Transformative Approaches for Sustainable Weed Management: The Power of Gene Drive and CRISPR-Cas9
Abstract
:1. Introduction
2. Gene Drive Systems
2.1. Transposable Elements
2.2. Medea
2.3. Meiotic Drive
2.4. Homing Endonuclease Genes (HEGs)
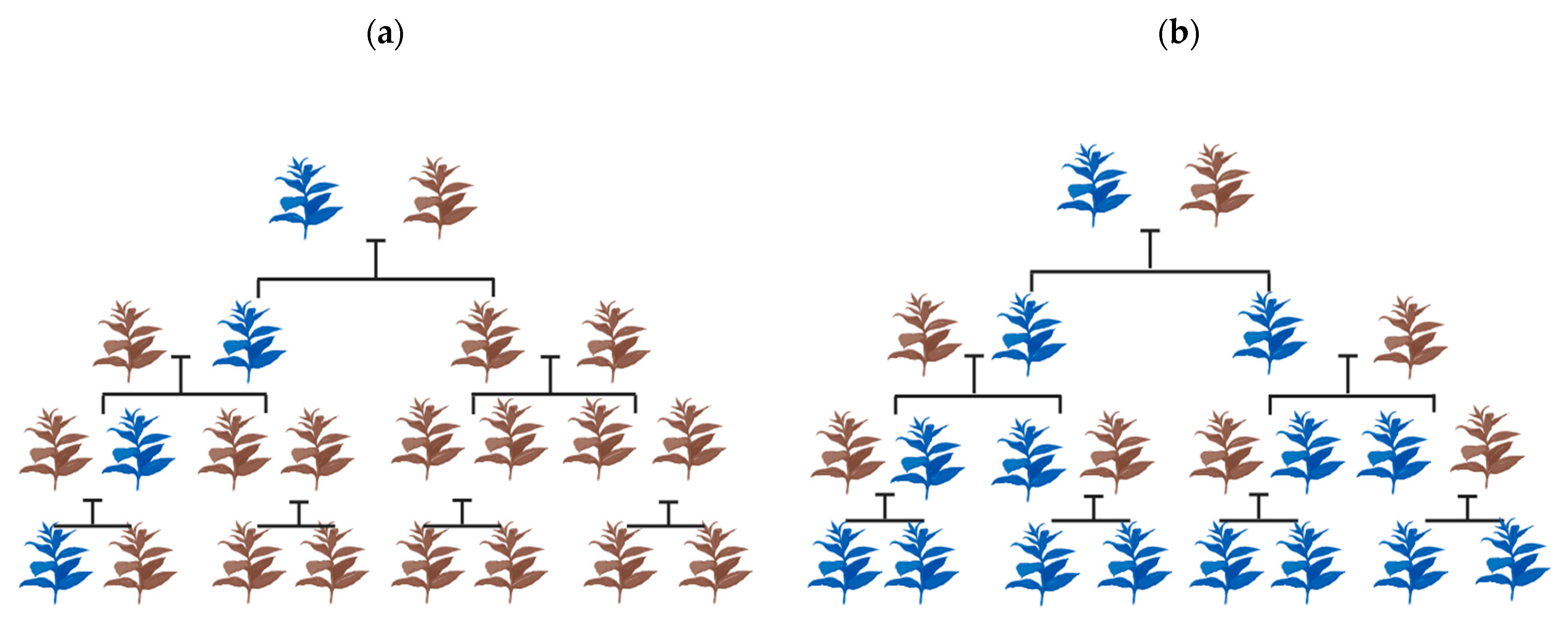
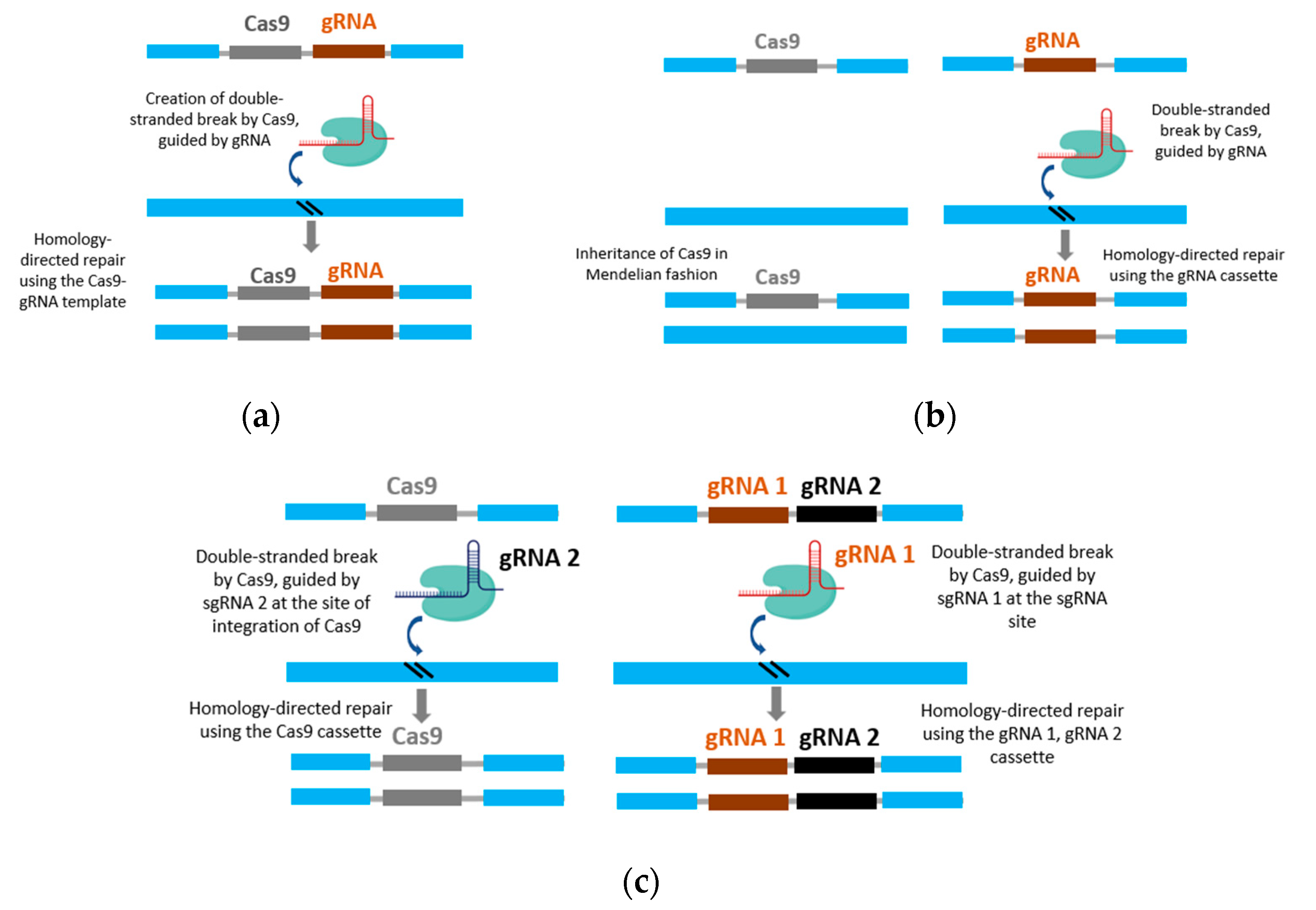
3. CRISPR: A Biotechnological Breakthrough in Artificial Gene Drive
4. Factors Governing the Success of Gene Drive for Weed Management
4.1. Mode of Reproduction in Weed Species
4.2. Establishment of the International Weed Genomics Consortium and the Genomic Database of Weeds
5. CRISPR-Cas9 Gene Drive for Weed Control
6. CRISPR-Modified Crop Plants for Herbicide Tolerance
7. Risk Aspects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef]
- Heap Current Status of the International Herbicide-Resistant Weed Database. 2023. Available online: www.weedscience.org (accessed on 22 November 2023).
- Bajwa, A.A. Sustainable Weed Management in Conservation Agriculture. Crop Prot. 2014, 65, 105–113. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the Water? A Review of the Existing Products for Integrated Weed Management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic Interactions and Allelochemicals: New Possibilities for Sustainable Weed Management. CRC Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Tesio, F.; Ferrero, A. Allelopathy, a Chance for Sustainable Weed Management. Int. J. Sustain. Dev. World Ecol. 2010, 17, 377–389. [Google Scholar] [CrossRef]
- Baltzegar, J.; Cavin Barnes, J.; Elsensohn, J.E.; Gutzmann, N.; Jones, M.S.; King, S.; Sudweeks, J. Anticipating Complexity in the Deployment of Gene Drive Insects in Agriculture. J. Responsible Innov. 2018, 5, S81–S97. [Google Scholar] [CrossRef]
- Khanh, T.D.; Xuan, T.D.; Chung, I.M. Rice Allelopathy and the Possibility for Weed Management. Ann. Appl. Biol. 2007, 151, 325–339. [Google Scholar] [CrossRef]
- Auld, B.A.; Hetherington, S.D.; Smith, H.E. Advances in Bioherbicide Formulation. Weed Biol. Manag. 2003, 3, 61–67. [Google Scholar] [CrossRef]
- Kremer, R.J. The Role of Bioherbicides in Weed Management. Biopestic. Int. 2005, 1, 127–141. [Google Scholar]
- Bhowmik, P.C. Inderjit Challenges and Opportunities in Implementing Allelopathy for Natural Weed Management. Crop Prot. 2003, 22, 661–671. [Google Scholar] [CrossRef]
- Curtis, C.F. Genetic Control of Insect Pests: Growth Industry or Lead Balloon? Biol. J. Linn. Soc. 1985, 26, 359–374. [Google Scholar] [CrossRef]
- Gould, F. Broadening the Application of Evolutionarily Based Genetic Pest Management. Evolution 2008, 62, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Neve, P. Gene Drive Systems: Do They Have a Place in Agricultural Weed Management? Pest Manag. Sci. 2018, 74, 2671–2679. [Google Scholar] [CrossRef]
- Barrett, L.G.; Legros, M.; Kumaran, N.; Glassop, D.; Raghu, S.; Gardiner, D.M. Gene Drives in Plants: Opportunities and Challenges for Weed Control and Engineered Resilience. Proc. R. Soc. B Biol. Sci. 2019, 286, 1–9. [Google Scholar] [CrossRef]
- Champer, J.; Buchman, A.; Akbari, O.S. Cheating Evolution: Engineering Gene Drives to Manipulate the Fate of Wild Populations. Nat. Rev. Genet. 2016, 17, 146–159. [Google Scholar] [CrossRef]
- Kumaran, N.; Choudhary, A.; Legros, M.; Sheppard, A.W.; Barrett, L.G.; Gardiner, D.M.; Raghu, S. Gene Technologies in Weed Management: A Technical Feasibility Analysis. Curr. Opin. Insect Sci. 2020, 38, 6–14. [Google Scholar] [CrossRef]
- Burt, A. Site-Specific Selfish Genes as Tools for the Control and Genetic Engineering of Natural Populations. Proc. R. Soc. B Biol. Sci. 2003, 270, 921–928. [Google Scholar] [CrossRef]
- Wedell, N.; Price, T.A.R.; Lindholm, A.K. Gene Drive: Progress and Prospects. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192709. [Google Scholar] [CrossRef]
- Bull, J.J.; Malik, H.S. The Gene Drive Bubble: New Realities. PLoS Genet. 2017, 13, e1006850. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles Gambiae Mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1071. [Google Scholar] [CrossRef]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F.; Church, G.M. Concerning RNA-Guided Gene Drives for the Alteration of Wild Populations. eLife 2014, 3, e03401. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito Vector Anopheles Gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, J.E.; Chavez, A.; Dietz, S.L.; Esvelt, K.M.; Church, G.M. Safeguarding CRISPR-Cas9 Gene Drives in Yeast. Nat. Biotechnol. 2015, 33, 1250–1255. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Werren, J.H. The Role of Selfish Genetic Elements in Eukaryotic Evolution. Nat. Rev. Genet. 2001, 2, 597–606. [Google Scholar] [CrossRef]
- Werren, J.H.; Nur, U.; Wu, C.I. Selfish Genetic Elements. Trends Ecol. Evol. 1988, 3, 297–302. [Google Scholar] [CrossRef]
- Werren, J.H. Selfish Genetic Elements, Genetic Conflict, and Evolutionary Innovation. Proc. Natl. Acad. Sci. USA 2011, 108, 10863–10870. [Google Scholar] [CrossRef] [PubMed]
- Burt, A.; Crisanti, A. Gene Drive: Evolved and Synthetic. ACS Chem. Biol. 2018, 13, 343–346. [Google Scholar] [CrossRef]
- Feschotte, C.; Jiang, N.; Wessler, S.R. Plant Transposable Elements: Where Genetics Meets Genomics. Nat. Rev. Genet. 2002, 3, 329–341. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Kidwell, M.G. Transposable Elements as Population Drive Mechanisms: Specification of Critical Parameter Values. J. Med. Entomol. 1994, 31, 10–16. [Google Scholar] [CrossRef]
- Carareto, C.M.A.; Kim, W.; Wojciechowski, M.F.; O’Grady, P.; Prokchorova, A.V.; Silva, J.C.; Kidwell, M.G. Testing Transposable Elements as Genetic Drive Mechanisms Using Drosophila P Element Constructs as a Model System. Genetica 1997, 101, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, J.L.; Gould, F. Transposable Element Insertion Location Bias and the Dynamics of Gene Drive in Mosquito Populations. Insect Mol. Biol. 2005, 14, 493–500. [Google Scholar] [CrossRef]
- Marshall, J.M. The Impact of Dissociation on Transposon-Mediated Disease Control Strategies. Genetics 2008, 178, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Beeman, R.W.; Friesen, K.S.; Denell, R.E. Maternal-Effect Selfish Genes in Flour Beetles. Science 1992, 256, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Beeman, R.W.; Friesen, K.S. Properties and Natural Occurrence of Maternal-Effect Selfish Genes (“Medea” Factors) in the Red Flour Beetle, Tribolium Castaneum. Heredity 1999, 82, 529–534. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, H.; Ward, C.M.; Su, J.T.; Schaeffer, L.V.; Guo, M.; Hay, B.A. A Synthetic Maternal-Effect Selfish Genetic Element Drives Population Replacement in Drosophila. Science 2007, 316, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.M.; Su, J.T.; Huang, Y.; Lloyd, A.L.; Gould, F.; Hay, B.A. Medea Selfish Genetic Elements as Tools for Altering Traits of Wild Populations: A Theoretical Analysis. Evolution 2011, 65, 1149–1162. [Google Scholar] [CrossRef]
- Buchman, A.; Marshall, J.M.; Ostrovski, D.; Yang, T.; Akbari, O.S. Synthetically Engineered Medea Gene Drive System in the Worldwide Crop Pest Drosophila Suzukii. Proc. Natl. Acad. Sci. USA 2018, 115, 4725–4730. [Google Scholar] [CrossRef]
- Cash, S.A.; Lorenzen, M.D.; Gould, F. The Distribution and Spread of Naturally Occurring Medea Selfish Genetic Elements in the United States. Ecol. Evol. 2019, 9, 14407–14416. [Google Scholar] [CrossRef]
- Akbari, O.S.; Chen, C.-H.; Marshall, J.M.; Huang, H.; Antoshechkin, I.; Hay, B.A. Novel Synthetic Medea Selfish Genetic Elements Drive Population Replacement in Drosophila; a Theoretical Exploration of Medea-Dependent Population Suppression. ACS Synth. Biol. 2014, 3, 915–928. [Google Scholar] [CrossRef]
- Lindholm, A.K.; Dyer, K.A.; Firman, R.C.; Fishman, L.; Forstmeier, W.; Holman, L.; Johannesson, H.; Knief, U.; Kokko, H.; Larracuente, A.M.; et al. The Ecology and Evolutionary Dynamics of Meiotic Drive. Trends Ecol. Evol. 2016, 31, 315–326. [Google Scholar] [CrossRef]
- Jones, R.N. B Chromosomes in Plants. New Phytol. 1995, 131, 411–434. [Google Scholar] [CrossRef]
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S.; Timmis, J.N. Evolution and Biology of Supernumerary B Chromosomes. Cell. Mol. Life Sci. 2014, 71, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kitano, J. The Contribution of Female Meiotic Drive to the Evolution of Neo-Sex Chromosomes. Evolution 2012, 66, 3198–3208. [Google Scholar] [CrossRef]
- Presgraves, D. Drive and Sperm: The evolution and genetics of male meiotic drive. In Sperm Biology: An Evolutionary Perspective, 1st ed.; Birkhead, T.R., Hosken, D.J., Pitnick, S., Eds.; Elsevier: Burlington, MA, USA, 2009; pp. 471–506. ISBN 9780123725684. [Google Scholar]
- Raju, N.B. Ascomycete Spore Killers: Chromosomal Elements that Distort Genetic Ratios among the Products of Meiosis. Mycologia 1994, 86, 461–473. [Google Scholar] [CrossRef]
- Úbeda, F.; Normark, B.B. Male Killers and the Origins of Paternal Genome Elimination. Theor. Popul. Biol. 2006, 70, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Angélica, M.; Núñez, B.; Nuckolls, N.L.; Zanders, S.E. Genetic Villains: Killer Meiotic Drivers. Trends Genet. 2019, 34, 424–433. [Google Scholar] [CrossRef]
- Zimmering, S.; Sandler, L.; Nicoletti, B. Mechanisms of Meiotic Drive. Annu. Rev. Genet. 1970, 4, 409–436. [Google Scholar] [CrossRef]
- Courret, C.; Chang, C.H.; Wei, K.H.C.; Montchamp-Moreau, C.; Larracuente, A.M. Meiotic Drive Mechanisms: Lessons from Drosophila. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191430. [Google Scholar] [CrossRef]
- Yang, W.-C.; Shi, D.-Q.; Chen, Y.-H. Female Gametophyte Development in Flowering Plants. Annu. Rev. Plant Biol. 2010, 61, 89–108. [Google Scholar] [CrossRef]
- Dawe, R.K.; Lowry, E.G.; Gent, J.I.; Stitzer, M.C.; Swentowsky, K.W.; Higgins, D.M.; Ross-Ibarra, J.; Wallace, J.G.; Kanizay, L.B.; Alabady, M. A Kinesin-14 Motor Activates Neocentromeres to Promote Meiotic Drive in Maize. Cell 2018, 173, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Buckler, E.S.; Phelps-Durr, T.L.; Buckler, C.S.K.; Dawe, R.K.; Doebley, J.F.; Holtsford, T.P. Meiotic Drive of Chromosomal Knobs Reshaped the Maize Genome. Genetics 1999, 153, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Swentowsky, K.W.; Gent, J.I.; Lowry, E.G.; Schubert, V.; Ran, X.; Tseng, K.-F.; Harkess, A.E.; Qiu, W.; Dawe, R.K. Distinct Kinesin Motors Drive Two Types of Maize Neocentromeres. Genes Dev. 2020, 34, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Fishman, L.; Willis, J.H. A Cytonuclear Incompatibility Causes Anther Sterility in Mimulus Hybrids. Evolution 2006, 60, 1372–1381. [Google Scholar] [CrossRef]
- Sandler, L.; Hiraizumi, Y.; Sandler, I. Meiotic Drive in Natural Populations of Drosophila Melanogaster. I. The Cytogenetic Basis of Segregation-Distortion. Genetics 1959, 44, 233. [Google Scholar] [CrossRef]
- Ganetzky, B. On the Components of Segregation Distortion in Drosophila Melanogaster. Genetics 1977, 86, 321–355. [Google Scholar] [CrossRef]
- Larracuente, A.M.; Presgraves, D.C. The Selfish Segregation Distorter Gene Complex of Drosophila melanogaster. Genetics 2012, 192, 33–53. [Google Scholar] [CrossRef]
- Sandler, L.; Golic, K. Segregation Distortion in Drosophila. Trends Genet. 1985, 1, 181–185. [Google Scholar] [CrossRef]
- Jaenike, J. Sex Chromosome Meiotic Drive. Annu. Rev. Ecol. Syst. 2001, 32, 25–49. [Google Scholar] [CrossRef]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A Synthetic Sex Ratio Distortion System for the Control of the Human Malaria Mosquito. Nat. Commun. 2014, 5, 3977. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Zheng, X.; Zhou, J.; Kong, W.; Wang, P.; Bai, W.; Zheng, H.; Zhang, H.; Li, J.; et al. A Selfish Genetic Element Confers Non-Mendelian Inheritance in Rice. Science 2018, 360, 1130–1132. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Lu, J.; Xiong, Y.; Zhao, Z.; Yu, X.; Zheng, X.; Li, J.; Lin, Q.; Ren, Y. A Natural Gene Drive System Confers Reproductive Isolation in Rice. Cell 2023, 186, 3577–3592. [Google Scholar] [CrossRef]
- Belfort, M.; Roberts, R.J. Homing Endonucleases: Keeping the House in Order. Nucleic Acids Res. 1997, 25, 3379–3388. [Google Scholar] [CrossRef]
- Stoddard, B.L. Homing Endonucleases: From Microbial Genetic Invaders to Reagents for Targeted DNA Modification. Structure 2011, 19, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.S.; Stoddard, B.L. Homing Endonucleases: Structural and Functional Insight into the Catalysts of Intron/Intein Mobility. Nucleic Acids Res. 2001, 29, 3757–3774. [Google Scholar] [CrossRef] [PubMed]
- Windbichler, N.; Menichelli, M.; Papathanos, P.A.; Thyme, S.B.; Li, H.; Ulge, U.Y.; Hovde, B.T.; Baker, D.; Monnat, R.J.; Burt, A.; et al. A Synthetic Homing Endonuclease-Based Gene Drive System in the Human Malaria Mosquito. Nature 2011, 473, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Gogarten, J.P.; Senejani, A.G.; Zhaxybayeva, O.; Olendzenski, L.; Hilario, E. Inteins: Structure, Function, and Evolution. Annu. Rev. Microbiol. 2002, 56, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Lambert, A.R.; Mak, A.N.-S.; Jacoby, K.; Dickson, R.J.; Gloor, G.B.; Scharenberg, A.M.; Edgell, D.R.; Stoddard, B.L. Tapping Natural Reservoirs of Homing Endonucleases for Targeted Gene Modification. Proc. Natl. Acad. Sci. USA 2011, 108, 13077–13082. [Google Scholar] [CrossRef]
- Seligman, L.M.; Chisholm, K.M.; Chevalier, B.S.; Chadsey, M.S.; Edwards, S.T.; Savage, J.H.; Veillet, A.L. Mutations Altering the Cleavage Specificity of a Homing Endonuclease. Nucleic Acids Res. 2002, 30, 3870–3879. [Google Scholar] [CrossRef]
- Taylor, G.K.; Petrucci, L.H.; Lambert, A.R.; Baxter, S.K.; Jarjour, J.; Stoddard, B.L. LAHEDES: The LAGLIDADG Homing Endonuclease Database and Engineering Server. Nucleic Acids Res. 2012, 40, W110–W116. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-Guided Genetic Silencing Systems in Bacteria and Archaea. Nature 2012, 482, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Mohanraju, P.; Makarova, K.S.; Zetsche, B.; Zhang, F.; Koonin, E.V.; van der Oost, J. Diverse Evolutionary Roots and Mechanistic Variations of the CRISPR-Cas Systems. Science 2016, 353, aad5147. [Google Scholar] [CrossRef] [PubMed]
- Schiml, S.; Puchta, H. Revolutionizing Plant Biology: Multiple Ways of Genome Engineering by CRISPR/Cas. Plant Methods 2016, 12, 8. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, Mechanisms and Relevance. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Shabbir, M.Z.; Wu, Q.; Mahmood, S.; Sajid, A.; Maan, M.K.; Ahmed, S.; Naveed, U.; Hao, H.; Yuan, Z. CRISPR-Cas System: Biological Function in Microbes and Its Use to Treat Antimicrobial Resistant Pathogens. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 21. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR—Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–531. [Google Scholar] [CrossRef]
- McFarlane, G.R.; Whitelaw, C.B.A.; Lillico, S.G. CRISPR-Based Gene Drives for Pest Control. Trends Biotechnol. 2018, 36, 130–133. [Google Scholar] [CrossRef]
- Noble, C.; Olejarz, J.; Esvelt, K.M.; Church, G.M.; Nowak, M.A. Evolutionary Dynamics of CRISPR Gene Drives. Sci. Adv. 2017, 3, e1601964. [Google Scholar] [CrossRef]
- Gantz, V.M.; Bier, E. The Dawn of Active Genetics. Bioessays 2016, 38, 50–63. [Google Scholar] [CrossRef]
- Gantz, V.M.; Bier, E. Active Genetics Comes Alive: Exploring the Broad Applications of CRISPR-based Selfish Genetic Elements (or Gene-drives). BioEssays 2022, 44, 2100279. [Google Scholar] [CrossRef]
- López Del Amo, V.; Bishop, A.L.; Sánchez, C.H.M.; Bennett, J.B.; Feng, X.; Marshall, J.M.; Bier, E.; Gantz, V.M. A Transcomplementing Gene Drive Provides a Flexible Platform for Laboratory Investigation and Potential Field Deployment. Nat. Commun. 2020, 11, 352. [Google Scholar] [CrossRef]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, T.; Kandul, N.P.; Bui, M.; Gamez, S.; Raban, R.; Bennett, J.; Sánchez, C.H.M.; Lanzaro, G.C.; Schmidt, H.; et al. Development of a Confinable Gene Drive System in the Human Disease Vector Aedes Aegypti. eLife 2020, 9, e51701. [Google Scholar] [CrossRef] [PubMed]
- Galizi, R.; Hammond, A.; Kyrou, K.; Taxiarchi, C.; Bernardini, F.; O’Loughlin, S.M.; Papathanos, P.-A.; Nolan, T.; Windbichler, N.; Crisanti, A. A CRISPR-Cas9 Sex-Ratio Distortion System for Genetic Control. Sci. Rep. 2016, 6, 31139. [Google Scholar] [CrossRef] [PubMed]
- Champer, J.; Kim, I.; Champer, S.E.; Clark, A.G.; Messer, P.W. Performance Analysis of Novel Toxin-Antidote CRISPR Gene Drive Systems. bioRxiv 2019, 628362. [Google Scholar] [CrossRef] [PubMed]
- Unckless, R.L.; Messer, P.W.; Connallon, T.; Clark, A.G. Modeling the Manipulation of Natural Populations by the Mutagenic Chain Reaction. Genetics 2015, 201, 425–431. [Google Scholar] [CrossRef]
- Owen, M. Diverse Approaches to Herbicide-Resistant Weed Management. Weed Sci. 2016, 64, 570–584. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Weed Biology: Importance to Weed Management. Weed Sci. 1997, 45, 349–356. [Google Scholar] [CrossRef]
- Verma, P.; Reeves, R.G.; Simon, S.; Otto, M.; Gokhale, C.S. The Effect of Mating Complexity on Gene Drive Dynamics. Am. Nat. 2022, 201, E1–E22. [Google Scholar] [CrossRef]
- Moro, D.; Byrne, M.; Kennedy, M.; Campbell, S.; Tizard, M. Identifying Knowledge Gaps for Gene Drive Research to Control Invasive Animal Species: The next CRISPR Step. Glob. Ecol. Conserv. 2018, 13, e00363. [Google Scholar] [CrossRef]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The Biology of Invasive Alien Plants in Canada. 3. Amaranthus tuberculatus (Moq.) Sauer Var. Rudis (Sauer) Costea & Tardif. Can. J. Plant Sci. 2005, 85, 507–522. [Google Scholar] [CrossRef]
- Friesen, L.F.; Beckie, H.J.; Warwick, S.I.; Van Acker, R.C. The Biology of Canadian Weeds. 138. Kochia scoparia (L.) Schrad. Can. J. Plant Sci. 2009, 89, 141–167. [Google Scholar] [CrossRef]
- Keeley, P.E.; Carter, C.H.; Thullen, R.J. Influence of Planting Date on Growth of Palmer Amaranth (Amaranthus palmeri). Weed Sci. 1987, 35, 199–204. [Google Scholar] [CrossRef]
- Montgomery, J.S.; Sadeque, A.; Giacomini, D.A.; Brown, P.J.; Tranel, P.J. Sex-Specific Markers for Waterhemp (Amaranthus Tuberculatus) and Palmer Amaranth (Amaranthus Palmeri). Weed Sci. 2019, 67, 412–418. [Google Scholar] [CrossRef]
- Montgomery, J.S.; Giacomini, D.A.; Weigel, D.; Tranel, P.J. Male-Specific Y-Chromosomal Regions in Waterhemp (Amaranthus tuberculatus) and Palmer Amaranth (Amaranthus palmeri). New Phytol. 2021, 229, 3522–3533. [Google Scholar] [CrossRef]
- Rode, N.O.; Estoup, A.; Bourguet, D.; Courtier-Orgogozo, V.; Débarre, F. Population Management Using Gene Drive: Molecular Design, Models of Spread Dynamics and Assessment of Ecological Risks. Conserv. Genet. 2019, 20, 671–690. [Google Scholar] [CrossRef]
- Cavers, P.B. Seed Demography. Can. J. Bot. 1983, 61, 3578–3590. [Google Scholar] [CrossRef]
- Mithila, J.G.A. Understanding Genetics of Herbicide Resistance in Weeds: Implications for Weed Management. Adv. Crop Sci. Technol. 2013, 1, 3–5. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of Evolved Herbicide Resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Hirschberg, J.; McIntosh, L. Molecular Basis of Herbicide Resistance in Plants in Amaranthus hybridus. Science 1983, 222, 1346–1349. [Google Scholar] [CrossRef]
- Gronwald, J.W. Resistance to Photosystem II Inhibiting Herbicides. In Herbicide Resistance in Plants: Biology and Biochemistry, 1st ed.; Powles, S.B., Holtum, J.A.M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 27–60. [Google Scholar]
- Cui, Y.; Xu, J.; Cheng, M.; Liao, X.; Peng, S. Review of CRISPR/Cas9 SgRNA Design Tools. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 455–465. [Google Scholar] [CrossRef]
- Unckless, R.L.; Clark, A.G.; Messer, P.W. Evolution of Resistance against CRISPR/Cas9 Gene Drive. Genetics 2017, 205, 827–841. [Google Scholar] [CrossRef]
- Noble, C.; Min, J.; Olejarz, J.; Buchthal, J.; Chavez, A.; Smidler, A.L.; DeBenedictis, E.A.; Church, G.M.; Nowak, M.A.; Esvelt, K.M. Daisy-Chain Gene Drives for the Alteration of Local Populations. Proc. Natl. Acad. Sci. USA 2019, 116, 8275–8282. [Google Scholar] [CrossRef]
- Backus, G.A.; Delborne, J.A. Threshold-Dependent Gene Drives in the Wild: Spread, Controllability, and Ecological Uncertainty. Bioscience 2019, 69, 900–907. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, J.M.; Stinchcombe, J.R.; Wright, S.I. Population Genomics of Herbicide Resistance: Adaptation via Evolutionary Rescue. Annu. Rev. Plant Biol. 2018, 69, 611–635. [Google Scholar] [CrossRef]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted Mutagenesis, Precise Gene Editing, and Site-Specific Gene Insertion in Maize Using Cas9 and Guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef]
- Butt, H.; Rao, G.S.; Sedeek, K.; Aman, R.; Kamel, R.; Mahfouz, M. Engineering Herbicide Resistance via Prime Editing in Rice. Plant Biotechnol. J. 2020, 18, 2370–2372. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, C.; Xian, G.; Liu, D.; Lin, L.; Yin, S.; Sun, Q.; Fang, Y.; Wang, Y. Engineering Herbicide-Resistant Oilseed Rape by CRISPR/Cas9-Mediated Cytosine Base-Editing. Plant Biotechnol. J. 2020, 18, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Sony, S.K.; Kaul, T.; Motelb, K.F.A.; Thangaraj, A.; Bharti, J.; Kaul, R.; Verma, R.; Nehra, M. CRISPR/Cas9-mediated Homology Donor Repair Base Editing Confers Glyphosate Resistance to Rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1122926. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Jiang, L.; You, X.; Ma, P.; Xi, Z.; Wang, N.N. Generation of Herbicide-Resistant Soybean by Base Editing. Biology 2023, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, N.; Cai, Y.; Yang, Q.; Yu, L.; Chen, Z.; Shi, W.; Liu, J.; Pan, C.; Li, Y. CRISPR-Cas9-Mediated Editing of the OsHPPD 3′ UTR Confers Enhanced Resistance to HPPD-Inhibiting Herbicides in Rice. Plant Commun. 2023, 4, 100605. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted Base Editing in Rice and Tomato Using a CRISPR-Cas9 Cytidine Deaminase Fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Owen, M.D.K. Herbicide-Resistant Crops: Utilities and Limitations for Herbicide-Resistant Weed Management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef] [PubMed]
- Champer, J.; Reeves, R.; Yeon Oh, S.; Liu, C.; Liu, J.; Clark, A.G.; Messer, P.W. Novel CRISPR/Cas9 Gene Drive Constructs in Drosophila Reveal Insights into Mechanisms of Resistance Allele Formation and Drive Efficiency in Genetically Diverse Populations. bioRxiv 2017, 13, 1006796. [Google Scholar] [CrossRef]
- Gantz, V.M.; Bier, E. The Mutagenic Chain Reaction: A Method for Converting Heterozygous to Homozygous Mutations. Science 2015, 348, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Gene Drives Gaining Speed. Nat. Rev. Genet. 2022, 23, 5–22. [Google Scholar] [CrossRef]
- Courtier-Orgogozo, V.; Danchin, A.; Gouyon, P.; Boëte, C. Evaluating the Probability of CRISPR-based Gene Drive Contaminating Another Species. Evol. Appl. 2020, 13, 1888–1905. [Google Scholar] [CrossRef]
- Crow, J.F. Why Is Mendelian Segregation so Exact? BioEssays 1991, 13, 305–312. [Google Scholar] [CrossRef]
- Simon, S.; Otto, M.; Engelhard, M. Synthetic Gene Drive: Between Continuity and Novelty: Crucial Differences between Gene Drive and Genetically Modified Organisms Require an Adapted Risk Assessment for Their Use. EMBO Rep. 2018, 19, e45760. [Google Scholar] [CrossRef]
- James, A.A. Gene Drive Systems in Mosquitoes: Rules of the Road. Trends Parasitol. 2005, 21, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.; Windbichler, N.; Wenger, E.A.; Bever, C.A.; Selvaraj, P. Population Replacement Gene Drive Characteristics for Malaria Elimination in a Range of Seasonal Transmission Settings: A Modelling Study. Malar. J. 2022, 21, 226. [Google Scholar] [CrossRef]
- Kormos, A.; Lanzaro, G.C.; Bier, E.; Dimopoulos, G.; Marshall, J.M.; Pinto, J.; Dos Santos, A.A.; Bacar, A.; Rompão, H.S.P.S.; James, A.A. Application of the Relationship-Based Model to Engagement for Field Trials of Genetically Engineered Malaria Vectors. Am. J. Trop. Med. Hyg. 2021, 104, 805. [Google Scholar] [CrossRef] [PubMed]
- Annas, G.J.; Beisel, C.L.; Clement, K.; Crisanti, A.; Francis, S.; Galardini, M.; Galizi, R.; Grünewald, J.; Immobile, G.; Khalil, A.S. A Code of Ethics for Gene Drive Research. Cris. J. 2021, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome Edited Crops Touch the Market: A View on the Global Development and Regulatory Environment. Front. Plant Sci. 2020, 11, 1525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumam, Y.; Trick, H.N.; Vara Prasad, P.V.; Jugulam, M. Transformative Approaches for Sustainable Weed Management: The Power of Gene Drive and CRISPR-Cas9. Genes 2023, 14, 2176. https://doi.org/10.3390/genes14122176
Kumam Y, Trick HN, Vara Prasad PV, Jugulam M. Transformative Approaches for Sustainable Weed Management: The Power of Gene Drive and CRISPR-Cas9. Genes. 2023; 14(12):2176. https://doi.org/10.3390/genes14122176
Chicago/Turabian StyleKumam, Yaiphabi, Harold N Trick, P.V. Vara Prasad, and Mithila Jugulam. 2023. "Transformative Approaches for Sustainable Weed Management: The Power of Gene Drive and CRISPR-Cas9" Genes 14, no. 12: 2176. https://doi.org/10.3390/genes14122176
APA StyleKumam, Y., Trick, H. N., Vara Prasad, P. V., & Jugulam, M. (2023). Transformative Approaches for Sustainable Weed Management: The Power of Gene Drive and CRISPR-Cas9. Genes, 14(12), 2176. https://doi.org/10.3390/genes14122176







