Abstract
Background: Traumatic spinal cord injury (SCI) is a disabling condition that affects millions of people around the world. Currently, no clinical treatment can restore spinal cord function. Comparison of molecular responses in regenerating to non-regenerating vertebrates can shed light on neural restoration. The axolotl (Ambystoma mexicanum) is an amphibian that regenerates regions of the brain or spinal cord after damage. Methods: In this study, we compared the transcriptomes after SCI at acute (1–2 days after SCI) and sub-acute (6–7 days post-SCI) periods through the analysis of RNA-seq public datasets from axolotl and non-regenerating rodents. Results: Genes related to wound healing and immune responses were upregulated in axolotls, rats, and mice after SCI; however, the immune-related processes were more prevalent in rodents. In the acute phase of SCI in the axolotl, the molecular pathways and genes associated with early development were upregulated, while processes related to neuronal function were downregulated. Importantly, the downregulation of processes related to sensorial and motor functions was observed only in rodents. This analysis also revealed that genes related to pluripotency, cytoskeleton rearrangement, and transposable elements (e.g., Sox2, Krt5, and LOC100130764) were among the most upregulated in the axolotl. Finally, gene regulatory networks in axolotls revealed the early activation of genes related to neurogenesis, including Atf3/4 and Foxa2. Conclusions: Immune-related processes are upregulated shortly after SCI in axolotls and rodents; however, a strong immune response is more noticeable in rodents. Genes related to early development and neurogenesis are upregulated beginning in the acute stage of SCI in axolotls, while the loss of motor and sensory functions is detected only in rodents during the sub-acute period of SCI. The approach employed in this study might be useful for designing and establishing regenerative therapies after SCI in mammals, including humans.
1. Introduction
Spinal cord injury (SCI) is a disturbing event that causes profound changes in the structure and function of this organ. Moreover, depending on the type of injury and the extent of the damage, these changes often result in temporary or permanent sensory and motor disabilities in the individuals who suffer from it [1,2]. Traumatic injury due to external physical impacts (e.g., falls or car accidents) is the leading cause of SCI; nonetheless, SCI can also occur from non-traumatic events, such as tumor development or inflammatory processes derived from infectious conditions [3,4]. The pathophysiological events of traumatic SCI are temporally separated into the acute (<2 days), sub-acute (2–14 days), intermediate (14 days to 6 months), and chronic (>6 months) phases [1]. During the acute phase, the initial damage induces neuronal death and an acute inflammatory response, that in turn induces glial scar formation during the subsequent phases. Glial scarring, together with the null capacity of neuronal turnover in mammals, drastically reduces the spinal cord recovery potential [5], triggering, in most cases, the neurological deficits that are observed in patients with chronic SCI, such as loss of motor control and alteration of sensation [6]. In the United States of America, it is estimated that approximately 450,000 people are permanently disabled due to traumatic SCI [7]; in Western Europe, the estimated incidence of traumatic SCI is 15 cases per million people, while the overall global incidence corresponds to 10.5 cases per 100,000 persons, with 768,473 new cases each year around the world [1,8].
Animal models for traumatic SCI, typically in rodents, have provided significant information about the pathological processes that occur, as well as how recovery could be promoted after the insult [9]; however, to date, there is no effective therapy that reverses the histological changes induced in the damaged spinal cord, neither is there a clinical strategy capable of promoting the regeneration of the damaged neural circuits.
The axolotl is a salamander with an exceptional regenerative capacity. After tissue damage or amputation, axolotls can repair and replace entire anatomical parts, including several regions of the brain and spinal cord, restoring all the neuronal and glial cell types observed before damage [10,11]. Therefore, to comprehend the molecular differences with other vertebrate species that do not exhibit regeneration, such as mammals, several studies conducted to understand the regenerative capacity of the axolotl have focused on the identification of genes and molecular processes that lead to the repair and replacement of damaged tissues [12,13].
By analyzing RNA-seq data obtained from public datasets, in this study, we compared the transcriptomic responses of the spinal cords in axolotls and rodents after traumatic injury, to determine the gene expression differences between these species during the first days post-injury (dpi). With this approach, we aimed to identify transcriptional programs that allow the regeneration of the spinal cord in axolotls, as well as to understand the molecular processes that inhibit the repair of the damaged spinal cord in mammals. Such analysis could provide clues for therapeutic targets to be applied in regenerative medicine.
2. Materials and Methods
2.1. Data Collection
After a preliminary screening, three RNA-seq datasets derived from different species (axolotl, rat, and mouse) with traumatic SCI were analyzed and compared in this study. Datasets were collected from the European Nucleotide Archive (ENA) [14] under the following accession numbers: axolotl [PRJNA378982] [15], rat [PRJNA760277] [16], and mouse [PRJNA193596] [17]. The specifications of each dataset are described in Table 1.

Table 1.
Specifications of the RNA-seq datasets.
2.2. Data Pre-Processing
The quality of the obtained raw sequence reads in FASTQ format from the RNA-seq datasets were verified using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 18 April 2023). Then, the sequencing datasets were aligned with their respective reference genomes using the program HISAT2 [18]. For axolotl, the reference genome was AmexG_V6.0-DD [19], while for rat and mouse were mRatBN7.2 (GenBank assembly accession: GCA_015227675.2) and GRCm39 (GCA_000001635.9), respectively. The resulting SAM files from the alignments were converted to BAM files using SAMtools (ver. 1.18) [20]. GTF annotations files were used to tag aligned reads using the R package Rsubread (ver. 2.16) [21]; a data matrix containing read counts was extracted using the function featureCounts also from Rsubread. For axolotl genes, gene names annotated also in rat or mouse GTF files were preferred, otherwise, gene names also annotated in other species with better genome description were used. If neither was present, the axolotl gene IDs from the current reference genome (Amex60DD) were used as the gene names. To complete three technical replicates from the axolotl data, bootstrap resampling was applied to the matrix read counts in R [22].
2.3. Differential Gene Expression Analysis
The matrices obtained in Rsubread containing the read counts (Table S1) were used as input for gene expression analyses. Differential expression of three groups (intact, 1–2 dpi, and 6–7 dpi; n = 3 for each condition) from each species was analyzed in R by using the package DEseq2 [23]. Read counts were normalized by library size and gene counts were modeled by negative binomial distribution. Differentially expressed genes (DEG) were detected by the Wald test using a threshold of false discovery rate (q-value) < 0.005 and a fold-change <−2 for downregulated genes and >2, for upregulated genes. Heatmaps were plotted with the R function pheatmap. Volcano plots, Venn diagrams, and bar plots were also plotted from DEG in R. Reactome and KEGG pathways were derived from DEG identified in heatmaps and Venn diagrams, respectively, using the package enrichR (ver. 3.2) setting a q-value of 0.005.
2.4. Gene Ontology Analysis
DEG obtained from DEseq2 was used to perform gene ontology (GO) enrichment analysis using the R package clusterProfiler (ver. 4.10) [24] for each time post-injury. GO annotations for biological processes were queried by gene symbols using the organism database packages (OrgDb) for rat and mouse. For axolotl, the human Org.db was used using the gene symbols identified as human orthologs provided in the axolotl reference genome. Benjamini–Hochberg correction (BH) was used for false discovery rate estimation during GO analysis while the cutoff for q-value was 0.005. Bar plots were used to visualize the top GO terms enriched by the upregulated and downregulated genes for each time post-injury. Gene regulatory networks showing the result of the enriched genes for representative GO annotations were plotted using the cnetplot function from the clusterProfiler package.
2.5. Code Availability
The code employed in this study for gene expression analysis is available on GitHub at link https://github.com/JCGO221/Axolotl_SCI_Comparatives/ (accessed on 26 November 2023).
3. Results and Discussion
3.1. Changes in the Global Gene Expression after Traumatic SCI Show Large Differences between Axolotls and Rodents
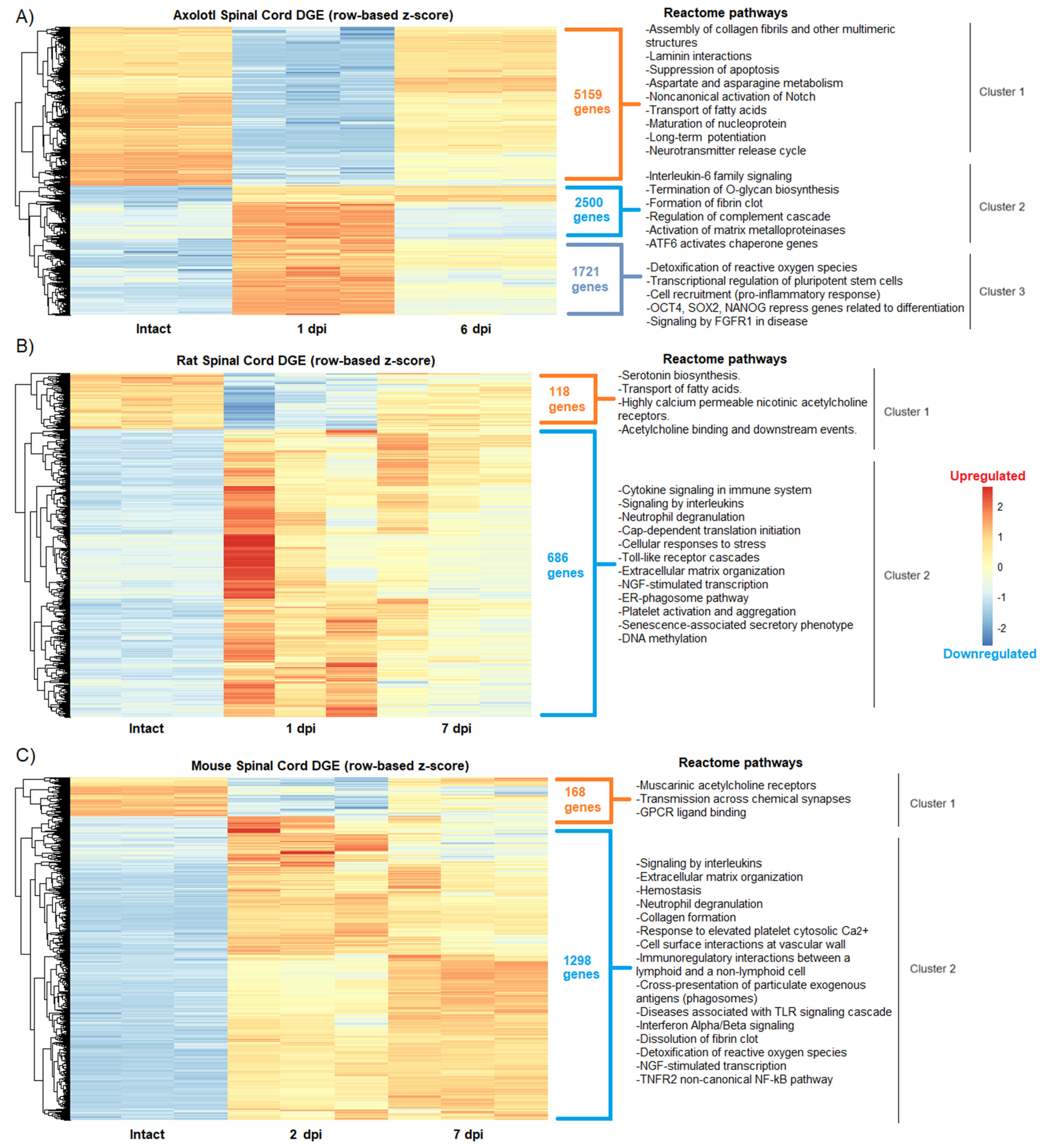
RNA-seq data from axolotls, rats, and mice with acute (1–2 dpi) or sub-acute (6–7 dpi) SCI were analyzed to determine changes in global gene expression after traumatic injury. First, and as was expected from published works [25,26,27], we observed in the hierarchical cluster analysis similarities, but also significant differences, in groups of genes that were upregulated and downregulated between the intact and SCI groups for the three species analyzed (Figure 1).
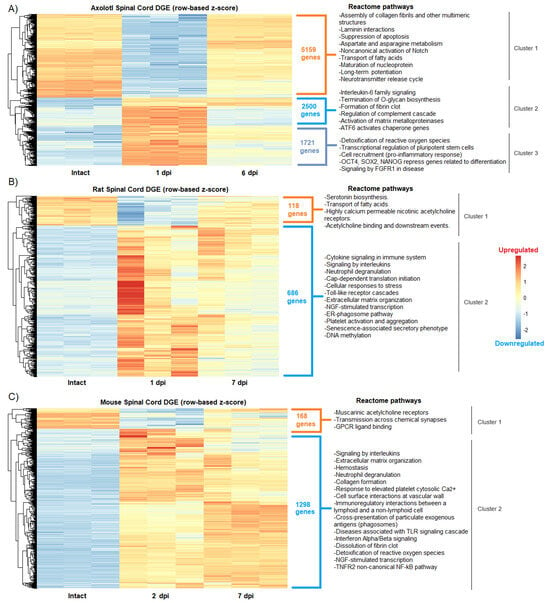
Figure 1.
Changes in the global gene expression during acute and sub-acute SCI phases in axolotl and rodents. Heatmaps show the differential gene expression (DGE) categorized by hierarchical clustering in (A) axolotl after 1 and 6 dpi; (B) rat after 1 and 7 dpi; and (C) mouse after 2 and 7 dpi. The intact spinal cord condition is shown for all species. Reactome pathways detected for each gene cluster in all species are depicted.
Particularly in axolotl, we found a cluster of genes (cluster 2) that were activated in the acute phase and that drastically decreased their expression levels in the sub-acute period, while others remained strongly upregulated (Figure 1A), an event that was not noticed in rodents. Furthermore, the enrichment analysis to depict Reactome pathways for each gene cluster showed in axolotl the upregulation of genes related to pluripotent stem cells and self-renewal, including Oct4, Sox2, and Nanog [28] at 1 dpi, as well as the enrichment of pathways associated to signaling of FGFR1, detoxification of reactive oxygen species (cluster 3,) and control of the quality of the proteins in response to stress by upregulation of ATF6 (cluster 2) (Figure 1A). Interestingly, ATF6 has been related to early neurodevelopment and the onset of myelination in mammals [29], while FGFR1 ligands have been proposed to restore spinal cord function after an insult [30].
These results demonstrate that protective and stem cell-related mechanisms are activated in axolotls very early in response to SCI, and not in rodents. Moreover, it was detected that neural functions associated with the activity of acetylcholine receptors were impaired in both rats and mice after injury (cluster 1 in both species) (Figure 1B,C), while in axolotl the downregulation of such functions was not detected by Reactome pathway analysis.
Nevertheless, molecular similarities were also identified among the studied species, particularly in the extracellular matrix and immune processes that were upregulated upon SCI. In agreement, a previous study where comparative transcriptomics between rats and axolotls after SCI was performed through microarray analysis, it was found that multiple extracellular matrix genes were upregulated in both species, thus highlighting the importance of extracellular matrix remodeling in response to SCI [31]. Despite this, and as was expected, the most common regulated processes between rodents and axolotls were those related to the activation of the immune system, particularly the activation of signaling by interleukins (cluster 2 in rats and mice), whose function has been extensively reviewed in SCI [32]; notably, interleukin-6 signaling was specifically detected in axolotl (cluster 2), which has recently been reported that its overexpression in rodents with SCI can substantially increase their functional recovery [33].
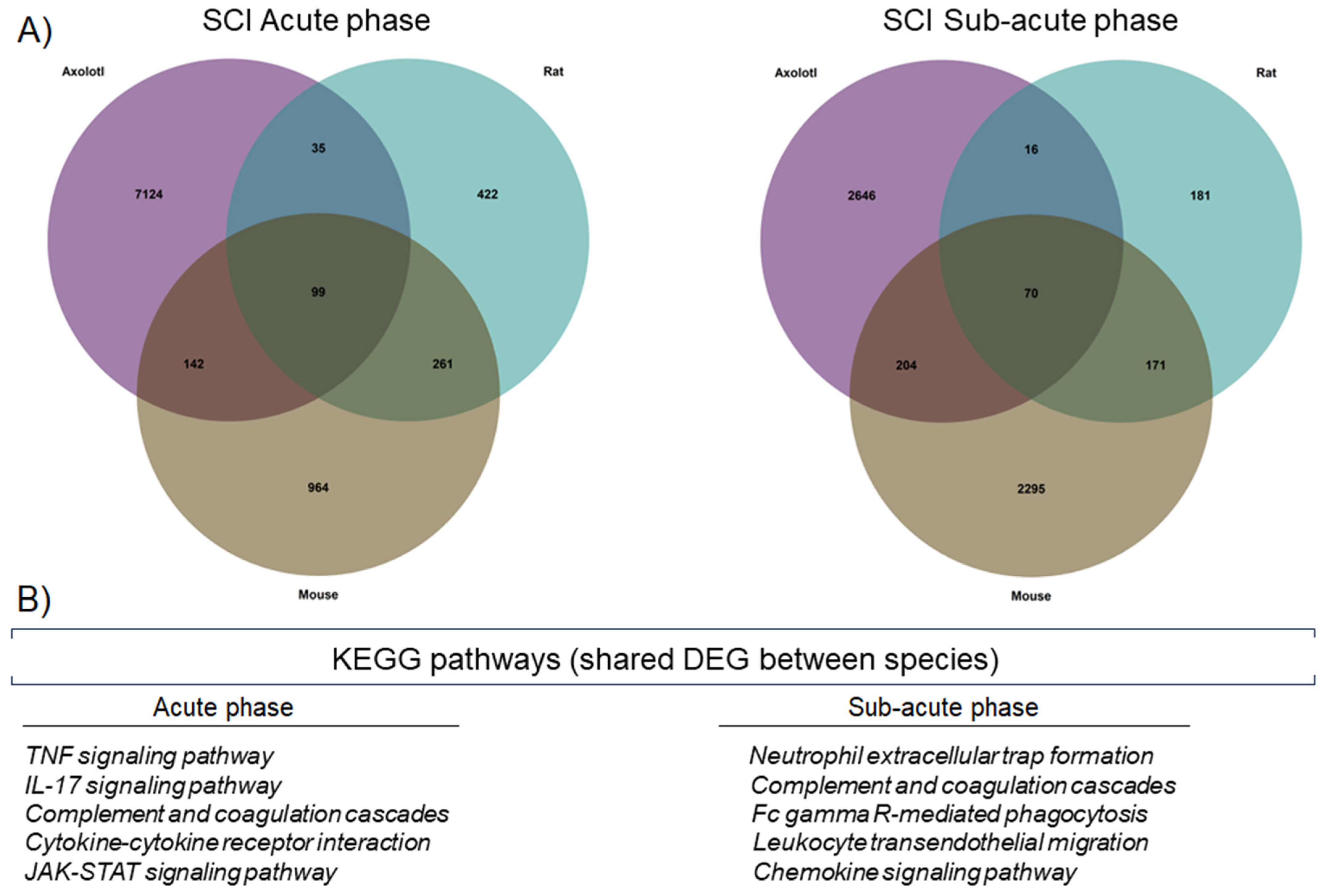
To further corroborate whether there were similar sets of genes that were regulated in the three species, at each stage of SCI analyzed, a Venn diagram was drawn up. Thus, we observed that 99 genes were shared in the acute stage, while 70 genes were shared during the sub-acute stage (Figure 2A). Importantly, the KEGG pathways detected for the genes shared between the species corresponded to immunological processes, indicating again that in axolotls and rodents, there are common genes and molecular pathways that are activated shortly, in response to SCI (Figure 2B).
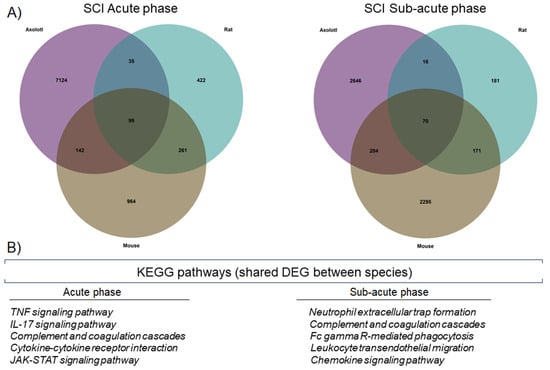
Figure 2.
Axolotls and rodents share specific molecular changes after SCI. (A) Venn diagrams for DEG in axolotl, rat, and mouse, during the acute and sub-acute phases of SCI. (B) KEGG pathways were detected by enrichment analysis of the shared DEG between the studied species.
Finally, DEG and cluster analysis also revealed that an elevated number of genes are significantly regulated in axolotl after SCI in comparison to rodents (Figures S1–S3), suggesting that a strong response at the level of gene expression is a trait of SCI in this species. This finding might be attributed to the size of the axolotl genome, which is much larger than that of rodents. Moreover, the axolotl genome is currently the largest ever sequenced and has a high number of repeated sequences product of transposon insertions, which are proposed to have some participation in the regeneration capacity of this animal [15,19].
3.2. Top Regulated Genes in Axolotl and Rodents in the Acute and Sub-Acute Stages of SCI
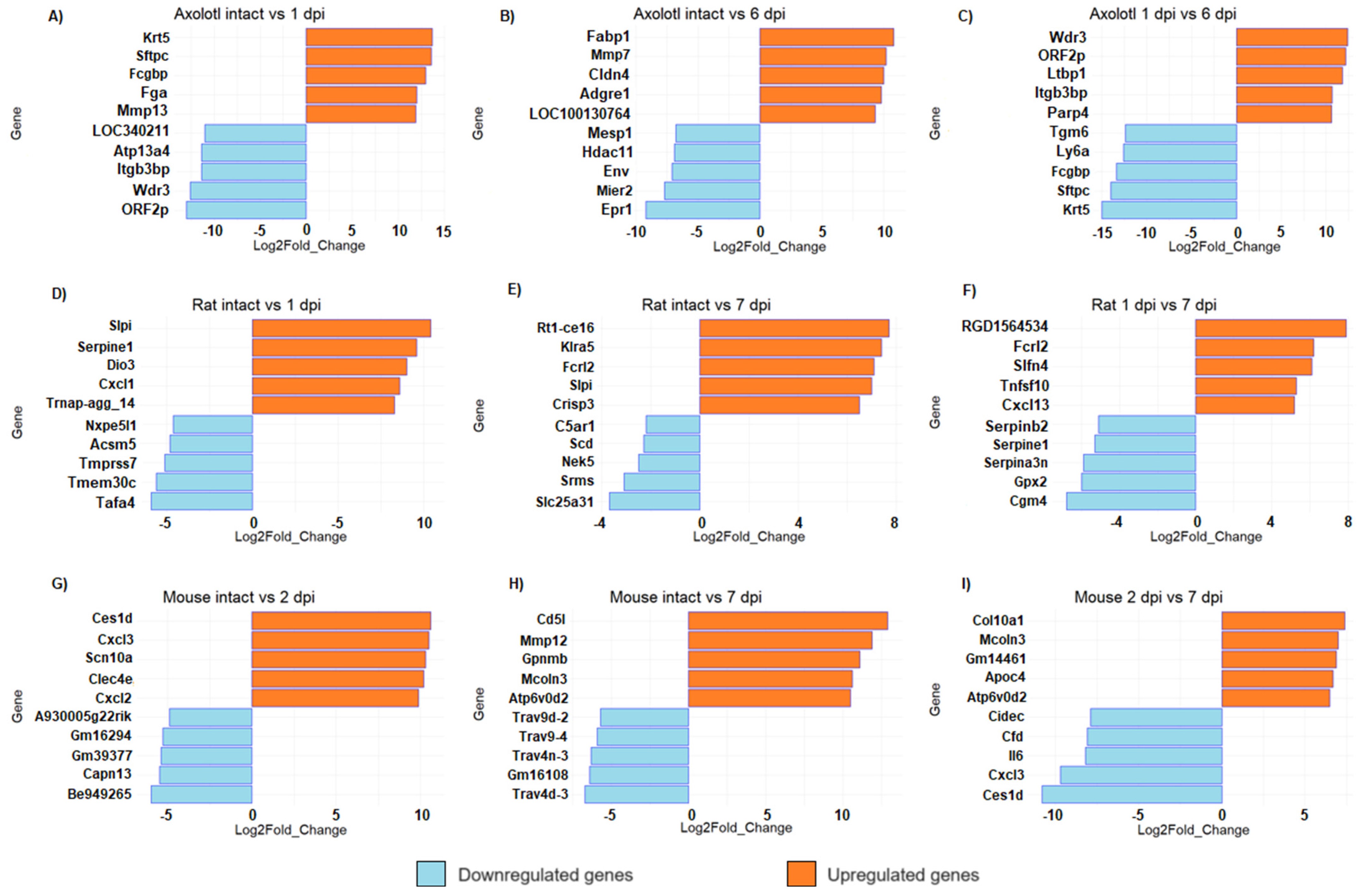
Unlike mammals, axolotls can fully regenerate the spinal cord after injury [11,34]. However, given the number of genes regulated in axolotl, it is interesting to analyze the molecular circuitry that is triggered early after SCI and that is required to initiate a pro-regenerative response during subsequent stages of SCI in this species. Then, to narrow the list of interesting genes, we performed a top list of DEG (Figure 3). We found that keratin 5 (Krt5) was the most upregulated gene in axolotl at 1 dpi (Figure 3A). Keratin intermediate filaments are key components of the cytoskeleton, they support cellular rigidity and stability and are also associated with cell adhesion and migration; also, Krt5 is a marker of basal and progenitor cells in mammalian epithelial tissue and has been identified as a regulator in the regeneration of limbs in axolotl; Randal Voss reported that it is highly upregulated during the 2–3 days post-amputation in forelimbs [35]. A gene related to blood clot (Fga), and genes related to the immune response and extracellular matrix organization (Fcgbp and Mmp13, respectively) were also detected among the most upregulated genes at 1 dpi in axolotl; particularly, it has been proposed that Mmp13 may promote a more permissive environment for axonal regeneration in mice with SCI through its action on infiltrating monocytes [36].
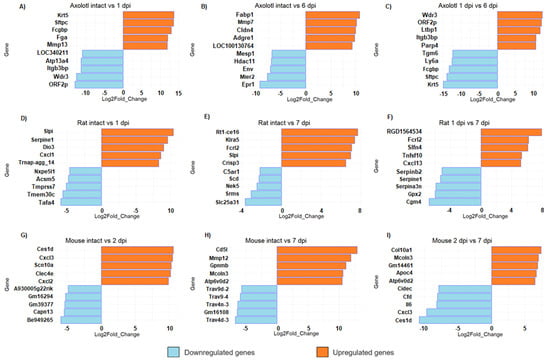
Figure 3.
Top list of upregulated and downregulated genes in axolotl (A,D,G), rat (B,E,H), and mouse (C,F,I) with acute or sub-acute SCI. For all comparisons, the represented change corresponds to the second condition, relative to the first. All panels show bar plots with the most upregulated and downregulated genes for each animal at the indicated timepoint according to its fold change (q-value < 0.005).
Interestingly, ORF2p and LOC340211 were detected among the top downregulated genes at 1 dpi, which are identified as genes of retroviral origin [37]. Meanwhile, at the sub-acute phase in axolotl (6 dpi), we found that Fabp1 was the top upregulated gene (Figure 3B); it is important to mention that a gene from the same family of fatty acid binding proteins, Fabp7, regulates inflammation and has a neuroprotective role in mice with autoimmune encephalomyelitis [38], so it would be important to study the effects of Fabp1 activation in rodents with SCI. Remarkably, LOC100130764 was among the top upregulated genes at 6 dpi in axolotl, and it is identified as LINE retrotransposon element (https://www.ncbi.nlm.nih.gov/gene/100130764, accessed on 20 August 2023); moreover, Env gene, which is also of retroviral origin [39], was a top downregulated gene at this SCI phase. Thus, it is worth studying the potential role of highly repeated sequences of retroviral origin in the regeneration of the spinal cord of the axolotl, and determining if the diminished number of these genetic elements in the mammalian genome is a crucial factor for the absence of regenerative processes in these species.
In rodents, among the genes that were most upregulated in the acute stage (1–2 dpi) were the CXC motif chemokine ligand family—Cxcl1, Cxcl2, and Cxcl3 (Figure 3D,G)—which are related to the inflammatory response, suggesting an acute immune response immediately after damage. It is known that CNS injury stimulates the expression of several proinflammatory chemokines and cytokines, including Cxcl1, and Cxcl2, which act to recruit leukocytes at lesion sites [40]. Interestingly, in rat, secretory leukocyte peptidase inhibitor (Slpi) was upregulated both in acute and sub-acute phases of SCI (Figure 3D,E). Slpi has anti-inflammatory properties, and it has been reported to promote wound healing [41]. Ghasemlou et al. reported in 2010 that Slpi has an early protective effect modulating the inflammatory response reducing NF-kB activation and TNF-a expression in the first few days after spinal cord injury [42]. Moreover, Slp1 has been reported to increase dramatically between two and five days after SCI in humans [43], so it would be interesting to verify whether Slp1 has also a protective effect in humans. Also, we found that Mmp12 was a top-upregulated gene at 7 dpi in mice (Figure 3H). It is important to note that Mmp12 has a negative effect on SCI in mice and contributes to the development of intermediate and chronic SCI [44].
Finally, the comparison between the acute and sub-acute phases in all species shows that some genes that were strongly upregulated during the acute phase are downregulated at the beginning of the sub-acute phase, and vice versa. For example, in axolotls, Krt5 and Sftpc are upregulated in the acute phase and then they were significantly downregulated in the sub-acute phase; conversely, Wdr5, ORF2p, and Itgb3bp were downregulated in the acute response but significantly increased in the sub-acute timepoint. These results indicate that there is a switch in the transcriptome signature between post-injury phases, each one characterized by its own gene expression profile (Figure 3C,F,I). The complete list of all DEG in each organism for all timepoints is provided in Tables S2–S10.
3.3. Gene Ontology Analysis Shows Differences and Similarities in Biological Processes between Axolotl and Rodents after Traumatic SCI
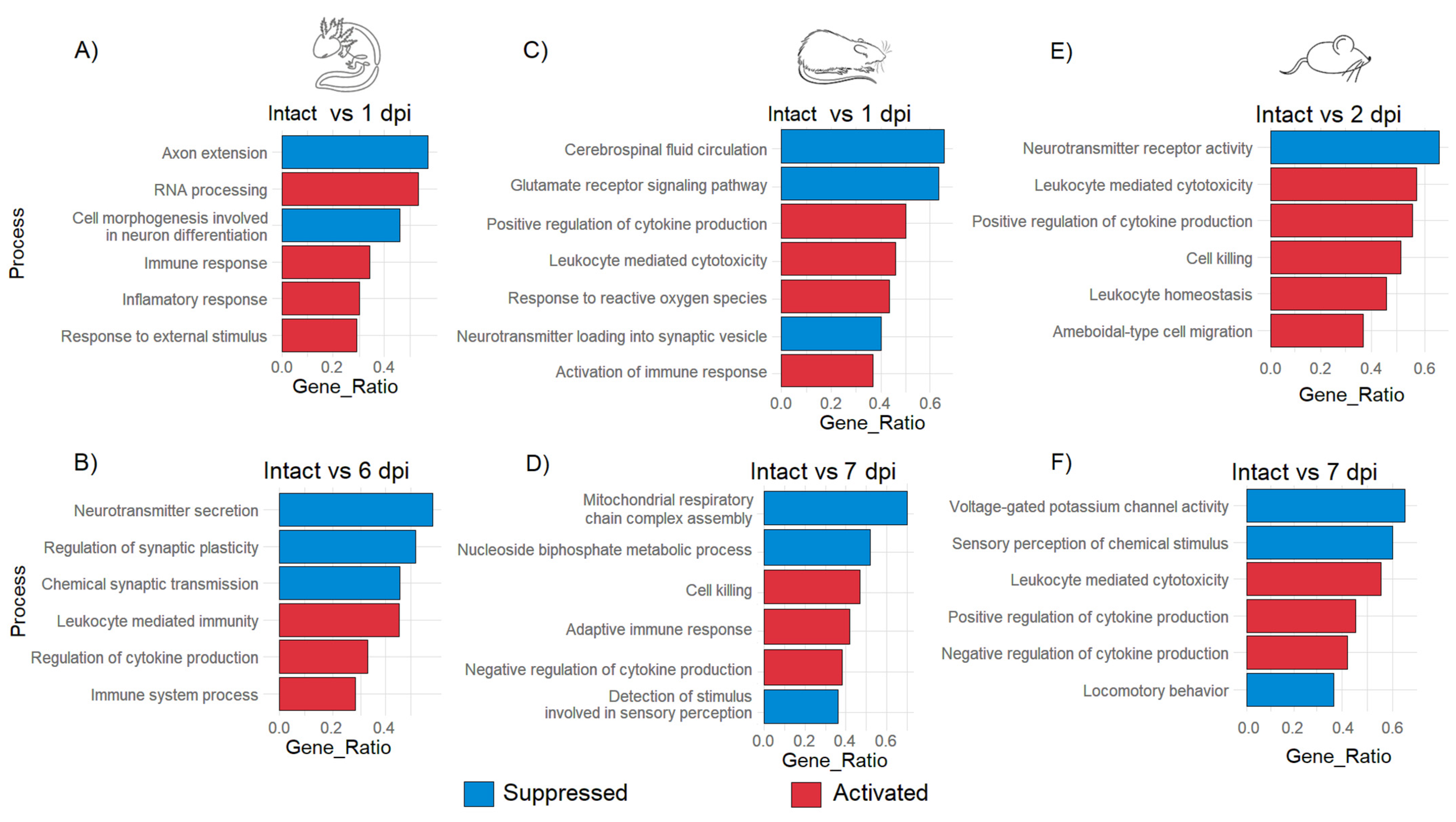
GO analysis was performed to detect the most enriched biological processes according to DEG at each timepoint after injury. After 1 dpi in the axolotl, it was observed that repair programs begin soon after the traumatic damage as indicated for the GO annotations “immune response” and “response to external stimulus” that were activated; these biological processes at this timepoint were also accompanied along with the annotation of “RNA processing”, which indicates again that a strong response at the level of gene expression is triggered after primary injury; meanwhile, “axon extension” and “cell morphogenesis involved in neuron differentiation” were suppressed at 1 dpi, suggesting an early activation of stem cells in the injury site as was observed in the hierarchical cluster analysis (Figure 4A and Figure S4A).
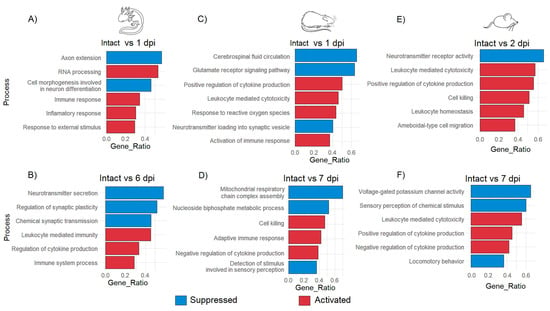
Figure 4.
GO enrichment analysis of biological processes in axolotl, rat, and mouse during acute and sub-acute SCI. Enriched biological processes for axolotl at 1 dpi (A) and 6 dpi (B); rat at 1 dpi (C) and 7 dpi (D); mouse at 2 dpi (E) and 7 dpi (F). The false discovery rate was estimated by BH correction. The cutoff for the q-value was 0.005.
Meanwhile, at 6 dpi, the suppression of the processes “neurotransmitter secretion” and “regulation of synaptic plasticity” was noticed in axolotls (Figure 4B). Regarding rat and mouse, it was detected that specific processes related to the acute immune response including “positive regulation of cytokine production”, “cell killing” and “leukocyte homeostasis” were predominant during the acute period of SCI, similar processes that were noticeable in the axolotl until the sub-acute phase. This finding was consistent with a previous study where it was shown that after a week, the injured spinal cord of the axolotl strongly upregulates genes related to the acute immune response, at the time in which visible regenerative tissue has been formed in the wound area [45].
Processes associated with neural functions like “cerebrospinal fluid circulation” or “neurotransmitter receptor activity” were immediately suppressed in rodents during the acute phase (Figure 4C,E); importantly, processes associated with the acute immune response were still observed during the sub-acute SCI in rodents. Also, the observed suppression of biological processes like “mitochondrial respiratory chain complex assembly” (Figure 4D) could suggest that induced oxidative stress is caused by the primary injury and that the acute immune response can increase the damage to the spinal cord in mammals instead of alleviating it. Moreover, the alteration of neurological functions was also noted in rat and mouse in the sub-acute phase since activities like “detection of stimulus involved in sensory perception”, “sensory perception of chemical stimulus”, or even “locomotory behavior” were suppressed (Figure 4D,F and Figure S4B,C). Therefore, these results indicate that the strong immune response triggered immediately by traumatic SCI in rodents might inhibit neurorepair mechanisms, and create a noxious environment that leads to the loss of the neurological functions associated with SCI starting at the sub-acute stage, while in axolotls a lax immune response from early phases of SCI could facilitate the triggering of molecular programs related to the repair and subsequent regeneration of the spinal cord.
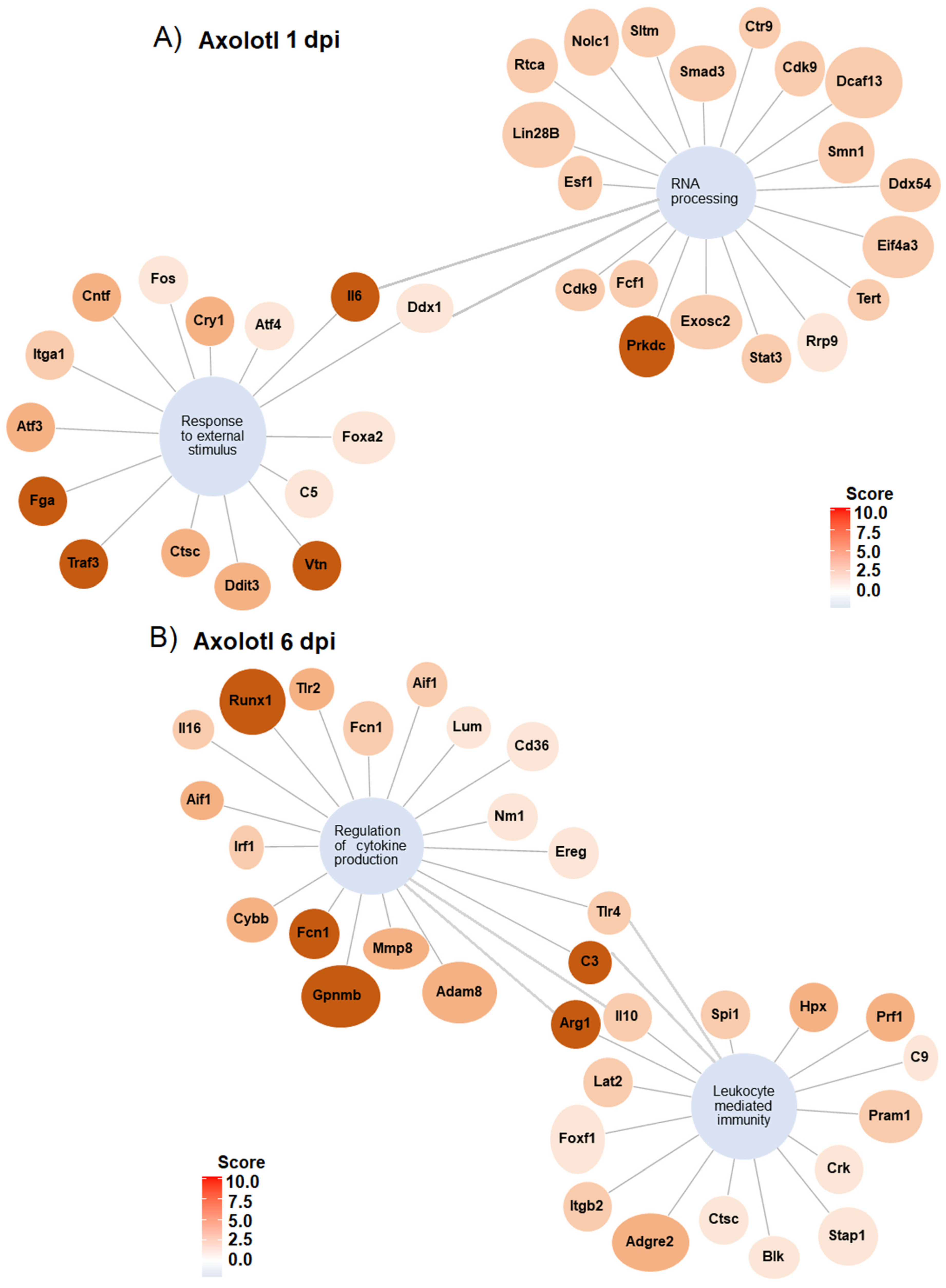
To gain insights into the networks that lead to the biological processes regulated in response to SCI in axolotl and rodents, gene regulatory networks were plotted to identify specific genes that were associated with the mark-enriched GO terms. Genes related to “response external stimulus” and “RNA processing” in axolotl at 1 dpi are connected through Il6 and Ddx1. Furthermore, detected genes for these GO terms include a few genes related to neurodevelopment and transcription/translation regulation (Figure 5A); for example, the gene Atf4 was upregulated along the GO term “Response to external stimulus”, whose activity has been related to cortical neurogenesis in mammals [46]. Likewise, Foxa2 is a gene associated with neuronal differentiation in mammals, particularly to dopaminergic differentiation during midbrain development. In addition, the genes Vtn, Atf3, and Cntf identified in our analysis are also related to the regulation of neurogenesis [47,48,49]; we propose to study all these genes to determine their potential role in spinal cord recovery in mammals. In addition, we found that the gene Tert, related to stem cells [50], was upregulated along the GO term “RNA processing”.
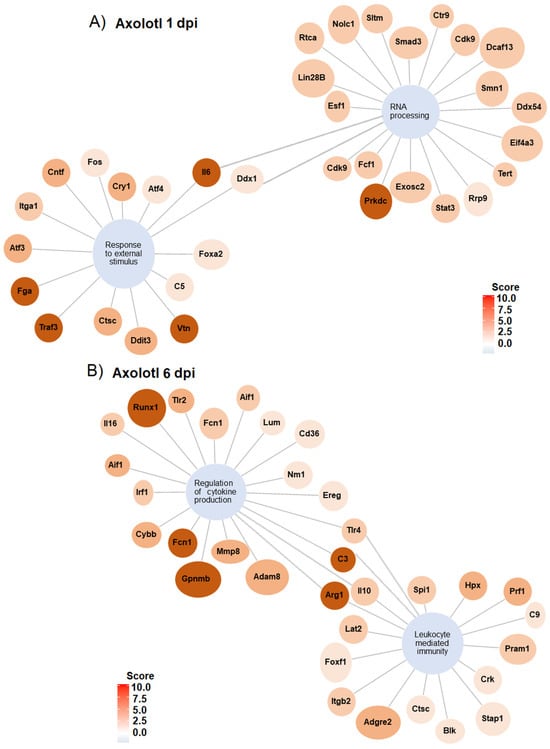
Figure 5.
Gene regulatory networks for GO terms in axolotl with SCI at 1 and 6 dpi. (A) Gene networks for GO enriched terms “RNA processing” and “response to external stimulus” at 1 dpi. (B) Gene network for GO enriched terms “regulation of cytokine production” and “leukocyte mediated immunity” at 6 dpi.
The gene regulatory network at 6 dpi shows GO terms related to the immunological response after an injury during the sub-acute phase in axolotl; among the most upregulated genes for these terms are included Runx1, Fcn1, C3, Arg1, and Gpnmb (Figure 5B). Whereas the gene networks for representative GO terms in rodents during the acute and sub-acute periods of SCI show a strong regulation of genes associated with the immune response, including the upregulation of genes associated with cell clearance, inflammation, and mobilization of immune cells (Figures S5 and S6).
Importantly, we identified that downregulation of the genes Cox14, Uqcc1, Coa8, Dmac1, or Bcs1l were related to the repression of the GO term “mitochondrial respiratory chain complex assembly” in rat, while the downregulated genes associated with “locomotory behavior” in mice were Lmx1b, Grin1, Gad1, Nrg1, Chat, Dscam, Abat, Pak6, Fgf12 and Hoxd9. Thus, it would be truly important to study whether the activation of any of these genes could have any positive effect on the functional recovery of mammals with SCI.
The importance of the immune system in regulating pro-regenerative responses in axolotl has been well documented during limb regeneration, where injury elicits an immediate wound healing response while in mammals promotes a strong innate immune response that triggers, in later phases of the injury, a fibrotic scarring program that limits the ability of the damaged tissue to regenerate [51]; additionally, several genes that contribute to the regeneration of amputated limbs in axolotls have been already identified, providing information about how regeneration could be elicited in species with limited tissue repair [13,52]. Therefore, the identification of genes and molecular programs that are specifically regulated in the injured spinal cord of the axolotl (and that are also conserved in mammals) could provide information for the study of potential regenerative therapies in humans affected by this condition.
The results obtained in our analysis show consistency with what was previously reported since the datasets used in this study were derived from the injured core tissue obtained from typical models of SCI induced in animals [9]; however, it is important to indicate that the nature of the sample obtained for analysis may influence the results. For example, it has been reported in rodents that the histopathology and gene expression profile in the injured spinal cord are not similar in the central zone than in the adjacent zone of injury [53,54]. Then, it would be interesting to study the changes in gene expression in the tissue adjacent to the lesion in the spinal cord of the axolotl and compare it with rodent models. On the other hand, in this study, the datasets used were obtained from adult animals, both neotenic axolotls and rodents, but some reports indicate that the regeneration capacity of axolotls decreases as age progresses [55]; thus, it would be important to analyze whether there are differences in the gene expression of the injured spinal cord between young and adult individuals. Lastly, another important consideration is that the expression patterns at the protein level may be different from what is observed at the transcript level, so it is important to study and compare the proteomics of SCI between axolotls and rodents to obtain a broader view of the regenerative mechanisms.
4. Conclusions
Through RNA-seq data analysis, we identified the molecular similarities and differences between rodents and axolotls after SCI. Immune-related pathways are upregulated in all studied species; however, a stronger immune response is noticeable at the level of gene expression in rodents in both acute and sub-acute periods of SCI. Differences were observed in genes related to self-renewal of stem cells (e.g., Sox2 and Tert), which were upregulated during the first days of SCI in axolotls, with the same scenario for genes related to neurodevelopment, including Foxa2, Vtn, Atf3, Atf6, and Cntf. In axolotls, at 1 dpi, the genes classified as “response external stimulus” and “RNA processing” were linked through Il6 and Ddx1. Although genes and molecular functions associated with neuron communication are suppressed in axolotl during SCI, it is only in rodents that we noticed the downregulation of processes related to sensory and motor functions. Thus, it is important to study in mammals the role of genes that are regulated in axolotls with SCI, to provide clues for the development of regenerative therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14122189/s1.
Author Contributions
J.C.G.-O. designed this project, performed the bioinformatic analysis, and contributed to writing the manuscript. I.E.-A. contributed to writing the manuscript. I.V. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONACYT 265793 (I.V).
Data Availability Statement
All data used in this study are publicly available at https://www.ncbi.nlm.nih.gov/bioproject/ (accessed on 3 March 2023) under the following accession numbers: PRJNA378982, PRJNA760277 and PRJNA193596.
Acknowledgments
We would like to thank Augusto César Poot Hernández and Carlos Alberto Peralta Álvarez from Unidad de Bioinformática y Manejo de la Información of Instituto de Fisiología Celular-UNAM for providing the computational resources where the analyzes present in this study were performed. We also thank Raquel Cuevas-Diaz Duran from Instituto Tecnológico de Monterrey for suggesting software to perform the analysis. González-Orozco thanks Dirección General de Asuntos del Personal Académico (DGAPA-UNAM) for his postdoctoral fellowship. We thank PAPIIT IN219122 for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Kooijmans, H.; Post, M.W.M.; Stam, H.J.; van der Woude, L.H.V.; Spijkerman, D.C.M.; Snoek, G.J.; Bongers-Janssen, H.M.H.; van Koppenhagen, C.F.; Twisk, J.W.; Bussmann, J.B.J. Effectiveness of a Self-Management Intervention to Promote an Active Lifestyle in Persons With Long-Term Spinal Cord Injury: The HABITS Randomized Clinical Trial. Neurorehabilit. Neural Repair 2017, 31, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Müller-Jensen, L.; Ploner, C.J.; Kroneberg, D.; Schmidt, W.U. Clinical Presentation and Causes of Non-Traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front. Neurol. 2021, 12, 701927. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Fauss, G.N.K.; Hudson, K.E.; Grau, J.W. Role of Descending Serotonergic Fibers in the Development of Pathophysiology after Spinal Cord Injury (SCI): Contribution to Chronic Pain, Spasticity, and Autonomic Dysreflexia. Biology 2022, 11, 234. [Google Scholar] [CrossRef]
- Wang, T.Y.; Park, C.; Zhang, H.; Rahimpour, S.; Murphy, K.R.; Goodwin, C.R.; Karikari, I.O.; Than, K.D.; Shaffrey, C.I.; Foster, N.; et al. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Front. Surg. 2021, 8, 698736. [Google Scholar] [CrossRef]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Safdarian, M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; et al. Animal Models of Spinal Cord Injury: A Systematic Review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Amamoto, R.; Huerta, V.G.L.; Takahashi, E.; Dai, G.; Grant, A.K.; Fu, Z.; Arlotta, P. Adult axolotls can regenerate original neuronal diversity in response to brain injury. eLife 2016, 5, e13998. [Google Scholar] [CrossRef] [PubMed]
- Mchedlishvili, L.; Epperlein, H.H.; Telzerow, A.; Tanaka, E.M. A Clonal Analysis of Neural Progenitors during Axolotl Spinal Cord Regeneration Reveals Evidence for Both Spatially Restricted and Multipotent Progenitors. Development 2007, 134, 2083–2093. [Google Scholar] [CrossRef]
- Haas, B.J.; Whited, J.L. Advances in Decoding Axolotl Limb Regeneration. Trends Genet. 2017, 33, 553–565. [Google Scholar] [CrossRef]
- Sibai, M.; Parlayan, C.; Tuğlu, P.; Öztürk, G.; Demircan, T. Integrative Analysis of Axolotl Gene Expression Data from Regenerative and Wound Healing Limb Tissues. Sci. Rep. 2019, 9, 20280. [Google Scholar] [CrossRef]
- Leinonen, R.; Akhtar, R.; Birney, E.; Bower, L.; Cerdeno-Tarraga, A.; Cheng, Y.; Cleland, I.; Faruque, N.; Goodgame, N.; Gibson, R.; et al. The European Nucleotide Archive. Nucleic Acids Res. 2011, 39, D28–D31. [Google Scholar] [CrossRef] [PubMed]
- Nowoshilow, S.; Schloissnig, S.; Fei, J.-F.; Dahl, A.; Pang, A.W.C.; Pippel, M.; Winkler, S.; Hastie, A.R.; Young, G.; Roscito, J.G.; et al. The Axolotl Genome and the Evolution of Key Tissue Formation Regulators. Nature 2018, 554, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhu, Z.; Liang, Z.; Zuo, X.; Ju, C.; Song, Z.; Li, X.; Hu, X.; Wang, Z. Photobiomodulation Promotes Repair Following Spinal Cord Injury by Regulating the Transformation of A1/A2 Reactive Astrocytes. Front. Neurosci. 2021, 15, 768262. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Deng, S.; Lu, H.; Zheng, Y.; Yang, G.; Kim, D.; Cao, Q.; Wu, J.Q. RNA-Seq Characterization of Spinal Cord Injury Transcriptome in Acute/Subacute Phases: A Resource for Understanding the Pathology at the Systems Level. PLoS ONE 2013, 8, e72567. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Schloissnig, S.; Kawaguchi, A.; Nowoshilow, S.; Falcon, F.; Otsuki, L.; Tardivo, P.; Timoshevskaya, N.; Keinath, M.C.; Smith, J.J.; Voss, S.R.; et al. The Giant Axolotl Genome Uncovers the Evolution, Scaling, and Transcriptional Control of Complex Gene Loci. Proc. Natl. Acad. Sci. USA 2021, 118, e2017176118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R Package Rsubread Is Easier, Faster, Cheaper and Better for Alignment and Quantification of RNA Sequencing Reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Saremi, B.; Gusmag, F.; Distl, O.; Schaarschmidt, F.; Metzger, J.; Becker, S.; Jung, K. A Comparison of Strategies for Generating Artificial Replicates in RNA-Seq Experiments. Sci. Rep. 2022, 12, 7170. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lv, J.; Huang, Y.-F.; Hao, D.-J.; Liu, J.-J. Bioinformatics analyses of differentially expressed genes associated with spinal cord injury: A microarray-based analysis in a mouse model. Neural Regen. Res. 2019, 14, 1262. [Google Scholar] [PubMed]
- Sabin, K.Z.; Jiang, P.; Gearhart, M.D.; Stewart, R.; Echeverri, K. AP-1cFos/JunB/miR-200a Regulate the pro-Regenerative Glial Cell Response during Axolotl Spinal Cord Regeneration. Commun. Biol. 2019, 2, 91. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-L.; Zhang, N.; Xie, X.-M.; Chen, Y.-J.; Wang, R.; Shen, L.; Zhou, J.-S.; Hu, J.-G.; Lü, H.-Z. Transcriptome Profile of Rat Genes in Injured Spinal Cord at Different Stages by RNA-Sequencing. BMC Genom. 2017, 18, 173. [Google Scholar] [CrossRef]
- Seymour, T.; Twigger, A.-J.; Kakulas, F. Pluripotency Genes and Their Functions in the Normal and Aberrant Breast and Brain. Int. J. Mol. Sci. 2015, 16, 27288–27301. [Google Scholar] [CrossRef]
- Hillary, R.F.; FitzGerald, U. A Lifetime of Stress: ATF6 in Development and Homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef]
- Haenzi, B.; Moon, L.D. The Function of FGFR1 Signalling in the Spinal Cord: Therapeutic Approaches Using FGFR1 Ligands after Spinal Cord Injury. Neural Plast. 2017, 2017, 2740768. [Google Scholar] [CrossRef]
- Tica, J.; Didangelos, A. Comparative Transcriptomics of Rat and Axolotl After Spinal Cord Injury Dissects Differences and Similarities in Inflammatory and Matrix Remodeling Gene Expression Patterns. Front. Neurosci. 2018, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflammation 2021, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, M.; Zeitler, C.; Gobrecht, P.; Andreadaki, A.; Gisselmann, G.; Fischer, D. Transneuronal Delivery of Hyper-Interleukin-6 Enables Functional Recovery after Severe Spinal Cord Injury in Mice. Nat. Commun. 2021, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, A.; Tanaka, E.M.; Fei, J.-F. Salamander Spinal Cord Regeneration: The Ultimate Positive Control in Vertebrate Spinal Cord Regeneration. Dev. Biol. 2017, 432, 63–71. [Google Scholar] [CrossRef]
- Randal Voss, S.; Murrugarra, D.; Jensen, T.B.; Monaghan, J.R. Transcriptional Correlates of Proximal-Distal Identify and Regeneration Timing in Axolotl Limbs. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 208, 53–63. [Google Scholar] [CrossRef]
- Shechter, R.; Raposo, C.; London, A.; Sagi, I.; Schwartz, M. The Glial Scar-Monocyte Interplay: A Pivotal Resolution Phase in Spinal Cord Repair. PLoS ONE 2011, 6, e27969. [Google Scholar] [CrossRef]
- Ardeljan, D.; Wang, X.; Oghbaie, M.; Taylor, M.S.; Husband, D.; Deshpande, V.; Steranka, J.P.; Gorbounov, M.; Yang, W.R.; Sie, B.; et al. LINE-1 ORF2p expression is nearly imperceptible in human cancers. Mob. DNA 2019, 11, 1. [Google Scholar] [CrossRef]
- Kamizato, K.; Sato, S.; Shil, S.K.; Umaru, B.A.; Kagawa, Y.; Yamamoto, Y.; Ogata, M.; Yasumoto, Y.; Okuyama, Y.; Ishii, N.; et al. The Role of Fatty Acid Binding Protein 7 in Spinal Cord Astrocytes in a Mouse Model of Experimental Autoimmune Encephalomyelitis. Neuroscience 2019, 409, 120–129. [Google Scholar] [CrossRef]
- Arrildt, K.T.; Joseph, S.B.; Swanstrom, R. The HIV-1 env protein: A coat of many colors. Curr. HIV/AIDS Rep. 2012, 9, 52–63. [Google Scholar] [CrossRef]
- Pineau, I.; Sun, L.; Bastien, D.; Lacroix, S. Astrocytes Initiate Inflammation in the Injured Mouse Spinal Cord by Promoting the Entry of Neutrophils and Inflammatory Monocytes in an IL-1 Receptor/MyD88-Dependent Fashion. Brain Behav. Immun. 2010, 24, 540–553. [Google Scholar] [CrossRef]
- Hannila, S.S. Secretory Leukocyte Protease Inhibitor (SLPI): Emerging Roles in CNS Trauma and Repair. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2015, 21, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, N.; Bouhy, D.; Yang, J.; López-Vales, R.; Haber, M.; Thuraisingam, T.; He, G.; Radzioch, D.; Ding, A.; David, S. Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury. Brain 2010, 133, 126–138. [Google Scholar] [CrossRef]
- Urso, M.L.; Chen, Y.; Scrimgeour, A.G.; Lee, P.C.; Lee, K.F.; Clarkson, P.M. Alterations in mRNA Expression and Protein Products Following Spinal Cord Injury in Humans. J. Physiol. 2007, 579, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.A.; Rice, T.K.; Nuttall, R.K.; Edwards, D.R.; Zekki, H.; Rivest, S.; Yong, V.W. An Adverse Role for Matrix Metalloproteinase 12 after Spinal Cord Injury in Mice. J. Neurosci. 2003, 23, 10107–10115. [Google Scholar] [CrossRef] [PubMed]
- Demircan, T. Dissecting the Molecular Signature of Spinal Cord Regeneration in the Axolotl Model. Cureus 2020, 12, e7014. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.L.; Ge, X.; Xie, Z.; Zhou, Y.; Tsai, L.H. Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J. Biol. Chem. 2010, 285, 33324–33337. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.-P.; Wang, G.; Han, S.-S.; Jin, Y.; Hu, Y.-X.; He, M.-Y.; Brand-Saberi, B.; Yang, X.; Liu, G.-S. CNTF and Nrf2 Are Coordinately Involved in Regulating Self-Renewal and Differentiation of Neural Stem Cell during Embryonic Development. iScience 2019, 19, 303–315. [Google Scholar] [CrossRef]
- Petrović, A.; Ban, J.; Ivaničić, M.; Tomljanović, I.; Mladinic, M. The Role of ATF3 in Neuronal Differentiation and Development of Neuronal Networks in Opossum Postnatal Cortical Cultures. Int. J. Mol. Sci. 2022, 23, 4964. [Google Scholar] [CrossRef]
- Ruzha, Y.; Ni, J.; Quan, Z.; Li, H.; Qing, H. Role of Vitronectin and Its Receptors in Neuronal Function and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 12387. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, D. Telomerase Reverse Transcriptase (TERT) in Action: Cross-Talking with Epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Tsai, S.L.; Baselga-Garriga, C.; Melton, D.A. Blastemal Progenitors Modulate Immune Signaling during Early Limb Regeneration. Development 2019, 146, dev169128. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.A.; Wells, K.M.; McCusker, C.D. Advancements to the Axolotl Model for Regeneration and Aging. Gerontology 2020, 66, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef]
- Sousounis, K.; Athippozhy, A.T.; Voss, S.R.; Tsonis, P.A. Plasticity for axolotl lens regeneration is associated with age-related changes in gene expression. Regeneration 2014, 1, 47–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).