Genome-Wide Identification of AhMDHs and Analysis of Gene Expression under Manganese Toxicity Stress in Arachis hypogaea
Abstract
:1. Introduction
2. Materials and Methods
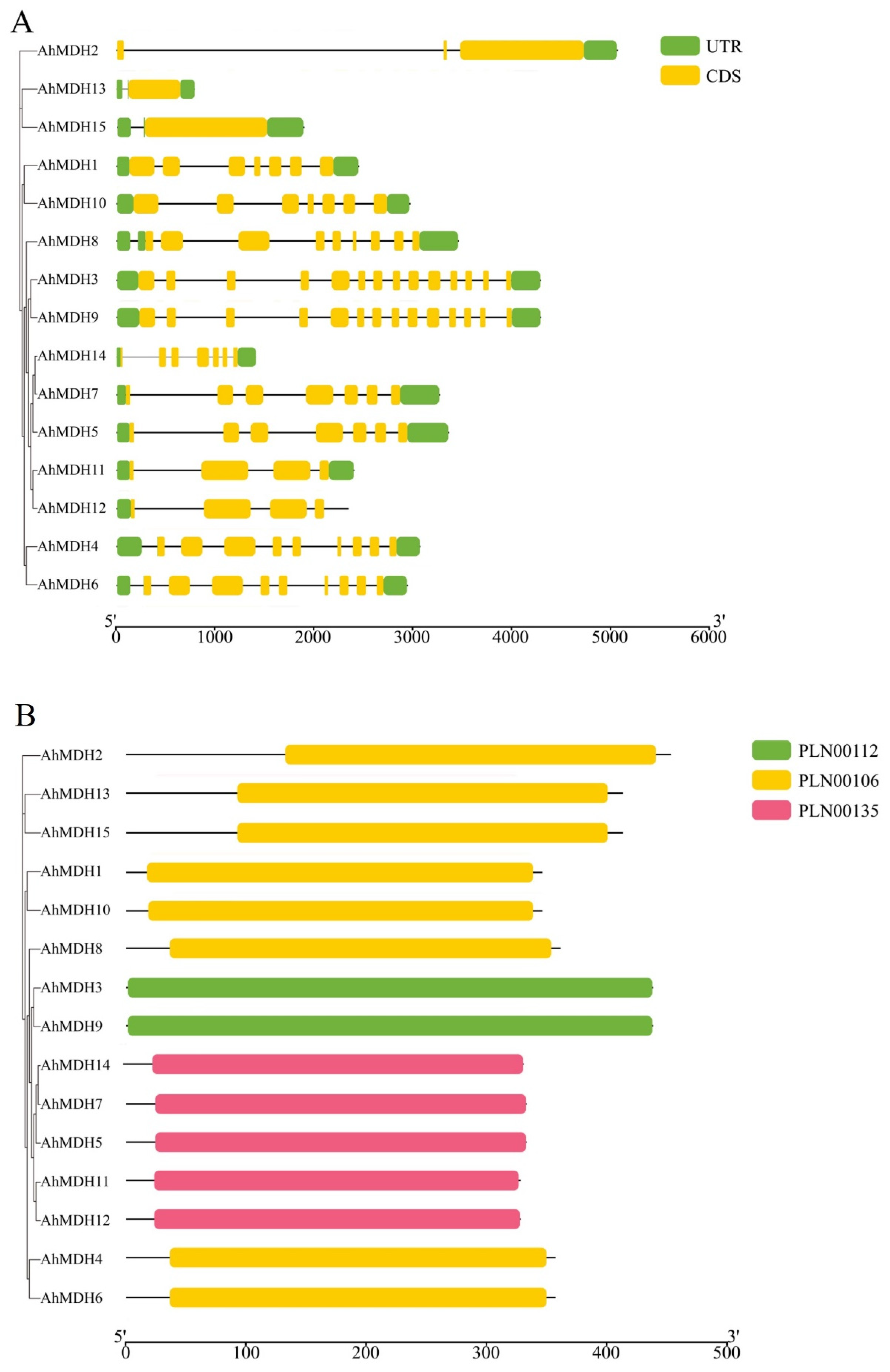
2.1. Analysis of AhMDH Structures
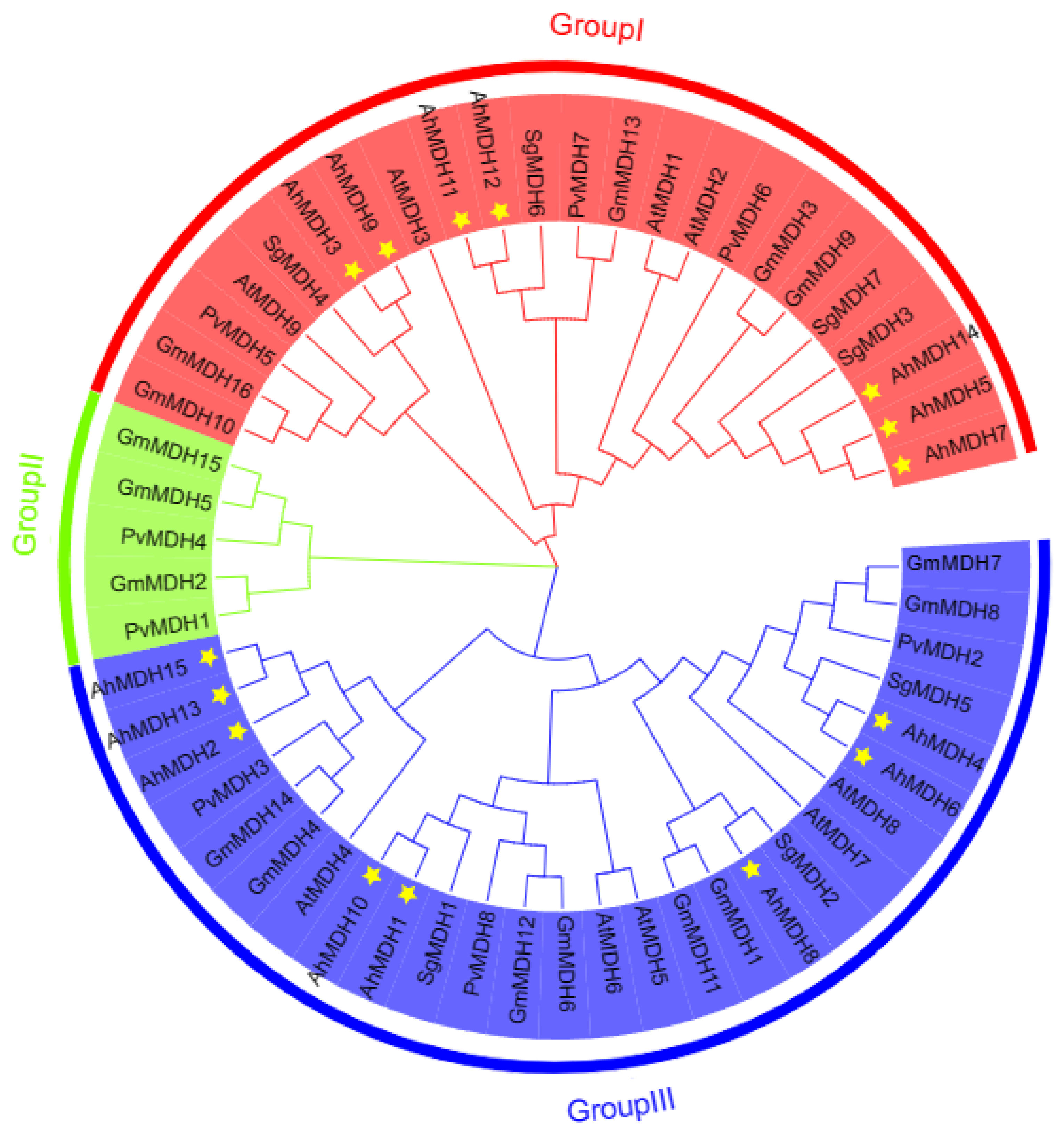
2.2. Constructing a Phylogeny Tree of the MDHs
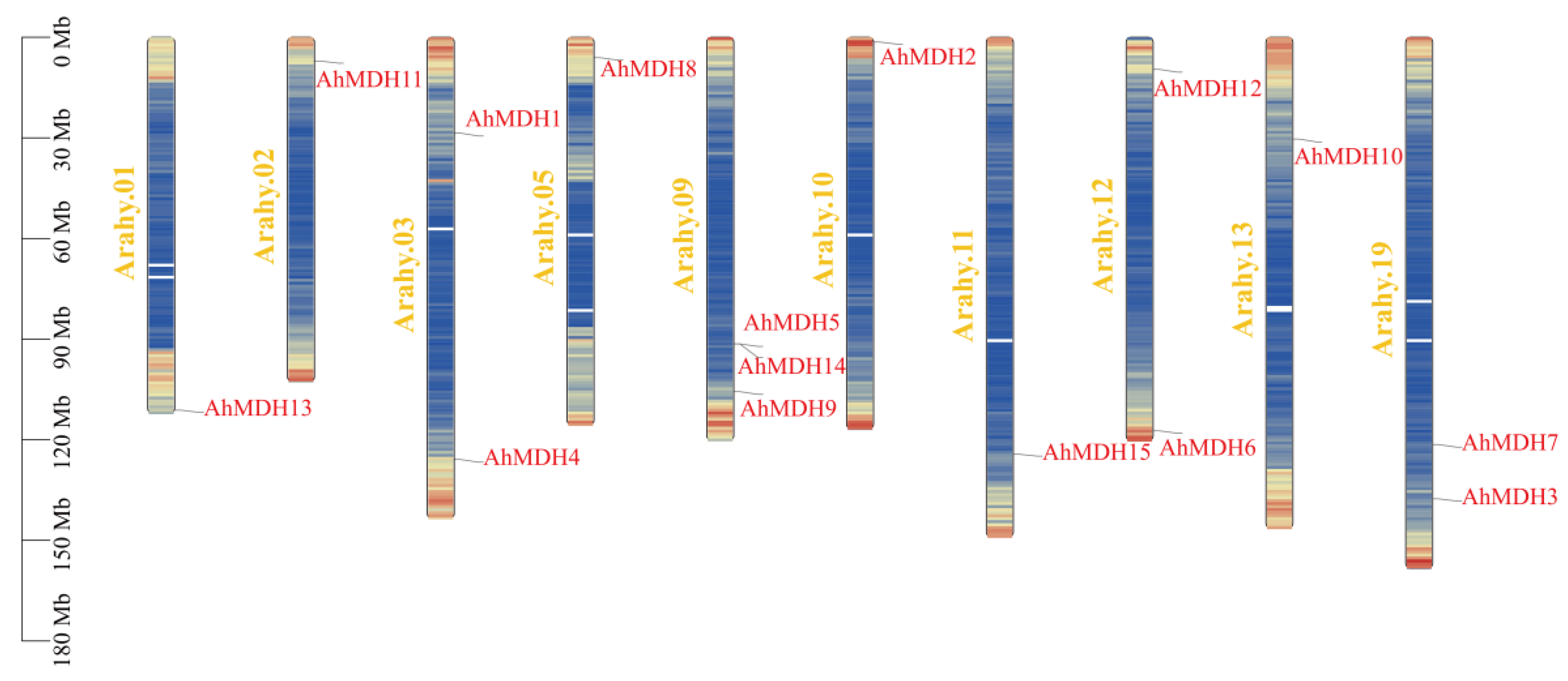
2.3. Chromosome Localization of the MDH Gene
2.4. MDH Conservative Motif Analysis
2.5. Cis-Acting Element Analysis
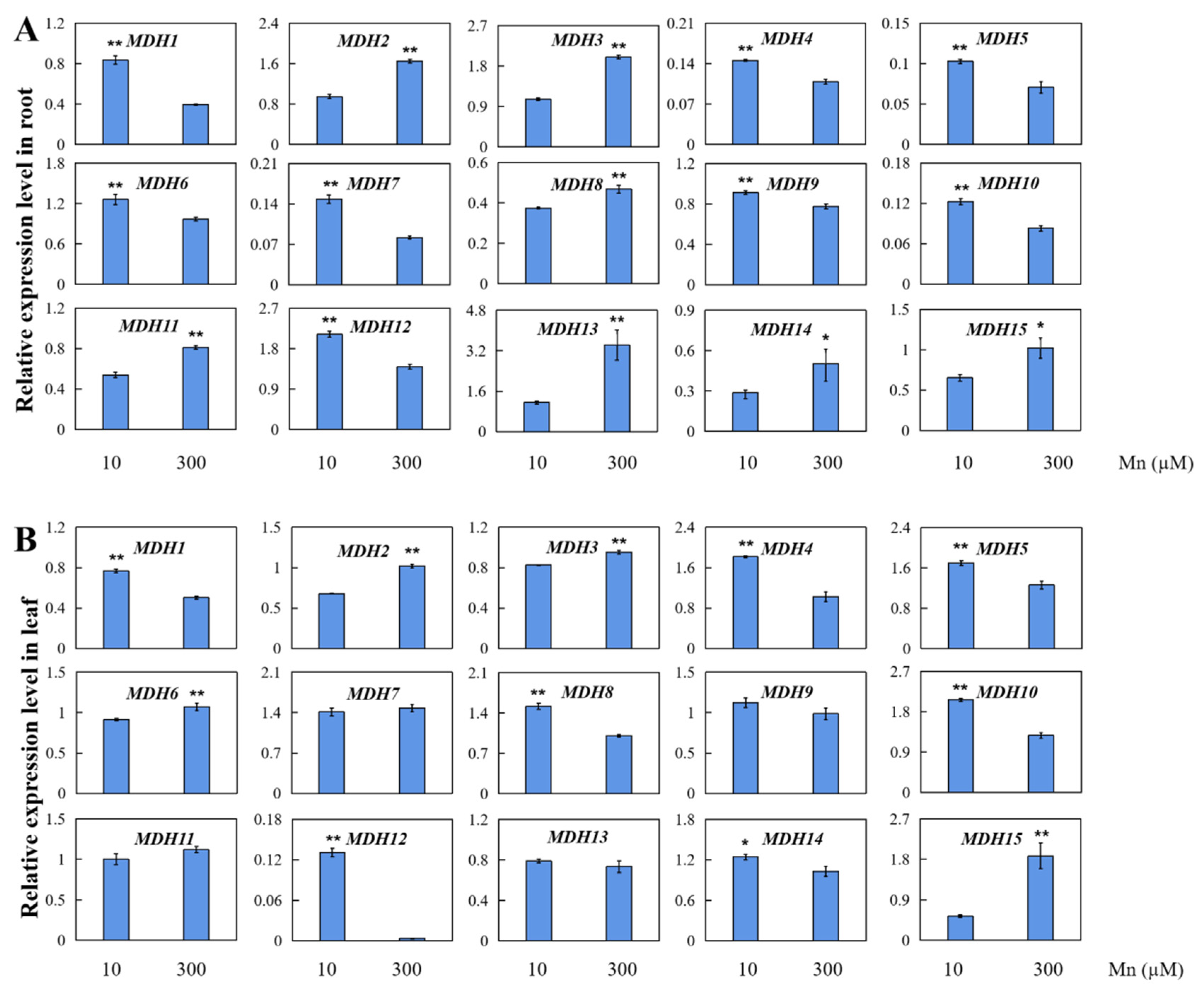
2.6. Transcriptome Sequencing Results of AhMDHs in Peanut Roots and Leaves under Manganese Toxicity Stress
2.7. Peanut Culture Conditions
2.8. qRT-PCR Analysis of MDH Gene Expression in Peanut
3. Results
3.1. Analysis of Basic Information of AhMDHs
3.2. Structural Analysis of AhMDHs
3.3. Evolutionary Tree Analysis of MDHs
3.4. Conservative Analysis of Peanut MDH
3.5. Chromosome Location of Peanut MDH
3.6. Identification of Cis-Acting Factors of the AhMDHs
3.7. Expression Profile of the Peanut MDH Gene
3.8. AhMDH Gene Expression Analysis under Mn Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yıldız, S.; Okay, A.; Büyük, İ. Defining the roles of PvMDH genes in response to salt stress and detailed characterization of the gene family. J. Plant Biochem. Biot. 2021, 31, 380–393. [Google Scholar] [CrossRef]
- Matthews, C.J.; Andrews, E.S.V.; Patrick, W.M. Enzyme-based amperometric biosensors for malic acid—A review. Anal. Chim. Acta 2021, 1156, 338218. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, J.H.; Chen, Y.R.; Xu, Q.; Song, P.; Li, Y.F.; Li, K.; Liu, H. Recent advances in microbial production of L-malic acid. Appl. Microbiol. Biot. 2022, 106, 7973–7992. [Google Scholar] [CrossRef]
- Minárik, P.; Tomaskova, N.; Kollarova, M.; Antalík, M. Malate dehydrogenases-structure and function. Gen. Physiol. Biophys. 2002, 21, 257–266. [Google Scholar]
- Pradedova, E.V.; Nimaeva, O.D.; Rakevich, A.L.; Salyaev, R.K. Comparative analyses of glutathione system of vacuoles and leucoplasts isolated from the storage parenchyma cells of dormant red beetroots (Beta vulgaris L.). Plant Physiol. Biochem. 2019, 145, 52–63. [Google Scholar] [CrossRef]
- Scheibe, R. Malate valves to balance cellular energy supply. Physiol Plant. 2004, 120, 21–26. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Mitochondria in photosynthetic cells: Coordinating redox control and energy balance. Plant Physiol. 2023, 191, 2104–2119. [Google Scholar] [CrossRef]
- Goward, C.R.; Nicholls, D.J. Malate dehydrogenase: A model for structure, evolution, and catalysis. Protein Sci. 1994, 3, 1883–1888. [Google Scholar] [CrossRef]
- Møller, I.M.; Rasmusson, A.G.; Aken, O.V. Plant mitochondria–past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Ashton, A.R.; Trevanion, S.J.; Carr, P.D.; Verger, D.; Ollis, D.L. Structural basis for the light regulation of chloroplast NADP malate dehydrogenase. Physiol. Plant. 2000, 110, 314–321. [Google Scholar] [CrossRef]
- Zhao, J.F.; Du, Y.W.; Yu, A.L. Study on abiotic stress response of MDH gene in millet. Chin. J. Nucl. Agric. 2020, 34, 2152–2160. (In Chinese) [Google Scholar]
- Shameer, S.; Ratcliffe, R.G.; Sweetlove, L.J. Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiol. 2019, 180, 1947–1961. [Google Scholar] [CrossRef]
- Liszka, A.; Schimpf, R.; Cartuche Zaruma, K.I.; Buhr, A.; Seidel, T.; Walter, S.; Knuesting, J.; Dreyer, A.; Dietz, K.J.; Scheibe, R.; et al. Three cytosolic NAD-malate dehydrogenase isoforms of Arabidopsis thaliana: On the crossroad between energy fluxes and redox signaling. Biochem. J. 2020, 477, 3673–3693. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chen, W.T.; Yang, Q.; Zhang, Y.J.; Ma, X.Q.; Li, M. Genome-wide characterization and gene expression analyses of malate dehydrogenase (MDH) genes in low-phosphorus stress tolerance of Chinese fir (Cunninghamia lanceolata). Int. J. Mol. Sci. 2023, 24, 4414. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, Y.L.; Sun, X.M.; Yuan, J.; Zhao, Z.Q.; Gao, J.; Wen, X.R.; Tang, F.; Kang, M.; Abliz, B.; et al. Genome-wide identification of MDH family genes and their association with salt tolerance in rice. Plants 2022, 11, 1498. [Google Scholar] [CrossRef]
- Zhu, S.N.; Chen, Z.J.; Xie, B.X.; Guo, Q.; Chen, M.H.; Liang, C.Y.; Bai, Z.L.; Wang, X.R.; Wang, H.C.; Liao, H.; et al. A phosphate starvation responsive malate dehydrogenase, GmMDH12 mediates malate synthesis and nodule size in soybean (Glycine max). Environ. Exp. Bot. 2021, 189, 104560. [Google Scholar] [CrossRef]
- Imran, M.; Tang, K.; Liu, J.Y. Comparative genome-wide analysis of the malate dehydrogenase gene families in cotton. PLoS ONE 2016, 11, e0166341. [Google Scholar] [CrossRef]
- Song, J.L.; Zou, X.Y.; Liu, P.D.; Cardoso, J.A.; Schultze-Kraft, R.; Liu, G.D.; Luo, L.J.; Chen, Z.J. Differential expressions and enzymatic properties of malate dehydrogenases in response to nutrient and metal stresses in Stylosanthes guianensis. Plant Physiol. Biochem. 2022, 170, 325–337. [Google Scholar] [CrossRef]
- Ma, B.Q.; Yuan, Y.Y.; Gao, M.; Xing, L.B.; Li, C.Y.; Li, M.J.; Ma, F.W. Genome-wide identification, classification, molecular evolution and expression analysis of malate dehydrogenases in apple. Int. J. Mol. Sci. 2018, 19, 3312. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhang, J.; Zhang, C.; Wang, S.J.; Yang, M.S. Genome-wide investigation of malate dehydrogenase gene family in poplar (Populus trichocarpa) and their expression analysis under salt stress. Acta Physiol. Plant. 2021, 43, 28. [Google Scholar] [CrossRef]
- Zhuang, W.J.; Varshney, R.K. Editorial: Peanut genomics and biotechnology in breeding applications. Front. Plant Sci. 2023, 14, 1226637. [Google Scholar] [CrossRef]
- Liao, B.S. Current situation and potential analysis of peanut production in China. Chin. J. Oil Crop. 2020, 42, 161–166. (In Chinese) [Google Scholar]
- Bonku, R.; Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Well. 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Li, M.R.; Guo, S.; Ho, C.T.; Bai, N.S. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). J. Food Biochem. 2022, 46, e14119. [Google Scholar] [CrossRef]
- Syed, F.; Arif, S.; Ahmed, I.; Khalid, N. Groundnut (Peanut) (Arachis hypogaea). In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 93–122. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Chen, J.; Wu, W.J.; Luo, J.H.; Long, T.; Wu, Q.S.; Wang, Q.L.; Zhen, J.B.; Zhao, Y.P.; Wang, Y.C.; et al. Detection of peanut seed vigor based on hyperspectral imaging and chemometrics. Front. Plant Sci. 2023, 14, 1127108. [Google Scholar] [CrossRef]
- Sew, Y.S.; Ströher, E.; Fenske, R.; Millar, A.H. Loss of mitochondrial malate dehydrogenase activity alters seed metabolism impairing seed maturation and post-germination growth in Arabidopsis. Plant Physiol. 2016, 171, 849–863. [Google Scholar] [CrossRef]
- Yin, G.K.; Whelan, J.; Wu, S.H.; Zhou, J.; Chen, B.Y.; Chen, X.L.; Zhang, J.M.; He, J.J.; Xin, X.; Lu, X.X. Comprehensive mitochondrial metabolic shift during the critical node of seed ageing in rice. PLoS ONE 2016, 11, e0148013. [Google Scholar] [CrossRef]
- Xia, F.S.; Cheng, H.; Chen, L.L.; Zhu, H.S.; Mao, P.S.; Wang, M.Y. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Li, P.X.; Tang, G.Y.; Xu, P.L.; Zhu, J.Q.; Shan, L.; Wan, S.B. Structure and expression analysis of mitochondrial malate dehydrogenase gene AhMMDH1 in peanut. Chin. J. Oil Crop. 2020, 42, 1090–1099. (In Chinese) [Google Scholar]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Skórka, M.; Sieprawska, A.; Telk, A. The implication of manganese surplus on plant cell homeostasis: A review. J. Plant Growth Regul. 2023, 42, 1327–1341. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, J.Y.; Pan, Y.H.; Chen, J.Y.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef]
- Li, J.F.; Jia, Y.D.; Dong, R.S.; Huang, R.; Liu, P.D.; Li, X.Y.; Wang, Z.Y.; Liu, G.D.; Chen, Z.J. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- Le¡ková, A.; Giehl, R.F.H.; Hartmann, A.; Farga¡ová, A.; Wirén, N. Heavy metals induce iron deficiency responses at different hierarchic and regulatory levels. Plant Physiol. 2017, 174, 1648–1668. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Chen, J.Y.; Yang, S.X.; Chen, J.P.; Xue, Y.B. Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under Mn toxicity stress. Int. J. Mol. Sci. 2023, 24, 1161. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Sheng, X.G.; Zhao, Z.Q.; Wang, J.S.; Yu, H.F.; Shen, Y.S.; Zeng, X.Y.; Gu, H.H. Genome wide analysis of MADS-box gene family in Brassica oleracea reveals conservation and variation in flower development. BMC Plant Biol. 2019, 19, 106. [Google Scholar] [CrossRef]
- Shen, G.; Jia, Y.; Wang, W.L. Evolutionary divergence of motifs in B-class MADS-box proteins of seed plants. J. Biol. Res. 2021, 28, 12. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Chen, X.J.; Wang, P.J.; Sun, Y.; Yue, C.; Ye, N.X. Genome-wide and expression pattern analysis of JAZ family involved in stress responses and postharvest processing treatments in Camellia sinensis. Sci. Rep. 2020, 10, 2792. [Google Scholar] [CrossRef]
- Shao, Z.W.; He, M.H.; Zeng, Z.P.; Chen, Y.Z.; Hanna, A.; Zhu, H.B. Genome-wide identification and expression analysis of the MADS-Box gene family in sweet potato [Ipomoea batatas (L.) Lam]. Front. Genet. 2021, 12, 750137. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Y.B.; Xie, B.X.; Zhu, S.N.; Lu, X.; Liang, C.Y.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Biochem. 2020, 155, 231–242. [Google Scholar] [CrossRef]
- Zhang, L.H.; Wang, C.Z.; Jia, R.P.; Yang, N.X.; Jin, L.; Zhu, L.C.; Ma, B.Q.; Yao, Y.X.; Ma, F.W.; Li, M.J. Malate metabolism mediated by the cytoplasmic malate dehydrogenase gene MdcyMDH affects sucrose synthesis in apple fruit. Hort. Res. 2022, 9, uhac194. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, R.; Yang, M.; Law, Y.S.; Sun, F.; Hon, N.L.; Ngai, S.M.; Lim, B.L. A balance between the activities of chloroplasts and mitochondria is crucial for optimal plant growth. Antioxidants 2021, 10, 935. [Google Scholar] [CrossRef]
- Wang, Z.A.; Li, Q.; Ge, X.Y.; Yang, C.L.; Luo, X.L.; Zhang, A.H.; Xiao, J.L.; Tian, Y.C.; Xia, G.X.; Chen, X.Y.; et al. The mitochondrial malate dehydrogenase 1 gene GhmMDH1 is involved in plant and root growth under phosphorus deficiency conditions in cotton. Sci. Rep. 2015, 5, 10343. [Google Scholar] [CrossRef]
- Yin, X.; Yuan, Y.; Han, X.; Han, S.; Li, Y.; Ma, D.; Fang, Z.; Gong, S.; Yin, J. Genome-wide identification, characterization, and expression profiling of TaDUF668 gene family in Triticum aestivum. Agronomy 2023, 13, 2178. [Google Scholar] [CrossRef]
- Beeler, S.; Liu, H.C.; Stadler, M.; Schreier, M.; Eicke, S.; Lue, W.L.; Truernit, E.; Zeeman, S.; Chen, J.; Kötting, O. Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis. Plant Physiol. 2014, 164, 1175–1190. [Google Scholar] [CrossRef]
- Menckhoff, L.; Mielke-Ehret, N.; Buck, F.; Vuletić, M.; Lüthje, S. Plasma membrane-associated malate dehydrogenase of maize (Zea mays L.) roots: Native versus recombinant protein. J. Proteom. 2013, 80, 66–77. [Google Scholar] [CrossRef]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Lindén, P.; Lee, C.P.; Carroll, A.; Ströher, E.; Smith, S.; Gardeström, P.; Millar, A.H. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef]
- Roussi, Z.; Ben Mrid, R.; Ennoury, A.; Nhhala, N.; Zouaoui, Z.; Omari, R.E.; Nhiri, M. Insight into Cistus salviifolius extract for potential biostimulant effects in modulating cadmium-induced stress in sorghum plant. Physiol. Mol. Biol. Plants 2022, 28, 1323–1334. [Google Scholar] [CrossRef]
- Crombie, A.T. The effect of lanthanum on growth and gene expression in a facultative methanotroph. Environ. Microbiol. 2022, 24, 596–613. [Google Scholar] [CrossRef]
- Chen, Z.J.; Sun, L.L.; Liu, P.D.; Liu, G.D.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef]
- Peng, W.M.H. Functional Study of Rice Malate Dehydrogenase Gene OsMDH; Yangzhou University: Yangzhou, China, 2022. (In Chinese) [Google Scholar]
- Wu, X.; Han, Y.Y.; Zhu, X.Y.; Shah, A.; Wang, W.; Sheng, Y.B.; Fan, T.T.; Cao, A.Q. Negative regulation of cadmium tolerance in Arabidopsis by MMDH2. Plant Mol. Biol. 2019, 101, 507–516. [Google Scholar] [CrossRef]
- Zhu, X.Y. Functional Analysis of mMDH2 Gene in Regulating Iron Deficiency Response of Arabidopsis Thaliana; Hefei University of Technology: Hefei, China, 2021. (In Chinese) [Google Scholar]



| Primer Name | Gene ID | Chr (Arahy) | CDS (bp) | Protein Length (aa) | MW (kDa) | Isoelectric Point (PI) | Subcellular Predicted |
|---|---|---|---|---|---|---|---|
| AhMDH1 | LOC112790043 | 03 | 1038 | 345 | 35.98 | 9.00 | Mitochondrion |
| AhMDH2 | LOC112718423 | 10 | 1359 | 452 | 47.55 | 8.37 | Chloroplast; mitochondrion |
| AhMDH3 | LOC112776877 | 19 | 1314 | 437 | 47.85 | 6.10 | Chloroplast |
| AhMDH4 | LOC112791339 | 03 | 1071 | 356 | 37.44 | 7.64 | Chloroplast; mitochondrion |
| AhMDH5 | LOC112711210 | 09 | 999 | 332 | 35.67 | 6.11 | Chloroplast; mitochondrion |
| AhMDH6 | LOC112728667 | 12 | 1071 | 356 | 37.50 | 6.97 | Chloroplast; mitochondrion |
| AhMDH7 | LOC112776383 | 19 | 999 | 332 | 35.63 | 6.11 | Chloroplast; mitochondrion |
| AhMDH8 | LOC112800443 | 05 | 1083 | 360 | 37.90 | 7.62 | Chloroplast; mitochondrion |
| AhMDH9 | LOC112711491 | 09 | 1314 | 437 | 47.91 | 6.10 | Chloroplast |
| AhMDH10 | LOC112737907 | 13 | 1038 | 345 | 36.10 | 8.89 | Mitochondrion |
| AhMDH11 | LOC112735613 | 02 | 984 | 327 | 35.45 | 6.09 | Chloroplast; mitochondrion |
| AhMDH12 | LOC112726743 | 12 | 984 | 327 | 35.43 | 6.01 | Chloroplast; mitochondrion |
| AhMDH13 | LOC112710233 | 01 | 1239 | 412 | 43.40 | 8.17 | Chloroplast; mitochondrion |
| AhMDH14 | LOC112711212 | 09 | 999 | 332 | 35.66 | 6.11 | Chloroplast |
| AhMDH15 | LOC112722502 | 11 | 1239 | 412 | 43.32 | 8.47 | Chloroplast; mitochondrion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, M.; Shi, J.; Yang, S.; Xue, Y. Genome-Wide Identification of AhMDHs and Analysis of Gene Expression under Manganese Toxicity Stress in Arachis hypogaea. Genes 2023, 14, 2109. https://doi.org/10.3390/genes14122109
Liu Y, Zhao M, Shi J, Yang S, Xue Y. Genome-Wide Identification of AhMDHs and Analysis of Gene Expression under Manganese Toxicity Stress in Arachis hypogaea. Genes. 2023; 14(12):2109. https://doi.org/10.3390/genes14122109
Chicago/Turabian StyleLiu, Ying, Min Zhao, Jianning Shi, Shaoxia Yang, and Yingbin Xue. 2023. "Genome-Wide Identification of AhMDHs and Analysis of Gene Expression under Manganese Toxicity Stress in Arachis hypogaea" Genes 14, no. 12: 2109. https://doi.org/10.3390/genes14122109




