrane domains (TMDs) II and III, and it contains the amino acids 519 to 586 (68 amino acids in total).
This is yet another reason for us to highlight its presence under some amplification conditions as it may confound the analysis of cDNA patterns in that region, with serious implications for the classification of pathogenic variants predicted to impact splicing.
3. Variants in the NPC1 Gene That Affect Splicing
Pathogenic variants that affect pre-mRNA splicing account for at least 15% of disease-causing mutations [49]. Most of these variants affect 5′ and 3′ss, the polypyrimidine tract, the branch-point sequence, and also cis-acting elements (exonic/intronic splicing enhancers and silencers). Other variants create novel splicing sequences deeply within introns, causing the abnormal inclusion of intron sequences. All of these variants lead to the production of abnormal transcripts that usually contain PTCs and are degraded by nonsense-mediated mRNA decay (NMD) [50]. Even exonic variants (missense and synonymous) may affect splicing, having a completely different effect from what was expected [51]. Therefore, both gDNA and cDNA should be analyzed.
The NPC1 gene contains 25 exons and spans 55 kb from the base pairs (23, 531, 442) to 23, 586, 506 on the reverse strand of chromosome 18 at 18q11.2 (NC_000018.10); here we report on 53 published pathogenic variants affecting splicing.
Seven of them are exonic, two are synonymous, five are missense (Table 1 and Table 2), and forty-six are intronic variants (Table 3), mainly affecting the 3′ss and the 5′ss; however, there are also variants reported in the branch point as well as deep-intronic variants.

Table 2.
Exonic splicing variants in the NPC1 gene.

Table 3.
Intronic splicing variants in NPC1 gene.
Altogether, these observations call attention to the need for extensive mRNA studies in NPC1, or even NPC2, to establish a definitive NPC diagnosis. In this context, the presence of an alternatively spliced transcript may be somewhat confusing and even mask or mimic a real pathogenic variant that impacts splicing only in NPC patients and NPC1 variant carriers.
As for tissue-specific differences in the relative abundance of the two NPC1 splice isoforms, we observed a higher expression of the spliced isoform in fibroblasts than in blood. In fact, in the genotype-tissue expression project (GTEx), which studies tissue-specific gene expression and regulation in fibroblasts, they quantified 30 fragments per kilobase of exon per million fragments mapped; meanwhile, in whole blood, only 17 fragments were quantified (https://www.genecards.org/cgi-bin/carddisp.pl?gene=NPC1&keywords=npc1#expression (accessed on 28 September 2023)).
4. Conclusions
Altogether, our data highlight the fact that a naturally occurring spliced form of NPC1 mRNA should be taken into consideration when analyzing the NPC1 cDNA amplicons, especially when considering fibroblast cell cultures. Even though the traditional diagnostic workflows rely almost exclusively on targeted genomic DNA sequencing, the study of mRNA processing is often critical to understanding the real impact of the genomic variant. Therefore, cDNA analysis is highly recommended as the presence of an alternative transcript can muddle the results. Thus, one should be aware that, under certain conditions relying on the amount of total RNA used for in vitro cDNA synthesis, the transcript here reported is naturally occurring and not related to disease.
For now, the physiological role of such a transcript can only be a speculative assumption. However, the biological role of the NPC1 protein is still not fully understood. Specific efforts are striving to better understand how this protein, and others (including NPC2), take part in the egress of unesterified cholesterol from the LE/L compartment. Moreover, other important roles for NPC1, such as being the receptor of the Ebola virus, have recently been uncovered. Therefore, this transcript, as well as the encoding, may have a biological role that merits deeper research far beyond this technical recommendation for the diagnosis of NPC disease.
Author Contributions
Conceptualization, M.E. and S.A.; methodology, M.E., I.R. and M.F.C.; validation, S.A. writing—original draft preparation, M.E.; writing—review and editing, M.F.C., I.R., S.A., H.D. and D.Q.; supervision, D.Q. and S.A.; funding acquisition, M.E, D.Q. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project EXPL/BTM-TEC/1477/2021. This work was also financially supported with funding from FCT/MCTES (UIDB/00211/2020) through national funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| CHX | Cycloheximide |
| ESE | Exonic splicing enhancer |
| ESS | Exonic splicing silencer |
| GTEx | Genotype-tissue expression project |
| HSF | Human Splicing Finder |
| LE/L | Late endosome/lysosome |
| LSD | Lysosome storage disorder |
| MLPA | Multiplex ligation-dependent probe amplification |
| MoM | Multiple of median |
| MLD3 | Middle luminal domain 3 |
| NGS | Next-generation sequencing |
| NPC | Niemann-Pick type C |
| NMD | Nonsense-mediated mRNA decay |
| PPCS | N-palmitoyl-O-phosphocholineserine |
| PTC | Premature termination codon |
| TMD | Transmembrane domain |
| VUS | Variant of unknown significance |
| VSGP | Vertical supranuclear gaze palsy |
| wt | Wild type |
References
- Beck, M. Treatment Strategies for Lysosomal Storage Disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef]
- Encarnação, M.; Coutinho, M.F.; Silva, L.; Ribeiro, D.; Ouesleti, S.; Campos, T.; Santos, H.; Martins, E.; Cardoso, M.T.; Vilarinho, L.; et al. Assessing Lysosomal Disorders in the NGS Era: Identification of Novel Rare Variants. Int. J. Mol. Sci. 2020, 21, 6355. [Google Scholar] [CrossRef]
- Millat, G.; Baïlo, N.; Molinero, S.; Rodriguez, C.; Chikh, K.; Vanier, M.T. Niemann-Pick C Disease: Use of Denaturing High Performance Liquid Chromatography for the Detection of NPC1 and NPC2 Genetic Variations and Impact on Management of Patients and Families. Mol. Genet. Metab. 2005, 86, 220–232. [Google Scholar] [CrossRef]
- Wassif, C.A.; Cross, J.L.; Iben, J.; Sanchez-Pulido, L.; Cougnoux, A.; Platt, F.M.; Ory, D.S.; Ponting, C.P.; Bailey-Wilson, J.E.; Biesecker, L.G.; et al. High Incidence of Unrecognized Visceral/Neurological Late-Onset Niemann-Pick Disease, Type C1, Predicted by Analysis of Massively Parallel Sequencing Data Sets. Genet. Med. 2016, 18, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Mengel, E.; Jahnová, H.; Héron, B.; Imrie, J.; Lourenço, C.M.; van der Linden, V.; Karimzadeh, P.; Valayannopoulos, V.; Jesina, P.; et al. A Suspicion Index to Aid Screening of Early-Onset Niemann-Pick Disease Type C (NP-C). BMC Pediatr. 2016, 16, 107. [Google Scholar] [CrossRef]
- Wijburg, F.A.; Sedel, F.; Pineda, M.; Hendriksz, C.J.; Fahey, M.; Walterfang, M.; Patterson, M.C.; Wraith, J.E.; Kolb, S.A. Development of a Suspicion Index to Aid Diagnosis of Niemann-Pick Disease Type C. Neurology 2012, 78, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S.; Cupp, J.E.; Khan, M.A. Method for Detection and Isolation of Cholesteryl Ester-containing “Foam” Cells Using Flow Cytometry. Cytometry 1987, 8, 146–152. [Google Scholar] [CrossRef]
- Boernig, H.; Geyer, G. Staining of Cholesterol with the Fluorescent Antibiotic “Filipin”. Acta Histochem. 1974, 50, 110–115. [Google Scholar]
- Vanier, M.T.; Latour, P. Laboratory Diagnosis of Niemann-Pick Disease Type C: The Filipin Staining Test. Methods Cell Biol. 2015, 126, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Probert, F.; Ruiz-Rodado, V.; Te Vruchte, D.; Nicoli, E.R.; Claridge, T.D.W.; Wassif, C.A.; Farhat, N.; Porter, F.D.; Platt, F.M.; Grootveld, M. NMR Analysis Reveals Significant Differences in the Plasma Metabolic Profiles of Niemann Pick C1 Patients, Heterozygous Carriers, and Healthy Controls. Sci. Rep. 2017, 7, 6320. [Google Scholar] [CrossRef]
- Jiang, X.; Sidhu, R.; Porter, F.D.; Yanjanin, N.M.; Speak, A.O.; Te Vruchte, D.T.; Platt, F.M.; Fujiwara, H.; Scherrer, D.E.; Zhang, J.; et al. A Sensitive and Specific LC-MS/MS Method for Rapid Diagnosis of Niemann-Pick C1 Disease from Human Plasma. J. Lipid Res. 2011, 52, 1435–1445. [Google Scholar] [CrossRef]
- Reunert, J.; Fobker, M.; Kannenberg, F.; Du Chesne, I.; Plate, M.; Wellhausen, J.; Rust, S.; Marquardt, T. Rapid Diagnosis of 83 Patients with Niemann Pick Type C Disease and Related Cholesterol Transport Disorders by Cholestantriol Screening. EBioMedicine 2015, 4, 170–175. [Google Scholar] [CrossRef]
- Boenzi, S.; Deodato, F.; Taurisano, R.; Martinelli, D.; Verrigni, D.; Carrozzo, R.; Bertini, E.; Pastore, A.; Dionisi-Vici, C.; Johnson, D.W. A New Simple and Rapid LC-ESI-MS/MS Method for Quantification of Plasma Oxysterols as Dimethylaminobutyrate Esters. Its Successful Use for the Diagnosis of Niemann-Pick Type C Disease. Clin. Chim. Acta 2014, 437, 93–100. [Google Scholar] [CrossRef]
- Giese, A.K.; Mascher, H.; Grittner, U.; Eichler, S.; Kramp, G.; Lukas, J.; Te Vruchte, D.; Al Eisa, N.; Cortina-Borja, M.; Porter, F.D.; et al. A Novel, Highly Sensitive and Specific Biomarker for Niemann-Pick Type C1 Disease. Orphanet J. Rare Dis. 2015, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Welford, R.W.D.; Garzotti, M.; Lourenço, C.M.; Mengel, E.; Marquardt, T.; Reunert, J.; Amraoui, Y.; Kolb, S.A.; Morand, O.; Groenen, P. Plasma Lysosphingomyelin Demonstrates Great Potential as a Diagnostic Biomarker for Niemann-Pick Disease Type C in a Retrospective Study. PLoS ONE 2014, 9, e114669. [Google Scholar] [CrossRef]
- Jiang, X.; Sidhu, R.; Mydock-McGrane, L.; Hsu, F.F.; Covey, D.F.; Scherrer, D.E.; Earley, B.; Gale, S.E.; Farhat, N.Y.; Porter, F.D.; et al. Development of a Bile Acid-Based Newborn Screen for Niemann-Pick Disease Type C. Sci. Transl. Med. 2016, 8, 337ra63. [Google Scholar] [CrossRef]
- Mazzacuva, F.; Mills, P.B.; Mills, K.; Platt, F.; Maekawa, M.; Porter, F.D.; Clayton, P.T. Identification of New Biomarkers Suitable for an Early Diagnosis of Niemann-Pick C1. Mol. Genet. Metab. 2016, 117, S78. [Google Scholar] [CrossRef]
- Patterson, M.C.; Clayton, P.; Gissen, P.; Anheim, M.; Bauer, P.; Bonnot, O.; Dardis, A.; Dionisi-Vici, C.; Klünemann, H.H.; Latour, P.; et al. Recommendations for the Detection and Diagnosis of Niemann-Pick Disease Type C: An Update. Neurol. Clin. Pract. 2017, 7, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Jinnoh, I.; Matsumoto, Y.; Narita, A.; Mashima, R.; Takahashi, H.; Iwahori, A.; Saigusa, D.; Fujii, K.; Abe, A.; et al. Structural Determination of Lysosphingomyelin-509 and Discovery of Novel Class Lipids from Patients with Niemann-Pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 5018. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Mondjinou, Y.; Qian, M.; Song, H.; Kumar, A.B.; Hong, X.; Hsu, F.-F.; Dietzen, D.J.; Yanjanin, N.M.; Porter, F.D.; et al. N-Acyl-O-Phosphocholineserines: Structures of a Novel Class of Lipids That Are Biomarkers for Niemann-Pick C1 Disease. J. Lipid Res. 2019, 60, 1410–1424. [Google Scholar] [CrossRef]
- Patterson, M.C.; Hendriksz, C.J.; Walterfang, M.; Sedel, F.; Vanier, M.T.; Wijburg, F. Recommendations for the Diagnosis and Management of Niemann-Pick Disease Type C: An Update. Mol. Genet. Metab. 2012, 106, 330–344. [Google Scholar] [CrossRef]
- Geberhiwot, T.; Moro, A.; Dardis, A.; Ramaswami, U.; Sirrs, S.; Marfa, M.P.; Vanier, M.T.; Walterfang, M.; Bolton, S.; Dawson, C.; et al. Consensus Clinical Management Guidelines for Niemann-Pick Disease Type C. Orphanet J. Rare Dis. 2018, 13, 50. [Google Scholar] [CrossRef]
- Shammas, H.; Kuech, E.M.; Rizk, S.; Das, A.M.; Naim, H.Y. Different Niemann-Pick C1 Genotypes Generate Protein Phenotypes That Vary in Their Intracellular Processing, Trafficking and Localization. Sci. Rep. 2019, 9, 5292. [Google Scholar] [CrossRef]
- Vanier, M.T. Niemann-Pick Disease Type C. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef]
- Millat, G.; Marçais, C.; Tomasetto, C.; Chikh, K.; Fensom, A.H.; Harzer, K.; Wenger, D.A.; Ohno, K.; Vanier, M.T. Niemann-Pick C1 Disease: Correlations between NPC1 Mutations, Levels of NPC1 Protein, and Phenotypes Emphasize the Functional Significance of the Putative Sterol-Sensing Domain and of the Cysteine-Rich Luminal Loop. Am. J. Hum. Genet. 2001, 68, 1373–1385. [Google Scholar] [CrossRef]
- Ribeiro, I.; Marcão, A.; Amaral, O.; Sá Miranda, M.C.; Vanier, M.T.; Millat, G. Niemann-Pick Type C Disease: NPC1 Mutations Associated with Severe and Mild Cellular Cholesterol Trafficking Alterations. Hum. Genet. 2001, 109, 24–32. [Google Scholar] [CrossRef]
- Guatibonza Moreno, P.; Pardo, L.M.; Pereira, C.; Schroeder, S.; Vagiri, D.; Almeida, L.S.; Juaristi, C.; Hosny, H.; Loh, C.C.Y.; Leubauer, A.; et al. At a Glance: The Largest Niemann-Pick Type C1 Cohort with 602 Patients Diagnosed over 15 Years. Eur. J. Hum. Genet. 2023, 31, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Park, W.D.; O’Brien, J.F.; Lundquist, P.A.; Kraft, D.L.; Vockley, C.W.; Karnes, P.S.; Patterson, M.C.; Snow, K. Identification of 58 Novel Mutations in Niemann-Pick Disease Type C: Correlation with Biochemical Phenotype and Importance of PTC1-like Domains in NPC1. Hum. Mutat. 2003, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Gieselmann, V. Cellular Pathophysiology of Lysosomal Storage Diseases. In Fabry Disease: Perspectives from 5 Years of FOS; Oxford PharmaGenesis: Oxford, UK, 2006; ISBN 190353903X. [Google Scholar]
- Polten, A.; Fluharty, A.L.; Fluharty, C.B.; Kappler, J.; von Figura, K.; Gieselmann, V. Molecular Basis of Different Forms of Metachromatic Leukodystrophy. N. Engl. J. Med. 1991, 324, 18–22. [Google Scholar] [CrossRef] [PubMed]
- McInnes, B.; Potier, M.; Wakamatsu, N.; Melancon, S.B.; Klavins, M.H.; Tsuji, S.; Mahuran, D.J. An Unusual Splicing Mutation in the HEXB Gene Is Associated with Dramatically Different Phenotypes in Patients from Different Racial Backgrounds. J. Clin. Investig. 1992, 90, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Paron, F.; Dardis, A.; Buratti, E. Pre-MRNA Splicing Defects and RNA Binding Protein Involvement in Niemann Pick Type C Disease. J. Biotechnol. 2020, 318, 20–30. [Google Scholar] [CrossRef]
- Millat, G.; Chikh, K.; Naureckiene, S.; Sleat, D.E.; Fensom, A.H.; Higaki, K.; Elleder, M.; Lobel, P.; Vanier, M.T. Niemann-Pick Disease Type C: Spectrum of HE1 Mutations and Genotype/Phenotype Correlations in the NPC2 Group. Am. J. Hum. Genet. 2001, 69, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Tarugi, P.; Ballarini, G.; Bembi, B.; Battisti, C.; Palmeri, S.; Panzani, F.; Di Leo, E.; Martini, C.; Federico, A.; Calandra, S. Niemann-Pick Type C Disease: Mutations of NPC1 Gene and Evidence of Abnormal Expression of Some Mutant Alleles in Fibroblasts. J. Lipid Res. 2002, 43, 1908–1919. [Google Scholar] [CrossRef]
- Macías-Vidal, J.; Gort, L.; Lluch, M.; Pineda, M.; Coll, M.J. Nonsense-Mediated MRNA Decay Process in Nine Alleles of Niemann-Pick Type C Patients from Spain. Mol. Genet. Metab. 2009, 97, 60–64. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nanba, E.; Ninomiya, H.; Higaki, K.; Taniguchi, M.; Zhang, H.; Akaboshi, S.; Watanabe, Y.; Takeshima, T.; Inui, K.; et al. NPC1 Gene Mutations in Japanese Patients with Niemann-Pick Disease Type C. Hum. Genet. 1999, 105, 10–16. [Google Scholar] [CrossRef]
- Encarnação, M.; Coutinho, M.F.; Cho, S.M.; Cardoso, M.T.; Ribeiro, I.; Chaves, P.; Santos, J.I.; Quelhas, D.; Lacerda, L.; Leão Teles, E.; et al. NPC1 Silent Variant Induces Skipping of Exon 11 (p.V562V) and Unfolded Protein Response Was Found in a Specific Niemann-Pick Type C Patient. Mol. Genet. Genom. Med. 2020, 8, e1451. [Google Scholar] [CrossRef]
- Fernandez-Valero, E.M.; Ballart, A.; Iturriaga, C.; Lluch, M.; Macias, J.; Vanier, M.T.; Pineda, M.; Coll, M.J. Identification of 25 New Mutations in 40 Unrelated Spanish Niemann-Pick Type C Patients: Genotype-Phenotype Correlations. Clin. Genet. 2005, 68, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.F.; Chasin, L.A. Computational Definition of Sequence Motifs Governing Constitutive Exon Splicing. Genes Dev. 2004, 18, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Ram, O.; Amit, M.; Keren, H.; Lev-Maor, G.; Vig, I.; Pupko, T.; Ast, G. Comparative Analysis Identifies Exonic Splicing Regulatory Sequences-The Complex Definition of Enhancers and Silencers. Mol. Cell 2006, 22, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Nadjar, Y.; Hütter-Moncada, A.L.; Latour, P.; Ayrignac, X.; Kaphan, E.; Tranchant, C.; Cintas, P.; Degardin, A.; Goizet, C.; Laurencin, C.; et al. Adult Niemann-Pick Disease Type C in France: Clinical Phenotypes and Long-Term Miglustat Treatment Effect. Orphanet J. Rare Dis. 2018, 13, 175. [Google Scholar] [CrossRef]
- Anheim, M.; Lagha-Boukbiza, O.; Fleury-Lesaunier, M.C.; Valenti-Hirsch, M.P.; Hirsch, E.; Gervais-Bernard, H.; Broussolle, E.; Thobois, S.; Vanier, M.T.; Latour, P.; et al. Heterogeneity and Frequency of Movement Disorders in Juvenile and Adult-Onset Niemann-Pick C Disease. J. Neurol. 2014, 261, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Suzuki, K. Multiple Abnormal Beta-Hexosaminidase Alpha Chain MRNAs in a Compound-Heterozygous Ashkenazi Jewish Patient with Tay-Sachs Disease. J. Biol. Chem. 1988, 263, 18563–18567. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Juríčková, K.; Karimzadeh, P.; Kolniková, M.; Malinová, V.; Torres, J.; Kolb, S.A. Evaluation of Different Suspicion Indices in Identifying Patients with Niemann-Pick Disease Type C in Clinical Practice: A Post Hoc Analysis of a Retrospective Chart Review. Orphanet J. Rare Dis. 2019, 14, 161. [Google Scholar] [CrossRef]
- Jang, W.; Park, J.; Chae, H.; Kim, M. Comparison of In Silico Tools for Splice-Altering Variant Prediction Using Established Spliceogenic Variants: An End-User’s Point of View. Int. J. Genom. 2022, 2022, 5265686. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Hoya, M.d.l.; Wiggins, G.A.R.; Lindy, A.; Vincent, L.M.; Parsons, M.T.; Canson, D.M.; Bis-Brewer, D.; Cass, A.; Tchourbanov, A.; et al. Using the ACMG/AMP Framework to Capture Evidence Related to Predicted and Observed Impact on Splicing: Recommendations from the ClinGen SVI Splicing Subgroup. Am. J. Hum. Genet. 2023, 110, 1046–1067. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Coutavas, E.; Shi, H.; Hao, Q.; Blobel, G. Structure of Human Niemann-Pick C1 Protein. Proc. Natl. Acad. Sci. USA 2016, 113, 8212–8217. [Google Scholar] [CrossRef]
- Gong, X.; Qian, H.; Zhou, X.; Wu, J.; Wan, T.; Cao, P.; Huang, W.; Zhao, X.; Wang, X.; Wang, P.; et al. Structural Insights into the Niemann-Pick C1 (NPC1)-Mediated Cholesterol Transfer and Ebola Infection. Cell 2016, 165, 1467–1478. [Google Scholar] [CrossRef]
- Caminsky, N.; Mucaki, E.J.; Rogan, P.K. Interpretation of MRNA Splicing Mutations in Genetic Disease: Review of the Literature and Guidelines for Information-Theoretical Analysis. F1000Research 2014, 3, 282. [Google Scholar] [CrossRef]
- Anna, A.; Monika, G. Splicing Mutations in Human Genetic Disorders: Examples, Detection, and Confirmation. J. Appl. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef]
- Sarkar, A.; Panati, K.; Narala, V.R. Code inside the Codon: The Role of Synonymous Mutations in Regulating Splicing Machinery and Its Impact on Disease. Mutat. Res. Rev. Mutat. Res. 2022, 790, 108444. [Google Scholar] [CrossRef]
- Macías-Vidal, J.; Rodríguez-Pascau, L.; Sánchez-Ollé, G.; Lluch, M.; Vilageliu, L.; Grinberg, D.; Coll, M.J. Molecular Analysis of 30 Niemann-Pick Type C Patients from Spain. Clin. Genet. 2011, 80, 39–49. [Google Scholar] [CrossRef]
- Marelli, C.; Guissart, C.; Hubsch, C.; Renaud, M.; Villemin, J.-P.; Larrieu, L.; Charles, P.; Ayrignac, X.; Sacconi, S.; Collignon, P.; et al. Mini-Exome Coupled to Read-Depth Based Copy Number Variation Analysis in Patients with Inherited Ataxias. Hum. Mutat. 2016, 37, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Dardis, A.; Zampieri, S.; Gellera, C.; Carrozzo, R.; Cattarossi, S.; Peruzzo, P.; Dariol, R.; Sechi, A.; Deodato, F.; Caccia, C.; et al. Molecular Genetics of Niemann–Pick Type C Disease in Italy: An Update on 105 Patients and Description of 18 NPC1 Novel Variants. J. Clin. Med. 2020, 9, 679. [Google Scholar] [CrossRef]
- Terada, C.; Mirarchi, F.; Marino, R.; Eiroa, H.; Berensztein, E. Molecular and Functional Study of Pediatric Patients with Niemann-Pick C in Argentina. Medicina 2022, 82, 308–312. [Google Scholar]
- Fancello, T.; Dardis, A.; Rosano, C.; Tarugi, P.; Tappino, B.; Zampieri, S.; Pinotti, E.; Corsolini, F.; Fecarotta, S.; D’Amico, A.; et al. Molecular Analysis of NPC1 and NPC2 Gene in 34 Niemann-Pick C Italian Patients: Identification and Structural Modeling of Novel Mutations. Neurogenetics 2009, 10, 229–239. [Google Scholar] [CrossRef]
- Imrie, J.; Heptinstall, L.; Knight, S.; Strong, K. Observational Cohort Study of the Natural History of Niemann-Pick Disease Type C in the UK: A 5-Year Update from the UK Clinical Database. BMC Neurol. 2015, 15, 257. [Google Scholar] [CrossRef]
- Di Leo, E.; Panico, F.; Tarugi, P.; Battisti, C.; Federico, A.; Calandra, S. A Point Mutation in the Lariat Branch Point of Intron 6 of NPC1 as the Cause of Abnormal Pre-MRNA Splicing in Niemann-Pick Type C Disease. Hum. Mutat. 2004, 24, 440. [Google Scholar] [CrossRef]
- Zhan, X.; Lin, N.; Zhang, H.W.; Gao, X.L.; Qiu, W.J.; Han, L.S.; Ye, J.; Gu, X.F. Blood 7-ketocholesterol level, clinical features and gene mutation analysis of 18 children with Niemann-Pick disease type C. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2016, 54, 419–423. [Google Scholar] [CrossRef]
- Tängemo, C.; Weber, D.; Theiss, S.; Mengel, E.; Runz, H. Niemann-Pick Type C Disease: Characterizing Lipid Levels in Patients with Variant Lysosomal Cholesterol Storage. J. Lipid Res. 2011, 52, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pascau, L.; Coll, M.J.; Vilageliu, L.; Grinberg, D. Antisense Oligonucleotide Treatment for a Pseudoexon-Generating Mutation in the NPC1 Gene Causing Niemann-Pick Type C Disease. Hum. Mutat. 2009, 30, E993–E1001. [Google Scholar] [CrossRef]
- Sun, X.; Marks, D.L.; Park, W.D.; Wheatley, C.L.; Puri, V.; O’Brien, J.F.; Kraft, D.L.; Lundquist, P.A.; Patterson, M.C.; Pagano, R.E.; et al. Niemann-Pick C Variant Detection by Altered Sphingolipid Trafficking and Correlation with Mutations within a Specific Domain of NPC1. Am. J. Hum. Genet. 2001, 68, 1361–1372. [Google Scholar] [CrossRef]
- Kılıç Yıldırım, G.; Yarar, C.; Şeker Yılmaz, B.; Ceylaner, S. Niemann-Pick Type C Disease with a Novel Intronic Mutation: Three Turkish Cases from the Same Family. J. Pediatr. Endocrinol. Metab. 2022, 35, 535–541. [Google Scholar] [CrossRef]
- Almeida, L.S.; Pereira, C.; Aanicai, R.; Schröder, S.; Bochinski, T.; Kaune, A.; Urzi, A.; Spohr, T.C.L.S.; Viceconte, N.; Oppermann, S.; et al. An Integrated Multiomic Approach as an Excellent Tool for the Diagnosis of Metabolic Diseases: Our First 3720 Patients. Eur. J. Hum. Genet. 2022, 30, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.C.; Tian, Z.X.; Deng, Y.X.; Wang, Y.J.; Wu, X.J.; Zhang, Y.Z.; Gao, B.Q. Clinical features and gene mutation analysis of patients with Niemann-Pick disease type C. Zhonghua Yi Xue Za Zhi 2018, 98, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Cougnoux, A.; Farhat, N.; Wassif, C.A.; Porter, F.D. Association of NPC1 Variant p.P237S with a Pathogenic Splice Variant in Two Niemann-Pick Disease Type C1 Patients. Am. J. Med. Genet. Part A 2017, 173, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Alfadhel, M.; Benmeakel, M.; Hossain, M.A.; Al Mutairi, F.; Al Othaim, A.; Alfares, A.A.; Al Balwi, M.; Alzaben, A.; Eyaid, W. Thirteen Year Retrospective Review of the Spectrum of Inborn Errors of Metabolism Presenting in a Tertiary Center in Saudi Arabia. Orphanet J. Rare Dis. 2016, 11, 126. [Google Scholar] [CrossRef]
- Héron, B.; Valayannopoulos, V.; Baruteau, J.; Chabrol, B.; Ogier, H.; Latour, P.; Dobbelaere, D.; Eyer, D.; Labarthe, F.; Maurey, H.; et al. Miglustat Therapy in the French Cohort of Paediatric Patients with Niemann-Pick Disease Type C. Orphanet J. Rare Dis. 2012, 7, 36. [Google Scholar] [CrossRef]
- Stranneheim, H.; Lagerstedt-Robinson, K.; Magnusson, M.; Kvarnung, M.; Nilsson, D.; Lesko, N.; Engvall, M.; Anderlid, B.-M.; Arnell, H.; Johansson, C.B.; et al. Integration of Whole Genome Sequencing into a Healthcare Setting: High Diagnostic Rates across Multiple Clinical Entities in 3219 Rare Disease Patients. Genome Med. 2021, 13, 40. [Google Scholar] [CrossRef]
- Ganapathy, A.; Mishra, A.; Soni, M.R.; Kumar, P.; Sadagopan, M.; Kanthi, A.V.; Patric, I.R.P.; George, S.; Sridharan, A.; Thyagarajan, T.C.; et al. Multi-Gene Testing in Neurological Disorders Showed an Improved Diagnostic Yield: Data from over 1000 Indian Patients. J. Neurol. 2019, 266, 1919–1926. [Google Scholar] [CrossRef]
- Kaur, P.; do Rosario, M.C.; Hebbar, M.; Sharma, S.; Kausthubham, N.; Nair, K.; Shrikiran, A.; Ramesh Bhat, Y.; Lewis, L.E.S.; Nampoothiri, S.; et al. Clinical and Genetic Spectrum of 104 Indian Families with Central Nervous System White Matter Abnormalities. Clin. Genet. 2021, 100, 542–550. [Google Scholar] [CrossRef]
- Zampieri, S.; Bembi, B.; Rosso, N.; Filocamo, M.; Dardis, A. Treatment of Human Fibroblasts Carrying NPC1 Missense Mutations with MG132 Leads to an Improvement of Intracellular Cholesterol Trafficking. JIMD Rep. 2012, 2, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Cáceres, C.; Villena-Ortiz, Y.; Castillo-Ribelles, L.; Barquín-Del-Pino, R.; Camprodon-Gomez, M.; Felipe-Rucián, A.; Moreno-Martínez, D.; Lucas-Del-Pozo, S.; Hernández-Vara, J.; García-Serra, A.; et al. Influence of Initial Clinical Suspicion on the Diagnostic Yield of Laboratory Enzymatic Testing in Lysosomal Storage Disorders. Experience from a Multispecialty Hospital. Blood Cells Mol. Dis. 2023, 98, 102704. [Google Scholar] [CrossRef]
- Kresojević, N.; Dobričić, V.; Lukić, M.J.; Tomić, A.; Petrović, I.; Dragašević, N.; Perović, I.; Marjanović, A.; Branković, M.; Janković, M.; et al. Genetic and Phenotypic Variability in Adult Patients with Niemann Pick Type C from Serbia: Single-Center Experience. J. Neurol. 2022, 269, 3167–3174. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lin, N.; Yang, R.; Qiu, W.; Han, L.; Ye, J.; Gu, X. Diagnosis of Niemann-Pick Disease Type C with 7-Ketocholesterol Screening Followed by NPC1/NPC2 Gene Mutation Confirmation in Chinese Patients. Orphanet J. Rare Dis. 2014, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kluenemann, H.H.; Nutt, J.G.; Davis, M.Y.; Bird, T.D. Parkinsonism Syndrome in Heterozygotes for Niemann-Pick C1. J. Neurol. Sci. 2013, 335, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Harmuth, F.; Stampfer, M.; Müller Vom Hagen, J.; Schöls, L.; Bauer, P. NPC1 Is Enriched in Unexplained Early Onset Ataxia: A Targeted High-Throughput Screening. J. Neurol. 2015, 262, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, P.J.; Aten, E.; Barge-Schaapveld, D.Q.C.M.; Bijlsma, E.K.; Bökenkamp-Gramann, R.; Donker Kaat, L.; van Doorn, R.; van de Putte, D.F.; van Haeringen, A.; ten Harkel, A.D.J.; et al. Putting Genome-Wide Sequencing in Neonates into Perspective. Genet. Med. 2019, 21, 1074–1082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).