Abstract
Translation initiation in eukaryotes is regulated at several steps, one of which involves the availability of the cap binding protein to participate in cap-dependent protein synthesis. Binding of eIF4E to translational repressors (eIF4E-binding proteins [4E-BPs]) suppresses translation and is used by cells to link extra- and intracellular cues to protein synthetic rates. The best studied of these interactions involves repression of translation by 4E-BP1 upon inhibition of the PI3K/mTOR signaling pathway. Herein, we characterize a novel 4E-BP, C8ORF88, whose expression is predominantly restricted to early spermatids. C8ORF88:eIF4E interaction is dependent on the canonical eIF4E binding motif (4E-BM) present in other 4E-BPs. Whereas 4E-BP1:eIF4E interaction is dependent on the phosphorylation of 4E-BP1, these sites are not conserved in C8ORF88 indicating a different mode of regulation.
1. Introduction
Cap-dependent translation is the predominant mechanism of translation initiation in eukaryotes [1]. The cap structure, m7GpppN (where N is any nucleotide and m is a methyl group), links a methyl modified guanosine via a 5′-5′ triphosphate bridge to the first mRNA-transcribed nucleotide [2,3]. The activity of the cap in translation is mediated by the eukaryotic initiation factor (eIF) 4F complex, which assembles on the 5′ cap of mRNA to recruit the ribosome and its associated factors. The eIF4F complex consists of the cap-binding protein eIF4E, the DEAD-box RNA helicase eIF4A, and the large scaffolding protein eIF4G. The rate-limiting factor in eIF4F assembly is eIF4E, and as such, its availability governs rates of translation initiation [4,5,6,7,8].
eIF4E also interacts with eIF4E-binding proteins (4E-BPs). There are three 4E-BP homologs in mammals that share ~60% identity, of which the best studied is 4E-BP1 [4]. There are several 4E-BPs in yeast [9,10]. The association of eIF4E with 4E-BPs is disrupted by the serine/threonine kinase mTOR, a master regulator of several cellular processes, such as protein synthesis, cell growth and proliferation, lipid metabolism, cytoskeletal organization, mitochondrial function, and autophagy [11,12,13,14]. Activation of mTOR results in stepwise phosphorylation of several conserved residues in 4E-BPs, decreasing 4E-BP1′s affinity for eIF4E and leading to increased eIF4F levels [15]. A canonical eIF4E binding motif (4EBM) interacts with a conserved hydrophobic pocket on the dorsal surface of eIF4E via the sequence YXXXXLΦ (where Y denotes tyrosine, X is any amino acid, L denotes leucine and Φ is a hydrophobic residue), which is critical for interacting with eIF4E [16]. A second site, referred to as a non-canonical 4EBM, is present 15–30 residues downstream of the canonical 4EBM and significantly increases the affinity of the 4E-BPs for eIF4E [17,18,19,20]. Recently, it was discovered that the conservation of 4E-BPs extends to plants and is controlled by TOR [21].
Dysregulation of 4E-BP family members has been associated with disease states. Overexpression of hyperphosphorylated 4E-BP1 has been extensively linked to different adverse outcome variables across a wide variety of cancers [22,23,24,25,26,27]. 4E-BP2 is the 4E-BP isoform predominantly expressed in the brain, and in recent years, loss of 4E-BP2 has been linked to the development of autism spectrum disorder (ASD)-associated behaviours in mice [28]. Lastly, 4E-BP3 was found to be important in maintaining translational repression during prolonged periods of mTORC1 inhibition [29,30,31].
In 2017, Sonenberg and colleagues published the results of a BioID assay [32] seeking to identify novel interacting partners of eIF4E and 4EHP, the latter being an eIF4E family member sharing 28% homology with eIF4E but acting as a translational repressor [33,34,35]. Among the datasets of the BioID assay, a protein of unknown function, C8ORF88, was found to be a high-confidence binding partner of eIF4E but not 4EHP [34]. Gene Ontology (GO) analysis predicted C8ORF88 to be an eIF4E binding protein and a negative regulator of translation. In this work, we characterize C8ORF88 and validate its interaction with eIF4E.
2. Materials and Methods
2.1. Sequence Alignments
All genomic DNA sequences were obtained from the NCBI Gene database [17]. Gene Accession numbers used were as follows: C8ORF88 (NM_001190972.2), 4E-BP1 (NP_004086.1), 4E-BP2 (NP_004087.1), 4E-BP3 (NP_003723.1). Amino acid sequence of C8ORF88 was obtained using the ExPASy Translation Tool [36]. Sequence alignments of C8ORF88 with the 4E-BP homologs were performed using the Clustal Omega Multiple Sequence Alignment Tool [37].
2.2. Northern Blot Analysis
Mouse tissue RNA was obtained from Zyagen. RNA (4 µg) or ssRNA Ladder (New England Biolabs, NEB) was prepared to a volume of 20 µL with loading buffer (50% deionized formamide, 6% formaldehyde, 20 mM sodium borate, 0.2 mM EDTA pH 8.3). Samples were denatured at 65 °C for 2 min directly prior to loading on a 1.2% agarose formaldehyde gel (6% formaldehyde, 20 mM sodium borate, 0.2 mM EDTA pH 8.3). The gel was run at 90V for 3 h with buffer recirculation, after which the ssRNA Ladder was cut from the gel and stained using SYBR™ Gold Nucleic Acid Gel Stain (Invitrogen, Carlsbad, CA, USA) followed by ultraviolet (UV) imaging. Samples were transferred to a Hybond N+ membrane (GE Healthcare, Aurora, OH, USA) through capillary action in 10X SSC Buffer (1.5 M NaCl, 150 mM sodium citrate) at room temperature for 48 h. The membrane was UV crosslinked using a Stratagene Stratalinker 2400 at a UV power of 1200.
The DNA probe was radiolabelled with α-32P-dATP (Perkin Elmer, Waltham, MA, USA) using the Takara Random Primer DNA Labeling Kit Ver.2.0 as per the manufacturer’s instructions. Unincorporated α-32P-dATP was removed by EZ-10 DNA spin column (Bio Basic, Markham, ON, Canada). C8Orf88 probe was generated from the full-length protein coding region of the cDNA, isolated through restriction digest of plasmid pGEX-6P1-Flag-mC8Orf88. An 18S rRNA probe was generated by PCR amplification of mouse tissue cDNA generated from mouse liver RNA using M-MuLV Reverse Transcriptase (New England Biolabs, NEB) with the following primers: 18S_rRNA_For, 5′AACTGTGGTAATTCTAGAGC3′, 18S_rRNA_Rev, 5′CCATCGAAAGTTGATAGGGC3′.
The membrane was blocked with 15mL hybridization buffer (50% formamide, 1× Denhardt’s solution, 0.8 M NaCl, 4% Dextran SO4, 1% sodium pyrophosphate, 50 mM Tris pH 7.5, 0.5% SDS, 1mg/mL salmon sperm DNA) at 42 °C overnight in a rotating hybridization oven. Following pre-hybridization, 1 × 106 cpm/mL of denatured radiolabelled probe was added directly to the hybridization buffer and incubated at 42 °C in a rotating hybridization oven for 16–24 h. The membrane was washed twice each for 30 min with decreasing concentrations of SSC buffer (2×, 1×, 0.5× and 0.2×) containing 0.1% SDS at 65 °C. Membranes were exposed at −80 °C to X-ray film for 30 min (rRNA probe) and 4 days (C8Orf88 probe).
2.3. RT-qPCR
Mouse tissue RNA was obtained from Zyagen. cDNA was generated from RNA (1 µg) using M-MuLV Reverse Transcriptase (New England Biolabs, NEB, Whitby, ON, Canada). cDNA was diluted 1:10 and 1 µL was used in a 10 µL qPCR reaction using SsoFast EvaGreen Supermix (Bio-Rad, Mississauga, ON, Canada) as per the manufacturer’s instructions. Raw cycle threshold (Ct) values were compared to assess expression levels.
Primers were designed using NCBI Primer-BLAST and were obtained from Integrated DNA Technologies (IDT):
- mC8Orf88_qPCR_For, 5′TGGGTCTTGAGGCGTATG3′
- mC8Orf88_qPCR_Rev, 5′ATCTGCCCACTCCACTTTGT3′
- β-actin_qPCR_For, 5′TTCCTTCTTGGGTATGGAATCC3′
- β-actin_qPCR_Rev, 5′AGGAGCAATGATCTTGATCTTC3′
2.4. Plasmids and Recombinant Proteins
pLeGo-Flag-C8ORF88 and pLeGo-Flag-C8ORF88(Δ6) were generated through restriction cloning of gBlocks™ Gene Fragments (Integrated DNA Technologies, Coralville, IA, USA) into the pLeGo-SP6 backbone. pLeGo-Flag-4E-BP [5A] was generated through PCR amplification of 4E-BP[5A] from pCMV-4E-BP[5A] using PCR primers containing an N-terminal Flag extension with the following primers:
Flag_4E-BP[5A]_For, 5′CCCCATATCATCGTGGGATCCCAATGGACTACAAGGACGACGACGATAAGATGTCGG3′
Flag_4E-BP[5A]_Rev, 5′CGAGATTTCACTGTTGAATTCTTAAAGTCCATCTCAAA3′
The PCR product was cloned into the pLeGo-SP6 backbone.
pGEX-C8ORF88 and pGEX-C8ORF88(Δ6) were generated through restriction cloning of gBlocks™ Gene Fragments (Integrated DNA Technologies) containing sequences codon optimized for Escherichia coli K12 into the pGEX-6P1 backbone. To generate recombinant GST-C8ORF88 and GST-C8ORF88(Δ6) protein, Rosetta™(DE3) Competent Cells (Sigma-Aldrich, Oakville, ON, Canada) were transformed with pGEX-C8ORF88 and pGEX-C8ORF88(Δ6) and induced with 0.5 mM IPTG for 3 h at 37 °C in a shaking incubator. A bacterial pellet was harvested via centrifugation, and a GST-based purification performed [38].
2.5. Cell Culture, Transfections and Lysate Preparation
HEK293T and HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine growth supplemented serum (BGSS), 1% penicillin-streptomycin antibiotics, and 2mM L-Glutamine at 37 °C and 5% CO2. Cells were transiently transfected with pLeGo-Flag-C8ORF88, pLeGo-Flag-4E-BP[5A], pLeGo-Flag-6aa-C8ORF88(Δ6), or empty pLeGo plasmid DNA in a 6-well plate (3.5 µg) or 10cm dish (10 µg). Transfections were performed using either polyethylenimine (PEI) [39] or calcium phosphate [40].
Two methods of cell lysate preparation were utilized. Firstly, for NP40 lysis cells were washed in ice cold PBS and scraped. Cells were then rinsed in PBS and lyzed in 400 µL NP40 lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 0.5% NP40, 2mM EDTA pH 8.3) supplemented with protease inhibitors (2 µg/mL leupeptin, 10 µg/mL aprotinin, 2.5 µM pepstatin A) for 10 min on ice. Lysates were cleared of cellular debris via centrifugation. Secondly, for RIPA lysis cells were washed in ice cold PBS and scraped. Cells were rinsed in PBS and lyzed in 100 µL RIPA lysis buffer (20 mM Tris. pH 7.6, 100 mM NaCl, 1 mM EDTA pH 8.0, 1 mM EGTA pH 8.0, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 10 mM NaF, 20 mM β-glycerophosphate, 1 mM PMSF) supplemented with protease inhibitors (2 µg/mL leupeptin, 10 µg/mL aprotinin, 2.5 µM pepstatin A) for 10 min on ice. Lysates were cleared of cellular debris via centrifugation.
2.6. Western Blot Analysis
Samples were resolved on either a 10% or 12.5% SDS-polyacrylamide gel (PAGE) and transferred to 0.2 µm Immun-Blot™ PVDF Membrane (BIO-RAD). Membranes were blocked for 1 h in 5% milk in Tris-buffered saline supplemented with 0.2% Tween 20 (TBS-T). The primary antibodies anti-DDDDK (Abcam, Waltham, MA, USA) (recognizes FLAG-tag sequence), anti-eIF4E (Cell Signaling Technology, Danvers, MA, USA) and anti-His (Cell Signaling Technologies) were diluted 1:1000 in 5% milk in TBS-T. The primary antibody anti-GST (Santa Cruz, Dallas, TX, USA) was diluted 1:500 in 5% milk in TBS-T. Membranes were incubated with the primary antibody for 1 h at room temperature and washed 3 times with TBS-T before addition of species-appropriate horseradish peroxidase (HRP) conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) in 5% milk in TBS-T. Membranes were washed 3 times with TBS-T before addition of ECL UltraScence Western Substrate (FroggaBio, Concord, ON, Canada) and exposed to Medical X-ray Blue film (Carestream, Rochester, NY, USA).
2.7. In Vitro Translation and m7GTP Cap-Affinity Chromatography
Capped mRNAs were synthesized using SP6 RNA polymerase (New England Biolabs, NEB) from linearized templates of pLeGo-Flag-C8ORF88, pLeGo-Flag-4E-BP[5A], pLeGo-Flag-C8ORF88(Δ6) and pLeGo-Flag-eIF4E in the presence of anti-reverse cap analog (ARCA) [41] and purified using a G50 Sephadex spin column. mRNAs encoding Flag-C8ORF88, Flag-4E-BP[5A] or Flag-C8ORF88(Δ6) (0.5 µg) were translated in conjunction with eIF4E (0.5 µg) in wheat germ extract (Promega, Madison, WI, USA). Each mRNA pair was translated in a 30 µL reaction for 1 h at 25 °C in the presence of 15 µCi EasyTag™ L-[35S]-methionine (PerkinElmer). Translations were then subjected to cap-affinity chromatography using a γ-amino phenyl m7GTP (C10 spacer) agarose (Jena Bioscience, Jena, Germany). Prior to use, beads were calibrated four times with LCB Buffer (20 mM Hepes pH 7.5, 0.1 M KCl, 0.2 mM EDTA pH 8.0). Translations were incubated on the resin for 2 h at 4 °C with end-over-end rotation. The resin was washed 3 times with LCB Buffer and 2 times with 500 µM GTP before elution in 500 µM m7GTP. For each sample, the input, second GTP wash and m7GTP elution were resolved on a 12.5% SDS-PAGE which was then treated with EN3HANCE™ (PerkinElmer) prior to drying and exposure to X-ray film (Carestream). For cap-pulldowns of recombinant His-eIF4E (2.5 µg) and GST-C8ORF88, GST-4E-BP1 or GST-C8ORF88(Δ6) (0.5 µg), the respective proteins were incubated in 50 µL LCB Buffer at RT for 1h followed by incubation on the resin, washing and elution as above. Input and m7GTP elutions were resolved on a 10% SDS-PAGE and analyzed by Western blotting using anti-GST (Santa Cruz) and anti-His (Cell Signaling Technologies) antibodies.
2.8. GST Pulldown
GST-C8ORF88, GST-4E-BP1 or GST-C8ORF88(Δ6) (2.5 µg) were incubated with 0.5 µg His-eIF4E or His-eIF4E(W73A) in 50 µL Binding Buffer (20 mM Tris pH 7.5, 100mM KCl, 10% glycerol, 0.1% NP-40) for 1 h at room temperature. Fifty microliters of a 50% Glutathione Sepharose® 4 Fast Flow (Cytiva, Vancouver, BC, Canada) slurry, that had been washed 3 times with Binding Buffer, was added to the protein mix and incubated for 1 h on ice. The beads were then washed 3 times with Binding Buffer prior to elution in 50 µL 10 mM Reduced Glutathione for 1 h on ice. Proteins were resolved on a 10% SDS-PAGE and analyzed by Western blotting using anti-GST (Santa Cruz) and anti-His (Cell Signaling Technologies) antibodies.
2.9. Co-Immunoprecipitation and m7GTP Cap-Affinity Chromatography
In co-immunoprecipitation experiments, transiently transfected HEK293T cells (10 cm dish) were lyzed in NP40 lysis buffer 24 h post-transfection and cleared of cellular debris as described above. Anti-FLAG® M2 magnetic beads (Millipore Sigma, Oakville, ON, Canada) were prepared by washing twice with NP40 lysis buffer. Lysates were immunoprecipitated with the antibody-coupled beads for 1 h on ice and were periodically agitated. After immunoprecipitation, beads were washed 5 times with NP40 lysis buffer and resuspended in 40 µL 1X SDS loading buffer. Samples were resolved on a 12.5% SDS-PAGE and analyzed by Western blotting using anti-DDDDK (Abcam) and anti-eIF4E (Cell Signaling Technology) antibodies.
For m7GTP cap-affinity chromatography, transfected HEK293T cells (10 cm dish) were lyzed in 400 µL NP40 lysis buffer 24 h post-transfection as described above. Lysates were subjected to cap-affinity chromatography using a γ-amino phenyl m7GTP (C10 spacer) agarose (Jena Bioscience), as described above. Samples were resolved on a 12.5% SDS-PAGE and analyzed by Western blotting using anti-DDDDK (Abcam) (recognizes FLAG-tag sequence) and anti-eIF4E (Cell Signaling Technology) antibodies.
2.10. L-[35S]-Methionine/Cysteine Labelled Protein Incorporation in Cells
HeLa cells were transiently transfected to express Flag-C8ORF88, Flag-C8ORF88(Δ6) or empty vector in a 6-well plate. Twenty-four hours post-transfection, cells were re-seeded in a 24-well plate, and 48 h post-transfection the media was changed to DMEM supplemented with 10% dialyzed FBS for 1 h. For the last 15 min, 150–225 μCi/mL of LLC EasyTag™ EXPRESS [35S]-methionine/cysteine Protein Labeling Mix (Perkin Elmer, Waltham, MA, USA) was added to each well. At the end of the incubation, media was removed, and cells were rinsed with cold PBS. Cells were lyzed in 40 µL of RIPA lysis buffer at 4 °C. Lysates were TCA precipitated on Whatman paper that had been pre-blocked with MEM Amino Acids Solution (50X) without methionine (Gibco/Fisher Scientific, Ottawa, ON, Canada). Radioactivity was measured via scintillation counting.
3. Results
3.1. Sequence Alignment Reveals Homology between C8ORF88 and the 4E-BP Homologs
C8ORF88 encodes a polypeptide of 117 amino acids (Figure 1a). Sequence alignment of C8ORF88 with 4E-BP revealed ~20–26% identity to the 4E-BP homologs and conservation of the 4EBM (Figure 1a). Three-dimensional structural alignment indicated that the C8ORF88 4EBM superimposed well onto the corresponding domain of eIF4E-bound 4E-BP1 (Figure 1b). Of the seven 4E-BP1 phosphorylation sites, two amino acids are also present in C8ORF88 (Figure 1a). However, analysis of the PhosphoSitePlus database indicated that neither of these are phosphorylated, but rather there is a minor site used at S70 (Figure 1a, downward arrow). Superimposition of the AlphaFold predicted C8ORF88 structure with 4E-BP1 in the eIF4E:4E-BP1 crystal structure showed an excellent fit across the 4EBM (Figure 1b).
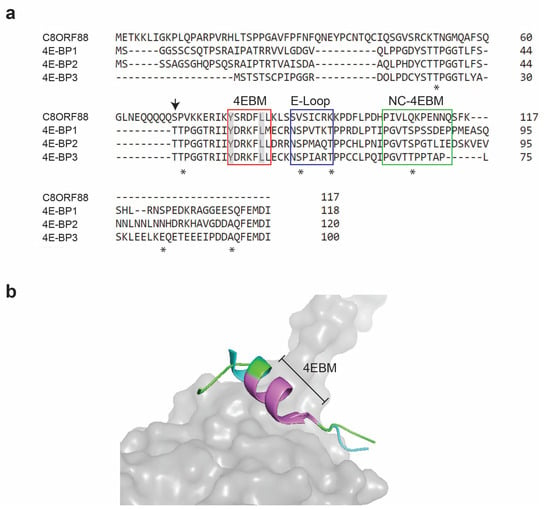
Figure 1.
C8ORF88 amino acid sequence alignment with 4E-BP homologs. (a) Conserved sequences in C8ORF88 and 4E-BPs implicated with eIF4E binding activity are identified with boxes and include the canonical 4EBM (C-4EBM) (red), the elbow loop domain (E-loop) (blue) and the non-canonical 4EBM (NC-4EBM) (green). The conserved tyrosine (Y) and leucine (L) of the canonical 4EBM sequence YXXXXLΦ are highlighted in grey. Sequence alignment was performed using the Clustal Omega Multiple Sequence Alignment Tool. Asterisks underneath the sequence denote phosphorylation sites previously identified in 4E-BP1 [15]. Downward arrow denotes C8ORF88 S70, which according to PhosphoSitePlus (https://www.phosphosite.org/proteinAction.action?id=35695900&showAllSites=true, accessed on 15 February 2022) represents a single minor phosphorylation site. (b) Superimposition of 4E-BP1 and C8ORF88. Predicted structure of C8ORF88 flanking 4EBM (https://alphafold.ebi.ac.uk/entry/P0DMB2, accessed on 20 July 2023) was superimposed on 4E-BP1:eIF4E crystal structure (PDB 1WKW) using PyMOL. C8ORF88 is in cyan, 4E-BP1 in green and eIF4E in grey. The canonical 4EBM is colored violet.
3.2. Expression Profile of C8Orf88
Perusal of the GTEx database portal shows that C8ORF88 expression was the highest in testis, with very little expression in other tissues (Figure 2a). This contrasts to levels of 4E-BP1 which are consistently higher across a broad tissue set (Figure S1a). These data were verified by RT-qPCR analysis of C8Orf88 mRNA levels across several tissue types (Figure 2b). Lastly, Northern blotting analysis indicated the presence of a single ~900 bp mRNA present in testis RNA (Figure 2c). RNA-Seq data derived from human tissue samples available through The Human Protein Atlas [42] maps expression of C8ORF88 to germ cells of testes. More specifically, RNA expression was highest in spermatocytes and early spermatids and diminished as they matured (Figure 2d). This is strikingly different from 4E-BP1 expression where expression was highest in late spermatids (Figure S1).
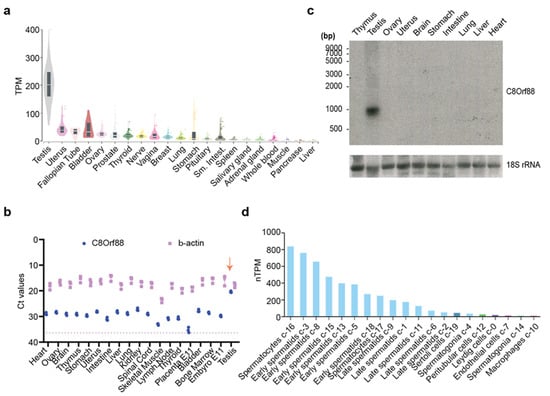
Figure 2.
Tissue expression pattern of C8ORF88. (a) Bulk tissue C8ORF88 expression from GTEx Portal (https://www.gtexportal.org/home/gene/C8ORF88, accessed on 15 July 2023). (b) Ct values obtained from C8Orf88 and β-actin amplification using RT-qPCR. The dotted line represents the Ct value of the background signal from β-actin’s blank control. The downward arrow identifies highest expression levels of C8Orf88 in testes. (c) Northern blot analysis of C8Orf88 mRNA from mouse tissues. (d) Single cell data showing C8ORF88 expression levels in testis as obtained from The Human Protein Atlas (https://www.proteinatlas.org/ENSG00000253250-C8orf88/single+cell+type, accessed on 15 July 2023).
3.3. In Vitro Association between C8ORF88 and eIF4E
To investigate the interaction between C8ORF88 and eIF4E, recombinant tagged proteins were purified and used in GST pulldown assays (Figure 3a). Here, GST-4E-BP1 was used as a positive control as it has previously been shown to interact with eIF4E in this assay. In addition to wild-type C8ORF88, we also expressed a mutant devoid of the canonical 4EBM by deletion of six amino acids YSRDFL (Δ6). Another control used was His-eIF4EW73A, which harbors a mutation in the conserved Trp73 on the dorsal surface of eIF4E and is essential for interaction with the canonical 4EBM [43]. Using a Glutathione Sepharose solid support matrix, all GST-tagged proteins (4E-BP1 and C8ORF88) were recovered (Figure 3a, lanes 5–8). His-eIF4E, but not His-eIF4EW73A, was detected in the pulldown fractions with GST-4EBP1, as expected. C8ORF88, but not C8ORF88(Δ6), was able to pulldown eIF4E (Figure 3a).
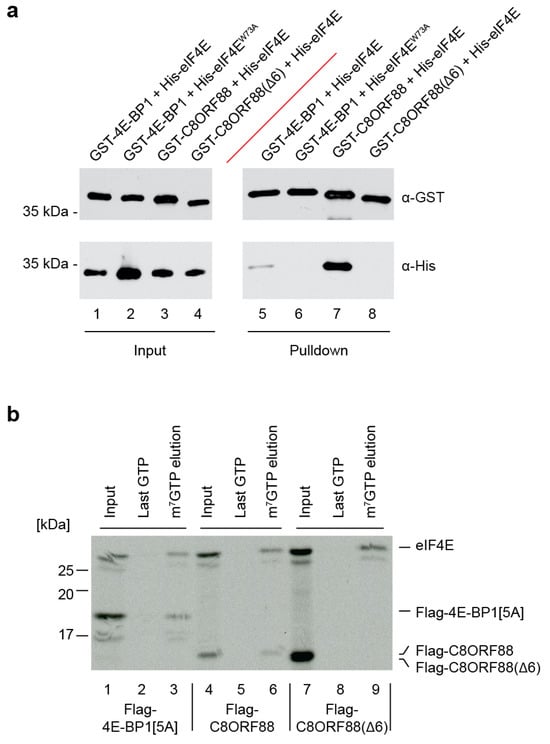
Figure 3.
In vitro association between C8ORF88 and eIF4E. (a) Pulldown of GST-tagged C8ORF88, 4E-BP1 and C8ORF88(Δ6) recombinant proteins in combination with His-4E or His-4EW73A. (b) Recombinant proteins were generated by in vitro translations in wheat germ extracts in the presence of [35S]-labelled methionine. Reactions were then incubated with m7GTP affinity resin. After binding, the resin was washed three times with LCB buffer and twice with 500 µM GTP before eluting with 500 µM m7GTP. For each reaction, the input, last GTP wash and m7GTP elution were resolved by SDS-PAGE, EN3HANCED, and visualized using autoradiography.
We next sought to determine whether binding of C8ORF88 to eIF4E would compromise eIF4E:cap interaction. To this end, we produced radiolabeled recombinant proteins in wheat germ extracts and undertook eIF4E pull down experiments using an m7GTP affinity matrix (Figure 3b). For these experiments, we used a mutant of 4E-BP1, Flag-4E-BP1[5A]. Flag-4E-BP1[5A] harbors alanine substitutions at five phosphorylation sites (T37, T46, S65, T70 and S83) and robustly interacts with eIF4E [43,44]. From extracts containing both eIF4E and Flag-4E-BP1[5A], both proteins were clearly present in the m7GTP elutions (Figure 3b, compare lane 3 to 2). Affinity chromatography from extracts harboring both eIF4E and C8ORF88, revealed that C8ORF88 was associated with cap-bound eIF4E (Figure 3b, compare lanes 6 to 5). In contrast, Flag-C8ORF88(Δ6) did not interact with cap-bound eIF4E (Figure 3b, compare lane 9 to 6). This was also validated utilizing the recombinant proteins tested in Figure 3a, as a reciprocal pulldown experiment performed using an m7GTP affinity column instead of Glutathione Sepharose solid support matrix (Figure S2). GST-4EBP1 and GST-C8ORF88 were detected in pulldown fractions with His-eIF4E, whereas His-eIF4E was not able to pulldown GST-C8ORF88(Δ6) (Figure S2).
These results indicate that C8ORF88 can interact with free and cap-bound eIF4E in vitro, and that the 4EBM is essential for this interaction.
3.4. C8ORF88 Interacts with Endogenous eIF4E in a Cultured Cell Line
To further investigate the interaction between C8ORF88 and eIF4E, the interaction was studied in a cultured cell line. HEK293T cells were transfected to express either Flag-4E-BP1[5A], Flag-C8ORF88 or Flag-C8ORF88(Δ6), and cell lysates were harvested 24 h later. Lysates were subjected to immunoprecipitation using anti-Flag conjugated magnetic beads (Figure 4a). Endogenous eIF4E co-immunoprecipitated with Flag-4E-BP1[5A] and Flag-C8ORF88 (Figure 4a, compare lanes 6 and 7 to 5). No interaction was observed in cells expressing Flag-C8ORF88(Δ6) (Figure 4a, lane 8).
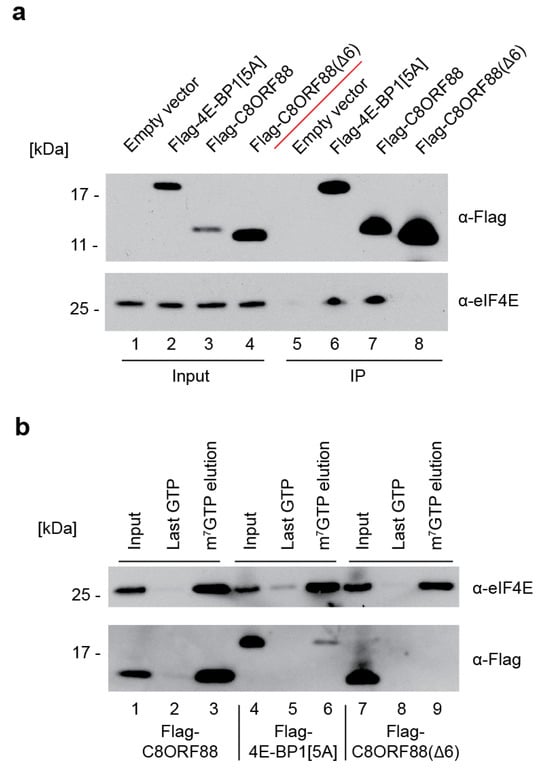
Figure 4.
Interaction between C8ORF88 and eIF4E in cell culture. (a) Immunoprecipitation of transfected HEK293T cell lysates with anti-Flag antibody conjugated magnetic beads. Samples were resolved by SDS-PAGE and analyzed by Western blotting. (b) m7GTP cap-affinity chromatography of HEK293T cell lysates. The resin was washed three times with LCB buffer and twice with 500 µM GTP before elution in 500 µM m7GTP. For each sample the input, last GTP wash and m7GTP elution were resolved by SDS-PAGE and analyzed by Western blotting.
Lysates prepared from HEK293T cells transfected to express the same three Flag-tagged proteins were also subjected to cap-affinity chromatography (Figure 4b). eIF4E was eluted from the cap column for all samples, indicating it successfully bound to the resin (Figure 4b, lanes 3, 6 and 9). C8ORF88 and Flag-4E-BP1[5A] eluted from the resin with endogenous eIF4E, as expected (Figure 4b, lanes 3 and 6). Flag-C8ORF88(Δ6) was not retained on the cap column, underscoring the importance of the canonical 4EBM for eIF4E interaction (Figure 4b, lane 9). Together, these data confirm the interaction between C8ORF88 and both free and cap-bound eIF4E.
3.5. Ectopic Expression of C8ORF88 Inhibits Translation in a Cultured Cell Line
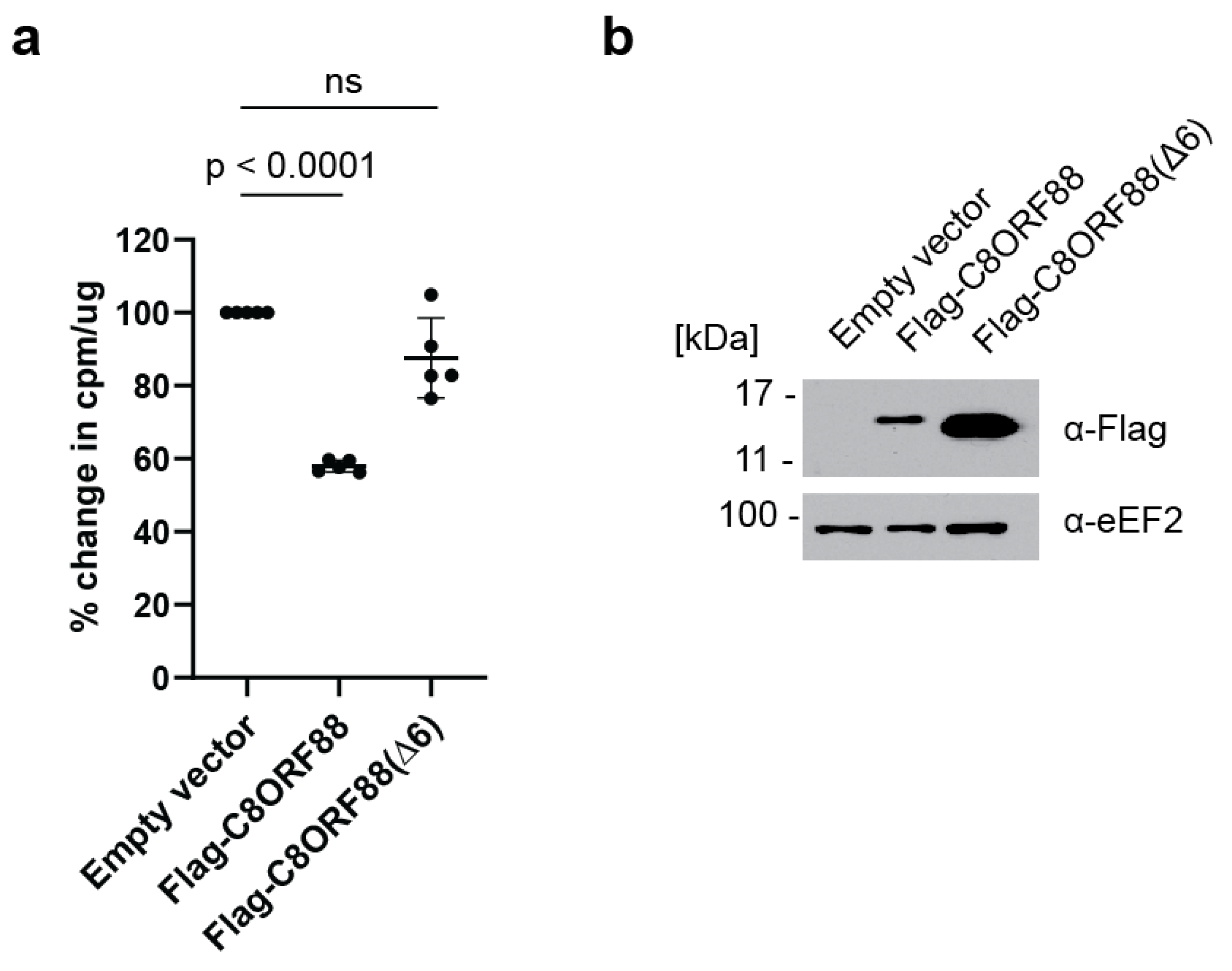
HeLa cells were transiently transfected to express Flag-C8ORF88 or Flag-C8ORF88(Δ6). Forty-eight hours post-transfection, cells were treated with [35S]-labelled Met/Cys for 1 h, lyzed, and radiolabel incorporation was measured via scintillation counting (Figure 5). A significant reduction (42%) in global translation rates was observed on Flag-C8ORF88 expressing cells, compared to control cells (Figure 5a). No significant difference in translation was observed between cells expressing Flag-C8ORF88(Δ6) or receiving empty vector (Figure 5a). Expression of the indicated Flag-tagged proteins was confirmed via Western blotting (Figure 5b). These data indicate that C8ORF88 is a negative regulator of translation.
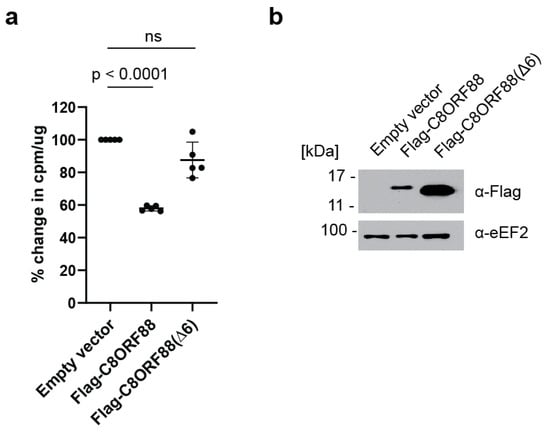
Figure 5.
Overexpression of C8ORF88 leads to a reduction in global translation rates. (a) HeLa cells transfected to overexpress Flag-C8ORF88, Flag-C8ORF88(Δ6) or empty vector, were treated with [35S]-labelled protein mix for 1 h, 48 h after transfection. Incorporation of [35S]-labelled proteins was assessed via scintillation counting and standardized to the empty vector control. p values were determined using Dunnett’s multiple comparison test. (b) Expression of Flag-tagged proteins confirmed by Western blotting.
4. Discussion
In this study, we provide an initial characterization of a novel testes-specific eIF4E binding protein. We found that expression of C8Orf88 was highest in testis, and this was congruent with available RNA-Seq data (Figure 2) [45,46]. The highest levels of C8ORF88 expression occur early in spermatogenesis (Figure 2d), in contrast to 4E-BP1 (Figure S1b), which is highest in late spermatids. Translational control during spermatogenesis plays critical roles in stem cell maintenance, meiotic entry, completion of meiosis, and gamete differentiation [47,48]. More specifically, recruitment of selective messenger ribonucleoproteins (mRNPs) to ribosomes by eIF4 factors is recognized to play a vital role in translational control during oocyte and embryonic development [49,50]. During the development of oocytes and embryos, mRNP granules control gene expression through precise, special-temporal regulation of mRNA expression [51,52]. This careful regulation is essential for the correct progression of the developmental program [51,53]. It was hypothesized that eIF4G dissociates 4E-BPs from repressed mRNPs to activate their translation, driving progression through the stages of development [54]. Furthermore, C8ORF88 is conserved across chordates [55,56,57]. The tissue-specific expression pattern, in addition to its conservation, suggests that C8ORF88 has a physiologically significant role in testes [58,59]. The identification of C8ORF88 as a novel germ cell-specific 4E-BP may thus have important implications for translational control during spermatogenesis and development.
Testicular cancer is the most common cancer in men aged 14–44 [60]. Cancers with germ-cell origins, such as testicular seminomas, comprise ~50% of all testicular cancer cases [61]. Given the extensively characterized role of 4E-BP1 dysregulation across many malignancies [22,62,63,64,65], and the mapping of C8ORF88 expression to the germ cells of testes, C8ORF88 might play a role in tumorigenesis and disease progression in cancers of germ cell origin. In a recent whole-exome and transcriptome sequence study of germ cell tumors (GCTs), p53 was found to be expressed in all samples sequenced [66]. This is a unique and unusual feature, as around half of other solid tumor types harbor mutations in p53 [67,68]. Expression of p53 is thought to be essential for cisplatin-induced apoptosis in GCTs [69,70]. 4E-BP activity has previously been linked to p53 expression in primary fibroblasts, where knock-down of 4E-BP1 and 4E-BP2 in conjunction with p53 expression led to resistance to oncogene-driven transformation and premature senescence [24]. As C8ORF88 is a germ cell-specific 4EBP, it may play a role in senescence and apoptosis as governed by p53 in GCTs.
We validated the interaction of C8ORF88 with eIF4E both in vitro and in cell culture (Figure 3 and Figure 4). Results obtained with C8ORF88(Δ6) highlighted the importance of the 4EBM domain for this interaction, whose function in turn was associated with inhibition of translation (Figure 5). Pull-down experiments using m7GTP affinity resin indicated that the C8ORF88-eIF4E interaction does not preclude eIF4E binding to the cap structure. The interaction between cap-bound eIF4E and C8ORF88 is of particular interest as it shows C8ORF88 to be a competitor with other endogenously expressed 4E-BPs. Binding of 4E-BP1 to eIF4E is highly regulated by mTORC1 and occurs through hierarchical phosphorylation of first Thr37 and Thr46 and, subsequently, Ser65, Thr70, and Ser83 [15] (Figure 1a, denoted by asterisks). We note that none of these phosphorylation sites are present in C8ORF88, precluding regulation of eIF4E binding by mTOR in a manner analogous to 4E-BP1:eIF4E. There has been one phosphorylation site identified in C8ORF88 (Ser70) (Figure 1a), the significance of which has yet to be determined and suggests an additional mechanism of regulation. Additionally, different RNA expression profiles were observed for C8ORF88 and 4E-BP1, where C8ORF88 was found to have greater expression in spermatocytes and early spermatids, while 4E-BP1 was found to have greater expression in late spermatids. This raises the question of whether there is differential translational control in the early and late stages of spermatids by C8ORF88 and 4E-BP1, which is yet to be determined. The data presented here, in conjunction with the recognized importance of 4E-BPs in translational control, provides rationale for further investigation into the physiological role of this protein.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14112076/s1, Figure S1: Protein expression levels of 4E-BP1; Figure S2: In vitro association between C8ORF88 and eIF4E.
Author Contributions
Formal analysis, L.P.; Funding acquisition, N.S. and J.P.; Methodology, L.P., A.Y., R.C. and J.P.; Project administration, J.P.; Resources, N.S. and J.P.; Software, S.K.N. and M.A.; Supervision, R.C., N.S. and J.P.; Validation, L.P.; Visualization, L.P., S.K.N. and J.P.; Writing—original draft, L.P., N.S. and J.P.; Writing—review and editing, L.P., N.S. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Canadian Institutes of Health Research, grant number FDN-148366, to J.P. A.Y. was employed by Arcalis Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: PhosphositePlus (https://www.phosphosite.org/proteinAction.action?id=35695900&showAllSites=true, accessed on 15 February 2022), AlphaFold (https://alphafold.ebi.ac.uk/entry/P0DMB2, accessed on 20 July 2023), GTEx Portal (https://www.gtexportal.org/home/gene/C8ORF88, accessed on 15 July 2023) and (https://gtexportal.org/home/gene/EIF4EBP1, accessed on 15 July 2023), The Human Protein Atlas (https://www.proteinatlas.org/ENSG00000253250-C8orf88/single+cell+type, accessed on 15 July 2023) and (https://www.proteinatlas.org/ENSG00000187840-EIF4EBP1/single+cell+type, accessed on 15 July 2023).
Acknowledgments
This work is dedicated to Jerry Pelletier who passed away during the writing of this manuscript. Jerry was a visionary and an inspirational leader who made seminal contributions to the field of mRNA translation. This body of work is a testament to his legacy, which will live on.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. A.Y. was employed by Arcalis Inc. at time of publication.
References
- Rhoads, R.E.; Joshi-Barve, S.; Rinker-Schaeffer, C. Mechanism of action and regulation of protein synthesis initiation factor 4E: Effects on mRNA discrimination, cellular growth rate, and oncogenesis. Prog. Nucleic Acid Res. Mol. Biol. 1993, 46, 183–219. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Schmeing, T.M.; Sonenberg, N. The multifaceted eukaryotic cap structure. Wiley Interdiscip. Rev. RNA 2021, 12, e1636. [Google Scholar] [CrossRef] [PubMed]
- Shatkin, A.J. Capping of eucaryotic mRNAs. Cell 1976, 9, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Sonenberg, N. The Organizing Principles of Eukaryotic Ribosome Recruitment. Annu. Rev. Biochem. 2019, 88, 307–335. [Google Scholar] [CrossRef] [PubMed]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, L.S.; Webb, N.R.; Rhoads, R.E. Immunological detection of the messenger RNA cap-binding protein. J. Biol. Chem. 1985, 260, 7843–7849. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Milburn, S.C.; Hershey, J.W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J. Biol. Chem. 1987, 262, 380–388. [Google Scholar] [CrossRef]
- Goss, D.J.; Carberry, S.E.; Dever, T.E.; Merrick, W.C.; Rhoads, R.E. Fluorescence study of the binding of m7GpppG and rabbit globin mRNA to protein synthesis initiation factors 4A, 4E, and 4F. Biochemistry 1990, 29, 5008–5012. [Google Scholar] [CrossRef]
- Altmann, M.; Schmitz, N.; Berset, C.; Trachsel, H. A novel inhibitor of cap-dependent translation initiation in yeast: p20 competes with eIF4G for binding to eIF4E. EMBO J. 1997, 16, 1114–1121. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Li, A.-M.; Vargas, C.A.; Riehman, K.; Watson, A.; Fridovich-Keil, J.L. Genetic and biochemical interactions between SCP160 and EAP1 in yeast. Nucleic Acids Res. 2003, 31, 5838–5847. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, N.; Sonenberg, N. Signalling to eIF4E in cancer. Biochem. Soc. Trans. 2015, 43, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.-C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999, 13, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Marcotrigiano, J.; Gingras, A.-C.; Sonenberg, N.; Burley, S.K. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell 1999, 3, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Igreja, C.; Weber, R.; Wohlbold, L.; Weiler, C.; Ebertsch, L.; Weichenrieder, O.; Izaurralde, E. Molecular architecture of 4E-BP translational inhibitors bound to eIF4E. Mol. Cell 2015, 57, 1074–1087. [Google Scholar] [CrossRef]
- Igreja, C.; Peter, D.; Weiler, C.; Izaurralde, E. 4E-BPs require non-canonical 4E-binding motifs and a lateral surface of eIF4E to repress translation. Nat. Commun. 2014, 5, 4790. [Google Scholar] [CrossRef]
- Kinkelin, K.; Veith, K.; Grünwald, M.; Bono, F. Crystal structure of a minimal eIF4E–Cup complex reveals a general mechanism of eIF4E regulation in translational repression. RNA 2012, 18, 1624–1634. [Google Scholar] [CrossRef]
- Grüner, S.; Peter, D.; Weber, R.; Wohlbold, L.; Chung, M.-Y.; Weichenrieder, O.; Valkov, E.; Igreja, C.; Izaurralde, E. The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation. Mol. Cell 2016, 64, 467–479. [Google Scholar] [CrossRef]
- Dong, Y.; Srour, O.; Lukhovitskaya, N.; Makarian, J.; Baumberger, N.; Galzitskaya, O.; Elser, D.; Schepetilnikov, M.; Ryabova, L.A. Functional analogs of mammalian 4E-BPs reveal a role for TOR in global plant translation. Cell Rep. 2023, 42, 112892. [Google Scholar] [CrossRef] [PubMed]
- Musa, J.; Orth, M.F.; Dallmayer, M.; Baldauf, M.; Pardo, C.; Rotblat, B.; Kirchner, T.; Leprivier, G.; Grünewald, T.G.P. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 2016, 35, 4675–4688. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.; Karpisheva, K.; Pola, C.; Goldberg, J.; Hochman, T.; Yee, H.; Cangiarella, J.; Arju, R.; Formenti, S.C.; Schneider, R.J. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 2007, 28, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Petroulakis, E.; Parsyan, A.; Dowling, R.J.; LeBacquer, O.; Martineau, Y.; Bidinosti, M.; Larsson, O.; Alain, T.; Rong, L.; Mamane, Y.; et al. p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell 2009, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.; Pérez-Tenorio, G.; Amin, R.; Bostner, J.; Skoog, L.; Fornander, T.; Sgroi, D.C.; Nordenskjöld, B.; Hallbeck, A.-L.; Stål, O. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed, and associated with a poor prognosis and endocrine resistance in breast cancer: A retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res. 2013, 15, R96. [Google Scholar] [CrossRef] [PubMed]
- Rutkovsky, A.C.; Yeh, E.S.; Guest, S.T.; Findlay, V.J.; Muise-Helmericks, R.C.; Armeson, K.; Ethier, S.P. Eukaryotic initiation factor 4E-binding protein as an oncogene in breast cancer. BMC Cancer 2019, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Maracci, C.; Motta, S.; Romagnoli, A.; Costantino, M.; Perego, P.; Di Marino, D. The mTOR/4E-BP1/eIF4E Signalling Pathway as a Source of Cancer Drug Targets. Curr. Med. Chem. 2022, 29, 3501–3529. [Google Scholar] [CrossRef]
- Gkogkas, C.G.; Khoutorsky, A.; Ran, I.; Rampakakis, E.; Nevarko, T.; Weatherill, D.B.; Vasuta, C.; Yee, S.; Truitt, M.; Dallaire, P.; et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 2013, 493, 371–377. [Google Scholar] [CrossRef]
- Tsukumo, Y.; Alain, T.; Fonseca, B.D.; Nadon, R.; Sonenberg, N. Translation control during prolonged mTORC1 inhibition mediated by 4E-BP3. Nat. Commun. 2016, 7, 11776. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Li, L.; Lim, J.-A.; Patange, S.; Raben, N.; Puertollano, R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Burke, B.; May, D.G. BioID: A Screen for Protein-Protein Interactions. Curr. Protoc. Protein Sci. 2018, 91, 19.23.1–19.23.15. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Cameron, A.; Jagus, R. Characterization of mammalian eIF4E-family members. Eur. J. Biochem. 2004, 271, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Chapat, C.; Jafarnejad, S.M.; Matta-Camacho, E.; Hesketh, G.G.; Gelbart, I.A.; Attig, J.; Gkogkas, C.G.; Alain, T.; Stern-Ginossar, N.; Fabian, M.R.; et al. Cap-binding protein 4EHP effects translation silencing by microRNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.; Igreja, C. eIF4E-homologous protein (4EHP): A multifarious cap-binding protein. FEBS J. 2021, 290, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinform. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef] [PubMed]
- Rebay, I.; Fehon, R.G. Preparation of insoluble GST fusion proteins. Cold Spring Harb. Protoc. 2009, 2009, pdb.prot4997. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Kingston, R.E.; Chen, C.A.; Rose, J.K. Calcium phosphate transfection. Curr. Protoc. Mol. Biol. 2003, 63, 9.1.1–9.1.11. [Google Scholar] [CrossRef]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Imataka, H.; Gingras, A.C.; Fukunaga, R.; Hunter, T.; Sonenberg, N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999, 18, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Rapley, J.; Oshiro, N.; Ortiz-Vega, S.; Avruch, J. The mechanism of insulin-stimulated 4E-BP protein binding to mammalian target of rapamycin (mTOR) complex 1 and its contribution to mTOR complex 1 signaling. J. Biol. Chem. 2011, 286, 38043–38053. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R.; et al. Gene: A gene-centered information resource at NCBI. Nucleic Acids Res. 2015, 43, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Friday, A.J.; Keiper, B.D. Positive mRNA Translational Control in Germ Cells by Initiation Factor Selectivity. Biomed Res. Int. 2015, 2015, 327963. [Google Scholar] [CrossRef] [PubMed]
- Ciosk, R.; DePalma, M.; Priess, J.R. Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 2006, 311, 851–853. [Google Scholar] [CrossRef]
- Henderson, M.A.; Cronland, E.; Dunkelbarger, S.; Contreras, V.; Strome, S.; Keiper, B.D. A germline-specific isoform of eIF4E (IFE-1) is required for efficient translation of stored mRNAs and maturation of both oocytes and sperm. J. Cell Sci. 2009, 122, 1529–1539. [Google Scholar] [CrossRef]
- Ghosh, S.; Lasko, P. Loss-of-function analysis reveals distinct requirements of the translation initiation factors eIF4E, eIF4E-3, eIF4G and eIF4G2 in Drosophila spermatogenesis. PLoS ONE 2015, 10, e0122519. [Google Scholar] [CrossRef]
- Buchan, J.R. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014, 11, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Huggins, H.P.; Keiper, B.D. Regulation of Germ Cell mRNPs by eIF4E:4EIP Complexes: Multiple Mechanisms, One Goal. Front. Cell Dev. Biol. 2020, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Voronina, A.S.; Pshennikova, E.S. mRNPs: Structure and role in development. Cell Biochem. Funct. 2021, 39, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Huggins, H.P.; Subash, J.S.; Stoffel, H.; Henderson, M.A.; Hoffman, J.L.; Buckner, D.S.; Sengupta, M.S.; Boag, P.R.; Lee, M.-H.; Keiper, B.D. Distinct roles of two eIF4E isoforms in the germline of Caenorhabditis elegans. J. Cell Sci. 2020, 133, jcs237990. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Abugessaisa, I., Kasukawa, T., Eds.; Springer Nature: Singapore, 2021; pp. 27–56. [Google Scholar]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Dowell, R.D. The similarity of gene expression between human and mouse tissues. Genome Biol. 2011, 12, 101. [Google Scholar] [CrossRef]
- Gu, X.; Su, Z. Tissue-driven hypothesis of genomic evolution and sequence-expression correlations. Proc. Natl. Acad. Sci. USA 2007, 104, 2779–2784. [Google Scholar] [CrossRef]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Primers 2018, 4, 29. [Google Scholar] [CrossRef]
- Daugaard, G.; Gundgaard, M.G.; Mortensen, M.S.; Agerbæk, M.; Holm, N.V.; Rørth, M.; von der Maase, H.; Christensen, I.J.; Lauritsen, J. Surveillance for stage I nonseminoma testicular cancer: Outcomes and long-term follow-up in a population-based cohort. J. Clin. Oncol. 2014, 32, 3817–3823. [Google Scholar] [CrossRef]
- De Benedetti, A.; Graff, J.R. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004, 23, 3189–3199. [Google Scholar] [CrossRef]
- Fingar, D.C.; Richardson, C.J.; Tee, A.R.; Cheatham, L.; Tsou, C.; Blenis, J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 2004, 24, 200–216. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ruggero, D. The Role of Translation Control in Tumorigenesis and Its Therapeutic Implications. Annu. Rev. Cancer Biol. 2020, 4, 437–457. [Google Scholar] [CrossRef]
- Taylor-Weiner, A.; Zack, T.; O’Donnell, E.; Guerriero, J.L.; Bernard, B.; Reddy, A.; Han, G.C.; AlDubayan, S.; Amin-Mansour, A.; Schumacher, S.E.; et al. Genomic evolution and chemoresistance in germ-cell tumours. Nature 2016, 540, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef] [PubMed]
- Lutzker, S.G. P53 tumour suppressor gene and germ cell neoplasia. APMIS 1998, 106, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.J.; Rossetti, S.; Conteduca, V.; Schepisi, G.; Cavaliere, C.; Di Franco, R.; La Mantia, E.; Castaldo, L.; Nocerino, F.; Ametrano, G.; et al. Role of DNA repair machinery and p53 in the testicular germ cell cancer: A review. Oncotarget 2016, 7, 85641–85649. [Google Scholar] [CrossRef]
- Shimada, T.; Yabuki, Y.; Noguchi, T.; Tsuchida, M.; Komatsu, R.; Hamano, S.; Yamada, M.; Ezaki, Y.; Hirata, Y.; Matsuzawa, A. The Distinct Roles of LKB1 and AMPK in p53-Dependent Apoptosis Induced by Cisplatin. Int. J. Mol. Sci. 2022, 23, 10064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).