Public Awareness and Acceptability of PGT-M in Cancer Predisposition Syndromes
Abstract
:1. Background
2. Cancer Predisposition Syndromes
- -
- -
- onset of tumors with specific histological characteristics (e.g., triple-negative breast cancer tumors and serous ovarian adenocarcinomas, both frequently associated with variants in BRCA1/2 [5]);
- -
- origin from specific geographical areas (e.g., the high prevalence of a specific variant in SDHD in Trentino, Italy [6]; updated clinical practice guidelines for Diffuse Gastric Cancer (DGC) recommend that all New Zealand Māori with a confirmed diagnosis of DGC should undergo genetic testing, given the high prevalence of CDH1 variants in this population [7]).
- -
3. Reproductive Choices Available to Patients Affected by CPSs
3.1. Preservation of Fertility
3.2. Invasive Prenatal Diagnosis
3.3. PGT-M
4. Knowledge of PGT
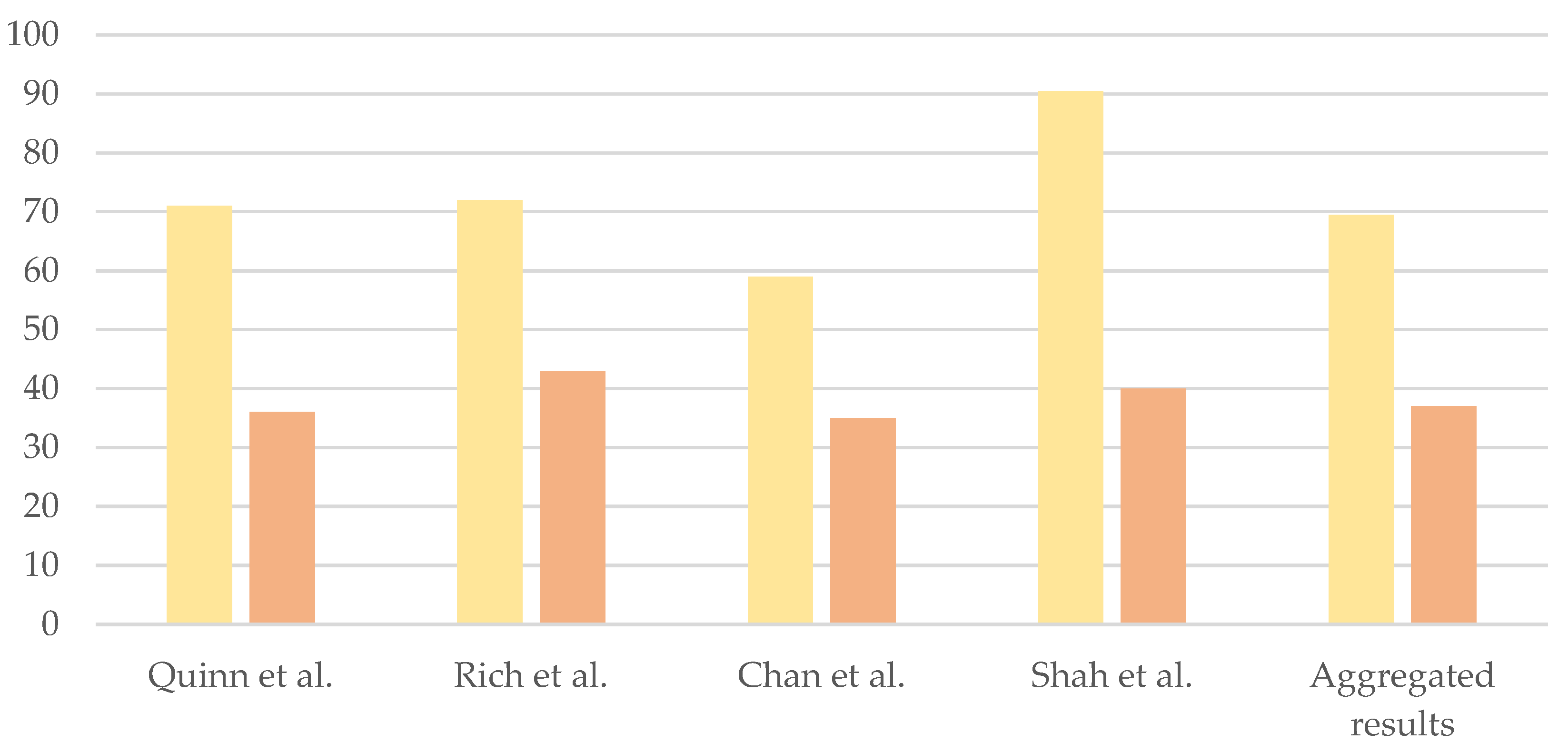
5. Acceptability of PGT
5.1. Healthcare Providers’ Opinion
5.2. Patients’ Opinion
5.2.1. Clinical Factors
5.2.2. Demographic Factors
5.2.3. Reproductive Factors
5.2.4. Socio-Cultural, Ethical and Psychological Factors
6. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Syndrome | Genes | H 1 | Prevalence | Tumor Site | Lifetime Risk | Mean Age Onset |
|---|---|---|---|---|---|---|
| Lynch | MLH1 MSH2 MSH6 PMS2 EPCAM | AD | 1:279 | Colorectal, endometrial, ovarian, gastric and duodenal, distal small bowel, urinary tract (renal pelvis, ureter, and/or bladder), pancreatic | 22–78% F 22–71% M | 43–69 |
| Constitutional Mismatch repair deficiency | MLH1 MSH2 MSH6 PMS2 | AR | - | Brain, digestive tract, hematological, Lynch syndrome associated, others | 33–50% | 1–65 |
| FAP | APC | AD | From 1:6850 to 1:31,250 live births | Colon-rectal, small bowel (duodenum, most often periampullary region, or distal to the duodenum), pancreatic, thyroid, CNS, liver, bile ducts, gastric | 70% | 34–43 |
| Peutz-Jeghers | STK11 | AD | From 1:25,000 to 1:280,000 | Colorectal, gastric, small bowel, breast, ovarian (mostly SCTAT), cervix (adenoma malignum), uterine, pancreatic, testicular (Sertoli cell tumor), lung | 7–54% | 6–59 |
| MUTYH associated polyposis | MUTYH | AR | 1–2% het. 1:20,000 to 1:60,000 biallelic | Colorectal, duodenal, ovarian, bladder, breast, endometrial, gastric, pancreatic, skin, thyroid | 80–90% colorectal 1–25% others | 38–61 |
| Hereditary diffuse gastric cancer | CDH1 | AD | 1–3% | Lobular breast cancer, colorectal (uncertain) | 56–70% hdgc 42% lbc in female | 14–69 (average 38) |
| HBOC | BRCA1 BRCA2 | AD | 1:400–1:500 | Breast, contralateral breast, ovarian, male breast, prostate, pancreatic, melanoma (cutaneous and ocular) | 1–3% for male breast and pancreas 21–72% others | 44–48 |
| VHL | VHL | AD | Between 1 in 31,000 to 1 in 91,000 | CNS hemangioblastoma, retinal hemangioblastoma, renal cell carcinoma, pheochromocytomas, pancreatic cyst and neuroendocrine tumors, endolymphatic sac tumors, epididymal or broad ligament papillary cyst adenomas | 10–70% | 1–78 |
| Li-Fraumeni | TP53 | AD | 1:3555 to 1:5476 data not well established | Adrenocortical, breast, CNS, osteosarcomas and soft-tissue sarcomas. Several additional cancers including leukemia, lymphoma, gastrointestinal cancers, cancers of head and neck, kidney, larynx, lung, skin (e.g., melanoma), ovary, pancreas, prostate, testis, and thyroid | ≥70% for men and ≥90% for women | Any age |
| NF1 | NF1 | AD | 1:2052 between ages 0 and 74 years | Optic pathway glioma, non-optic glioma, malignant peripheral nerve sheath tumor, Breast cancer, rhabdomyosarcomas, pheochromocytomas, paragangliomas, gastrointestinal stromal tumors, glomus tumors | 2–20% | Birth-any age |
| Schwannomatosis | SMARCB1 LZTR1 NF2 | AD | 1/70,000 | Schwannomas, meningiomas, MPNST | - | Any age |
| DICER1 Tumor Predisposition | DICER1 | AD | 1/5000 | Pleuropulmonary blastoma (PPB), pulmonary cysts, thyroid gland neoplasia, ovarian tumors | Lung cysts/type Ir PPB in 25–40%; PPB types I, II, & III in <10% | Any age |
| Cowden | PTEN | AD | Unknown but is estimated at 1/200,000 | Thyroid, breast, kidney, and endometrium | Breast cancer is 85%, thyroid cancer approximately 35% renal cell cancer 34% endometrial cancer 28% | 38 and 46 |
| PPGLs | MAX SDHA SDHAF2 SDHB SDHC SDHD TMEM127 | AD | 1-9/1,000,000 | Paragangliomas, pheochromocytomas, GISTs, pulmonary chondromas, renal clear cell carcinoma, papillary thyroid carcinoma, pituitary adenomas, and neuroendocrine tumors | - | Childhood |
| MEN1 | MEN1 | AD | Between 1:10,000 and 1:100,000 | Parathyroid tumors, pituitary tumors, well-differentiated endocrine tumors of the gastro-entero-pancreatic tract, carcinoid tumors, adrenocortical tumors | - | 20–25 |
| MEN2 | RET | AD | 1:35,000 | Medullary Thyroid carcinoma, pheochromocytoma, Parathyroid Disease | 20–100% | 30–70 |
| Retinoblastoma | RB1 | AD | 1:15,000 and 1:20,000 | Retinoblastoma, retinoma, pinealoblastomas, osteosarcomas, soft tissue sarcomas (mostly leiomyosarcomas and rhabdomyosarcomas), or melanomas | - | 1–5 |
| Gorlin | PTCH1 SUFU | AD | Nearer to 1:30,827 A study in Australia gave a minimum prevalence of 1:164,000 | Medulloblastoma, basal cell carcinoma, cardiac and ovarian fibromas, rhabdomyomas | 2–20% | Adolescence-30 |
| Carney complex | PRKAR1A | AD | Unknown | Myxomas, primary pigmented nodular adrenocortical disease (PPNAD), growth hormone (GH)-producing adenoma, large-cell calcifying Sertoli cell tumors (LCCSCT), thyroid adenoma or carcinoma, psammomatous melanotic schwannoma (PMS), breast ductal adenoma | - | Birth-4 decade |
| Familial melanoma syndrome | CDKN2 ACDK4 BAP1 POT1 TERF2IP ACD TERT MITF MC1R | AD/M 2 | Unknown | Pancreatic, melanoma, others | - | 30–40 |
| Birt-Hogg-Dubè | FLCN | AD | More than 400 affected families from various populations have been described | Cutaneous manifestations (fibrofolliculomas, acrochordons, angiofibromas, oral papules, cutaneous collagenomas, and epidermal cysts), pulmonary cysts/history of pneumothorax, and various types of renal tumors | - | Childhood-69 |
| Ataxia teleangectasia | ATM | AR | In the US is 1:40,000–1:100,000 | Leukemia, Lymphoma, ovarian cancer, breast cancer, gastric cancer, melanoma, leiomyomas, and sarcomas | 38% | Variabile |
| Hereditary leiomiomatosis | FH | AD | - | Uterine leiomyomata Uterine leiomyosarcoma Cutaneous leiomyomata Cutaneous leiomyosarcoma Renal cell carcinoma | 15–90% | - |
References
- Rich, T.A.; Liu, M.; Etzel, C.J.; Bannon, S.A.; Mork, M.E.; Ready, K.; Saraiya, D.S.; Grubbs, E.G.; Perrier, N.D.; Lu, K.H.; et al. Comparison of attitudes regarding preimplantation genetic diagnosis among patients with Cancer Predisposition Syndromes. Fam. Cancer 2014, 13, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Leeuwaarde RSVan Ahmad, S.; Links, T.P.; Giles, R.H. Von Hippel-Lindau Syndrome Summary. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1463/ (accessed on 1 July 2023).
- González, I.A.; Stewart, D.R.; Schultz, K.A.P.; Field, A.P.; Hill, D.A.; Dehner, L.P. DICER1 tumor predisposition syndrome: An evolving story initiated with the pleuropulmonary blastoma. Mod. Pathol. 2022, 35, 4–22. [Google Scholar] [CrossRef] [PubMed]
- GeneReviews—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 1 July 2023).
- Schiavi, F.; Demattè, S.; Cecchini, M.E.; Taschin, E.; Bobisse, S.; Del Piano, A.; Donner, D.; Barbareschi, M.; Manera, V.; Zovato, S.; et al. The endemic paraganglioma syndrome type 1: Origin, spread, and clinical expression. J. Clin. Endocrinol. Metab. 2012, 97, 637–641. [Google Scholar] [CrossRef]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef] [PubMed]
- Magaña, M.; Landeta-Sa, A.P.; López-Flores, Y. Cowden Disease: A Review. Am. J. Dermatopathol. 2022, 44, 705–717. [Google Scholar] [CrossRef] [PubMed]
- GeneReviews®—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1109/ (accessed on 1 July 2023).
- Rooney, M.; Miller, K.; Plichta, J. Genetics of Breast Cancer: Risk Models, who to Test, and Management Options. Surg. Clin. N. Am. 2023, 103, 35–47. [Google Scholar] [CrossRef]
- Aelvoet, A.S.; Buttitta, F. Management of familial adenomatous polyposis and MUTYH associated polyposis; new insight. Gastroenterol. BPRC 2022, 58–59, 101793. [Google Scholar] [CrossRef]
- Dziadkowiec, K.; Gasiorowska, E. PARP inhibitors: Review of mechanisms of action and BRCA1/2 mutation targeting. Prz. Menopauzalny 2016, 15, 215–219. [Google Scholar] [CrossRef]
- Rahner, N.; Steinke, V. Cancer Predisposition Syndromes. Dtsch. Arztebl. Int. 2008, 105, 706–714. [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Use of preimplantation genetic testing for monogenic defects (PGT-M) for adult-onset conditions: An Ethics Committee opinion. Fertil. Steril. 2018, 109, 989–992. [Google Scholar] [CrossRef]
- Julian-Reynier, C.; Chabal, F.; Frebourg, T.; Lemery, D.; Noguès, C.; Puech, F.; Stoppa-Lyonnet, D. Professionals assess the acceptability of preimplantation genetic diagnosis and prenatal diagnosis for managing inherited predisposition to cancer. J. Clin. Oncol. 2009, 27, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Costantini, M.P.; Filippi, F.; Terenziani, M.; Riccaboni, A.; Nicotra, V.; Rago, R.; Paffoni, A.; Mencaglia, L.; Magnolfi, S.; et al. Fertility counseling in women with Cancer Predisposition Syndromes. Crit. Rev. Oncol. Hematol. 2022, 171, 103604. [Google Scholar] [CrossRef] [PubMed]
- Esplen, M.J.; Bleiker, E.M.A. Screening and testing for genetic susceptibility to cancer. In Psycho-Oncology; Holland, J.C., Breitbart, W.S., Jacobsen, P.B., Loscalzo, M.J., McCorkle, R., Eds.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Hughes, T.; Bracewell-Milnes, T.; Saso, S.; Jones, B.P.; Almeida, P.A.; Maclaren, K.; Norman-Taylor, J.; Johnson, M.; Nikolaou, D. A review on the motivations, decision-making factors, attitudes and experiences of couples using pre-implantation genetic testing for inherited conditions. Hum. Reprod. Update 2021, 27, 944–966. [Google Scholar] [CrossRef]
- Vuković, P.; Peccatori, F.A.; Massarotti, C.; Miralles, M.S.; Beketić-Orešković, L.; Lambertini, M. Preimplantation genetic testing for carriers of BRCA1/2 pathogenic variants. Crit. Rev. Oncol. Hematol. 2021, 157, 103201. [Google Scholar] [CrossRef]
- Mertes, H. Let’s not forget that many prepubertal girls do have other options besides ovarian tissue cryopreservation. Hum. Reprod. 2015, 30, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Bedoschi, G.; Emirdar, V.; Moy, F.; Oktay, K. Ovarian Stimulation in Patients With Cancer: Impact of Letrozole and BRCA Mutations on Fertility Preservation Cycle Outcomes. Reprod. Sci. 2018, 25, 26–32. [Google Scholar] [CrossRef]
- Oktay, K.; Turan, V.; Bedoschi, G.; Pacheco, F.S.; Moy, F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J. Clin. Oncol. 2015, 33, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Calogero, A.E.; Krausz, C.; Lombardo, F.; Paoli, D.; Rago, R.; Scarica, C.; Simoni, M.; Foresta, C.; Rochira, V.; et al. Management of male factor infertility: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). J. Endocrinol. Investig. 2022, 45, 1085–1113. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Jindal, A.; Sharma, M. Amniocentesis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559247/ (accessed on 1 July 2023).
- Jones, T.; Montero, F. Chorionic Villus Sampling. StatPearls (Internet). Available online: https://www.ncbi.nlm.nih.gov/books/NBK563301/ (accessed on 1 July 2023).
- Caceres, V.; Murray, T.; Myers, C.; Parbhoo, K. Prenatal Genetic Testing and Screening: A Focused Review. Semin. Pediatr. Neurol. 2022, 42, 100976. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Handyside, A.; Kontogianni, E. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 1990, 344, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Albujja, M.H.; Al-Ghedan, M.; Dakshnamoorthy, L.; Pla Victori, J. Preimplantation genetic testing for embryos predisposed to hereditary cancer: Possibilities and challenges. Cancer Pathog. Ther. 2023; in press. [Google Scholar] [CrossRef]
- Harris, B.S.; Bishop, K.C.; Kuller, J.A.; Alkilany, S.; Price, T.M. Preimplantation Genetic Testing: A Review of Current Modalities. FS Rev. 2021, 2, 43–56. [Google Scholar] [CrossRef]
- Du, R.Q.; Zhao, D.D.; Kang, K.; Wang, F.; Xu, R.-X.; Chi, C.L.; Kong, L.-Y.; Liang, B. A review of pre-implantation genetic testing technologies and applications. Reprod. Dev. Med. 2023, 7, 20–31. [Google Scholar] [CrossRef]
- Verlinsky, Y.; Rechitsky, S.; Verlinsky, O.; Xu, K.; Schattman, G.; Masciangelo, C.; Ginberg, N.; Strom, C.; Rosenwaks, Z.; Kuliev, A. Preimplantation diagnosis for p53 tumour suppressor gene mutations. Reprod. Biomed. Online 2001, 2, 102–105. [Google Scholar] [CrossRef]
- Verlinsky, Y.; Rechitsky, S.; Verlinsky, O.; Chistokhina, A.; Sharapova, T.; Masciangelo, C.; Levy, M.; Kaplan, B.; Lederer, K.; Kuliev, A. Preimplantation diagnosis for neurofibromatosis. Reprod. Biomed. Online 2002, 4, 218–222. [Google Scholar] [CrossRef]
- Rechitsky, S.; Verlinsky, O.; Chistokhina, A.; Sharapova, T.; Ozen, S.; Masciangelo, C.; Kuliev, A.; Verlinsky, Y. Preimplantation genetic diagnosis for cancer predisposition. Reprod. Biomed. Online 2002, 5, 148–155. [Google Scholar] [CrossRef]
- van Montfoort, A.; Carvalho, F.; Coonen, E.; Kokkali, G.; Moutou, C.; Rubio, C.; Goossens, V.; De Rycke, M. ESHRE PGT Consortium data collection XIX–XX: PGT analyses from 2016 to 2017. Hum. Reprod. Open 2021, 2021, hoab024. [Google Scholar] [CrossRef]
- Spinella, F.; Bronet, F.; Carvalho, F.; Coonen, E.; De Rycke, M.; Rubio, C.; Goossens, V.; Van Montfoort, A. ESHRE PGT Consortium data collection XXI: PGT analyses in 2018. Hum. Reprod. Open 2023, 2023, hoad010. [Google Scholar] [CrossRef] [PubMed]
- Vriesen, N.; Carmany, E.P.; Natoli, J.L. Clinical outcomes of preimplantation genetic testing for Cancer Predisposition Syndromes: A systematic review. Prenat. Diagn. 2022, 42, 201–211. [Google Scholar] [CrossRef]
- Dallagiovanna, C.; Filippi, F.; Riccaboni, A.; Vigano’, P.; Martinelli, F.; Somigliana, E.; Ricci, M.T.; Vitellaro, M. The neglected role of preimplantation genetic testing for Lynch syndrome. Reprod. Biomed. Online 2023, 46, 421–423. [Google Scholar] [CrossRef]
- Villy, M.-C.; Frydman, N.; Moutou, C.; Thierry, G.; Raad, J.; Colas, C.; Steffann, J.; Metras, J.; Chabbert-Buffet, N.; Parc, Y.; et al. Preimplantation genetic testing in patients with genetic susceptibility to cancer. Fam. Cancer 2023, 22, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Pal, T.; Murphy, D.; Vadaparampil, S.T.; Kumar, A. High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: A systematic review and meta-analysis. Genet. Med. 2012, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Trumello, C.; Stuppia, L.; Antonucci, I.; Brandão, T.; Babore, A. BRCA1/2 pathogenetic variant carriers and reproductive decisions: Gender differences and factors associated with the choice of preimplantation genetic diagnosis (PGD) and prenatal diagnosis (PND). J. Assist. Reprod. Genet. 2022, 39, 1433–1443. [Google Scholar] [CrossRef]
- Brandt, A.C.; Tschirgi, M.L. Knowledge, attitudes and clinical experience of physicians regarding preimplantation genetic diagnosis for hereditary cancer predisposition syndromes. Fam. Cancer 2010, 9, 479–487. [Google Scholar] [CrossRef]
- Quinn, G.P.; Knapp, C. Knowledge and Educational Needs about Pre-Implantation Genetic Diagnosis (PGD) among Oncology Nurses. J. Clin. Med. 2014, 3, 632–645. [Google Scholar] [CrossRef]
- Gietel-Habets, J.; de Die-Smulders, C.; Tjan-Heijnen, V.; Derks-Smeets, I.; van Golde, R.; Gomez-Garcia, E.; van Osch, L. Professionals’ knowledge, attitude and referral behaviour of preimplantation genetic diagnosis for hereditary breast and ovarian cancer. Reprod. Biomed. Online 2018, 36, 137–144. [Google Scholar] [CrossRef]
- Chan, J.; Lnc, J.; Sammel, M.; DiGiovanni, L.; Voong, C. Reproductive Decision-Making in Women with BRCA1/2 Mutations. J. Genet. Couns. 2017, 26, 594–603. [Google Scholar] [CrossRef]
- Shah, I.H.; Salo-Mullen, E.E.; Amoroso, K.A.; Kelsen, D.; Stadler, Z.K.; Hamilton, J.G. Attitudes toward preimplantation genetic testing and quality of life among individuals with hereditary diffuse gastric cancer syndrome. Hered. Cancer Clin. Pract. 2022, 20, 31. [Google Scholar] [CrossRef]
- Würgler Hansen, A.; Sønderberg Roos, L.K.; Løssl, K.; Godballe, C.; Mathiesen, J.S. Preimplantation Genetic Testing of Multiple Endocrine Neoplasia Type 2A. Front. Endocrinol. 2020, 11, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Krones, T.; Schlüter, E.; Manolopoulos, K.; Bock, K.; Tinneberg, H.-R.; Koch, M.C.; Lindner, M.; Hoffmann, G.F.; Mayatepek, E.; Huels, G.; et al. Public, expert and patients’ opinions on preimplantation genetic diagnosis (PGD) in Germany. Reprod. Biomed. Online 2005, 10, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Marteau, T.; Michie, S.; Drake, H.; Bobrow, M. Public attitudes towards the selection of desirable characteristics in children. J. Med. Genet. 1996, 32, 796–798. [Google Scholar] [CrossRef]
- Menon, U.; Harper, J.; Sharma, A.; Fraser, L.; Burnell, M.; ElMasry, K.; Rodeck, C.; Jacobs, I. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum. Reprod. 2007, 22, 1573–1577. [Google Scholar] [CrossRef]
- Kastrinos, F.; Stoffel, E.M.; Balmaña, J.; Syngal, S. Attitudes toward prenatal genetic testing in patients with familial adenomatous polyposis. Am. J. Gastroenterol. 2007, 102, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Lammens, C.; Bleiker, E.; Aaronson, N.; Vriends, A.; Ausems, M.; Jansweijer, M.; Wagner, A.; Sijmons, R.; Ouweland, A.V.D.; van der Luijt, R.; et al. Attitude towards pre-implantation genetic diagnosis for hereditary cancer. Fam. Cancer 2009, 8, 457–464. [Google Scholar] [CrossRef]
- Derks-Smeets, I.A.P.; Gietel-Habets, J.J.G.; Tibben, A.; Tjan-Heijnen, V.C.G.; Meijer-Hoogeveen, M.; Geraedts, J.P.M.; van Golde, R.; Gomez-Garcia, E.; Bogaart, E.v.D.; van Hooijdonk, M.; et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: A challenge for couples with hereditary breast and ovarian cancer. Hum. Reprod. 2014, 29, 1103–1112. [Google Scholar] [CrossRef]
- Fortuny, D.; Balmaña, J.; Graña, B.; Torres, A.; Ramón y Cajal, T.; Darder, E.; Gadea, N.; Velasco, A.; López, C.; Sanz, J.; et al. Opinion about reproductive decision making among individuals undergoing BRCA1/2 genetic testing in a multicentre Spanish cohort. Hum. Reprod. 2009, 24, 1000–1006. [Google Scholar] [CrossRef]
- Ormondroyd, E.; Donnelly, L.; Moynihan, C.; Savona, C.; Bancroft, E.; Evans, D.G.; Eeles, R.; Lavery, S.; Watson, M. Attitudes to reproductive genetic testing in women who had a positive BRCA test before having children: A qualitative analysis. Eur. J. Hum. Genet. 2012, 20, 4–10. [Google Scholar] [CrossRef]
- Wells, S., Jr.; Asa, S. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M. Constitutional mismatch repair deficiency: Current problems and emerging therapeutic strategies. Oncotarget 2018, 9, 35458–35469. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): A review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.F.; Ponder, M.A. The clinical and screening age-at-onset distribution for the MEN-2 syndrome. Am. J. Hum. Genet. 1989, 44, 208–215. [Google Scholar]
- Orphanet. Available online: http://orpha.net (accessed on 1 July 2023).
- GeneReviews—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1116/ (accessed on 1 July 2023).
- National Cancer Istitute. Available online: http://www.cancer.gov (accessed on 1 July 2023).

| Clinical Factors | Demographic Factors | Reproductive Factors | Other Factors |
|---|---|---|---|
| Early age of onset | Male gender | Having previous children | Concerns for the newborn child’s health |
| High penetrance | Personal and familiar oncological history | ||
| High burden | |||
| Availability of primary prevention measures | Willingness to consider PND + VTP | Ethical/moral beliefs | |
| Availability of secondary prevention measures | Religious beliefs | ||
| Availability of effective treatment options | Concerns related to the procedure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calosci, D.; Passaglia, L.; Gabbiato, I.; Cartisano, F.; Affuso, R.; Sorrentino, U.; Zuccarello, D. Public Awareness and Acceptability of PGT-M in Cancer Predisposition Syndromes. Genes 2023, 14, 2069. https://doi.org/10.3390/genes14112069
Calosci D, Passaglia L, Gabbiato I, Cartisano F, Affuso R, Sorrentino U, Zuccarello D. Public Awareness and Acceptability of PGT-M in Cancer Predisposition Syndromes. Genes. 2023; 14(11):2069. https://doi.org/10.3390/genes14112069
Chicago/Turabian StyleCalosci, Davide, Lisa Passaglia, Ilaria Gabbiato, Francesca Cartisano, Rebecca Affuso, Ugo Sorrentino, and Daniela Zuccarello. 2023. "Public Awareness and Acceptability of PGT-M in Cancer Predisposition Syndromes" Genes 14, no. 11: 2069. https://doi.org/10.3390/genes14112069







