Expression of Long Noncoding RNAs in Fibroblasts from Mucopolysaccharidosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lines of Human Fibroblasts and Cell Cultures
2.2. Transcriptomic Analyses
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parenti, G.; Andria, G.; Ballabio, A. Lysosomal storage diseases: From pathophysiology to therapy. Annu. Rev. Med. 2015, 66, 471–486. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, M.T.; Gonçalves Pereira, V.; do Nascimento, C.C.; D’Almeida, V. The Underexploited Role of Non-Coding RNAs in Lysosomal Storage Diseases. Front. Endocrinol. 2016, 7, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeze, H.; Eklund, E.; Ng, B.; Patterson, M. Neurological aspects of human glycosylation disorders. Annu. Rev. Neurosci. 2015, 38, 105–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Węgrzyn, G.; Pierzynowska, K.; Pavone, L.M. Editorial: Molecular Aspects of Mucopolysaccharidoses. Front. Mol. Biosci. 2022, 9, 874267. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Wolski, J.; Gaffke, L.; Cyske, Z.; Pierzynowska, K.; Węgrzyn, G. Misdiagnosis in mucopolysaccharidoses. J. Appl. Genet. 2022, 63, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Lavery, C.; Giugliani, R.; Harmatz, P.; Scarpa, M.; Węgrzyn, G.; Orii, T. Mucopolysaccharidoses Update; Nova Science Publisher: New York, NY, USA, 2018; ISBN 978-1-53613-986-0. [Google Scholar]
- Cyske, Z.; Anikiej-Wiczenbach, P.; Wisniewska, K.; Gaffke, L.; Pierzynowska, K.; Mański, A.; Wegrzyn, G. Sanfilippo Syndrome: Optimizing Care with a Multidisciplinary Approach. J. Multidiscip. Healthc. 2022, 15, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- McBride, K.L.; Flanigan, K.M. Update in the Mucopolysaccharidoses. Semin. Pediatr. Neurol. 2021, 37, 100874. [Google Scholar] [CrossRef]
- Penon-Portmann, M.; Blair, D.R.; Harmatz, P. Current and new therapies for mucopolysaccharidoses. Pediatr. Neonatol. 2022. [Google Scholar] [CrossRef]
- Dornelles, A.; Artigalás, O.; da Silva, A.; Ardila, D.; Alegra, T.; Pereira, T.; Vairo, F.; Schwartz, I. Efficacy and safety of intravenous laronidase for mucopolysaccharidosis type I: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0184065. [Google Scholar] [CrossRef] [Green Version]
- Aldenhoven, M.; van den Broek, B.; Wynn, R.; O’Meara, A.; Veys, P.; Rovelli, A.; Jones, S.; Parini, R.; van Hasselt, P.; Renard, M.; et al. Quality of life of Hurler syndrome patients after successful hematopoietic stem cell transplantation. Blood Adv. 2017, 1, 2236–2242. [Google Scholar] [CrossRef]
- Parente, M.; Rozen, R.; Seeholzer, S.; Wolfe, J. Integrated analysis of proteome and transcriptome changes in the mucopolysaccharidosis type VII mouse hippocampus. Mol. Genet. Metab. 2016, 118, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessitore, A.; Pirozzim, M.; Auricchio, A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics 2009, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierzynowska, K.; Gaffke, L.; Podlacha, M.; Brokowska, J.; Węgrzyn, G. Mucopolysaccharidosis and Autophagy: Controversies on the Contribution of the Process to the Pathogenesis and Possible Therapeutic Applications. Neuromol. Med. 2020, 22, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffke, L.; Pierzynowska, K.; Podlacha, M.; Brokowska, J.; Węgrzyn, G. Changes in cellular processes occurring in mucopolysaccharidoses as underestimated pathomechanisms of these diseases. Cell Biol. Int. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Cyske, Z.; Gaffke, L.; Pierzynowska, K.; Węgrzyn, G. Complex Changes in the Efficiency of the Expression of Many Genes in Monogenic Diseases, Mucopolysaccharidoses, May Arise from Significant Disturbances in the Levels of Factors Involved in the Gene Expression Regulation Processes. Genes 2022, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Rintz, E.; Gaffke, L.; Podlacha, M.; Brokowska, J.; Cyske, Z.; Węgrzyn, G.; Pierzynowska, K. Transcriptomic Changes Related to Cellular Processes with Particular Emphasis on Cell Activation in Lysosomal Storage Diseases from the Group of Mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 21, 3194. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Jankowska, E.; Rintz, E.; Witkowska, J.; Wieczerzak, E.; Podlacha, M.; Węgrzyn, G. Proteasome Composition and Activity Changes in Cultured Fibroblasts Derived from Mucopolysaccharidoses Patients and Their Modulation by Genistein. Front. Cell Dev. Biol. 2020, 8, 540726. [Google Scholar] [CrossRef]
- Brokowska, J.; Pierzynowska, K.; Gaffke, L.; Rintz, E.; Węgrzyn, G. Expression of genes involved in apoptosis is dysregulated in mucopolysaccharidoses as revealed by pilot transcriptomic analyses. Cell Biol. Int. 2021, 45, 549–557. [Google Scholar] [CrossRef]
- Gaffke, L.; Pierzynowska, K.; Rintz, E.; Cyske, Z.; Giecewicz, I.; Węgrzyn, G. Gene Expression-Related Changes in Morphologies of Organelles and Cellular Component Organization in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021, 22, 2766. [Google Scholar] [CrossRef]
- Gaffke, L.; Szczudło, Z.; Podlacha, M.; Cyske, Z.; Rintz, E.; Mantej, J.; Krzelowska, K.; Węgrzyn, G.; Pierzynowska, K. Impaired ion homeostasis as a possible associate factor in mucopolysaccharidosis pathogenesis: Transcriptomic, cellular and animal studies. Metab. Brain Dis. 2022, 37, 299–310. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Żabińska, M.; Gaffke, L.; Cyske, Z.; Węgrzyn, G. Changes in expression of signal transduction-related genes, and formation of aggregates of GPER1 and OXTR receptors in mucopolysaccharidosis cells. Eur. J. Cell Biol. 2022, 101, 151232. [Google Scholar] [CrossRef] [PubMed]
- Brokowska, J.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Węgrzyn, G. Cell cycle disturbances in mucopolysaccharidoses: Transcriptomic and experimental studies on cellular models. Exp. Biol. Med. 2022, 247, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Lekka, E.; Hall, J. Noncoding RNAs in disease. FEBS Lett. 2018, 592, 2884–2900. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Fabbri, M.; Calin, G. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, K.; Kjems, J.; Hansen, T. Circular RNAs: Identification, biogenesis and function. Biochim. Biophys. Acta 2016, 1859, 163–168. [Google Scholar] [CrossRef]
- Yang, J.; Rastetter, R.; Wilhelm, D. Non-coding RNAs: An Introduction. Adv. Exp. Med. Biol. 2016, 886, 13–32. [Google Scholar]
- van Rooij, E.; Kauppinen, S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014, 6, 851–864. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- St Laurent, G.; Vyatkin, Y.; Antonets, D.; Ri, M.; Qi, Y.; Saik, O.; Shtokalo, D.; De Hoon, M.J.; Kawaji, H.; Itoh, M.; et al. Functional annotation of the vlinc class of non-coding RNAs using systems biology approach. Nucleic Acids Res. 2016, 44, 3233–3252. [Google Scholar] [CrossRef] [PubMed]
- Sauvageau, M. Diverging RNPs: Toward Understanding lncRNA-Protein Interactions and Functions. Adv. Exp. Med. Biol. 2019, 1203, 285–312. [Google Scholar] [PubMed]
- Chattopadhyay, P.; Mishra, P.; Mehta, P.; Soni, J.; Gupta, R.; Tarai, B.; Pandey, R. Transcriptomic study reveals lncRNA-mediated downregulation of innate immune and inflammatory response in the SARS-CoV-2 vaccination breakthrough infections. Front. Immunol. 2022, 13, 1035111. [Google Scholar] [CrossRef] [PubMed]
- Cortini, F.; Chiara, F.; Villa, C. Emerging roles of long non-coding RNAs in the pathogenesis of Alzheimer’s disease. Aging Res. Rev. 2019, 50, 19–26. [Google Scholar] [CrossRef]
- Elkouris, M.; Kouroupi, G.; Vourvoukelis, A.; Papagiannakis, N.; Kaltezioti, V.; Matsas, R.; Stefanis, L.; Xilouri, S.; Politis, P. Long Non-coding RNAs Associated with Neurodegeneration-Linked Genes Are Reduced in Parkinson’s Disease Patients. Front. Cell Neurosci. 2019, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R. Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol. Dis. 2012, 46, 245–254. [Google Scholar] [CrossRef]
- Riva, P.; Ratti, A.; Venturin, M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Curr. Alzheimer Res. 2016, 13, 1219–1231. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wu, W. Comprehensive landscape and future perspectives of long noncoding RNAs (lncRNAs) in colorectal cancer (CRC): Based on a bibliometric analysis. Noncoding RNA Res. 2022, 8, 33–52. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Wang, Y.; Wang, X.; Liu, N.; You, Y. Regulation of let-7 and its target oncogenes. Oncol. Lett. 2012, 3, 955–960. [Google Scholar] [CrossRef]
- Thakral, S.; Ghoshal, K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Curr. Gene Ther. 2015, 15, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Samra, M.; Srivastavam, K. Non-coding RNA and their potential role in cardiovascular diseases. Gene 2023, 851, 147011. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Duffield, J.S. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Sparber, P.; Filatova, A.; Khantemirova, M.; Skoblov, M. The role of long non-coding RNAs in the pathogenesis of hereditary diseases. BMC Med. Genom. 2019, 12 (Suppl. 2), 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsait, B.; Komurcu-Bayrak, E.; Levula, M.; Erginel-Unaltuna, N.; Kähönen, M.; Rai, M.; Lehtimäki, T.; Laaksonen, R. Niemann-Pick type C fibroblasts have a distinct microRNA profile related to lipid metabolism and certain cellular components. Biochem. Biophys. Res. Commun. 2010, 403, 316–321. [Google Scholar] [CrossRef]
- Arora, S.; Beaudry, C.; Bisanz, K.; Sima, C.; Kiefer, J.; Azorsa, D. A high-content RNAi-screening assay to identify modulators of cholesterol accumulation in Niemann-Pick type C cells. Assay Drug Dev. Technol. 2010, 8, 295–320. [Google Scholar] [CrossRef]
- Siebert, M.; Westbroek, W.; Chen, Y.; Moaven, N.; Li, Y.; Velayati, A.; Saraiva-Pereira, M.; Martin, S.; Sidransky, E. Identification of miRNAs that modulate glucocerebrosidase activity in Gaucher disease cells. RNA Biol. 2014, 11, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Ginns, E.; Mak, S.; Ko, N.; Karlgren, J.; Akbarian, S.; Chou, V.; Guo, Y.; Lim, A.; Samuelsson, S.; LaMarca, M.; et al. Neuroinflammation and α-synuclein accumulation in response to glucocerebrosidase deficiency are accompanied by synaptic dysfunction. Mol. Genet. Metab. 2014, 111, 152–162. [Google Scholar] [CrossRef]
- Matsui, M.; Corey, D. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.; Queiroz, M.; D’Almeida, V. Differential expression of microRNAs from miR-17 family in the cerebellum of mucopolysaccharidosis type I mice. Gene 2016, 595, 207–211. [Google Scholar] [CrossRef]
- Kreutz, F.; dos Santos Petry, F.; Camassola, M.; Schein, V.; Guma, F.; Nardi, N.; Trindade, V. Alterations of membrane lipids and in gene expression of ganglioside metabolism in different brain structures in a mouse model of mucopolysaccharidosis type I (MPS I). Gene 2013, 527, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Gaffke, L.; Pierzynowska, K.; Podlacha, M.; Hoinkis, D.; Rintz, E.; Brokowska, J.; Wegrzyn, G. Underestimated Aspect of Mucopolysaccharidosis Pathogenesis: Global Changes in Cellular Processes Revealed by Transcriptomic Studies. Int. J. Mol. Sci. 2020, 21, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.; Shen, Y.; Sun, C.; Hu, Q.; Jiang, L.; Du, Q. GAS5 attenuates the malignant progression of glioma stem-like cells by promoting E-cadherin. Cancer Gene Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, T.; Cheng, C.; Wang, J.; Wang, C.; Huang, H.; Li, Y. LncRNA GAS5 regulates the Wnt/β-catenin pathway through the miR-18a-5p/AXIN2/GSK3β axis to inhibit the proliferation and migration of bladder cancer cells. Carcinogenesis 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chun, R.F.; Lisse, T.S.; Garcia, A.J.; Xu, J.; Adams, J.S.; Hewison, M. Vitamin D and alternative splicing of RNA. J. Steroid Biochem. Mol. Biol. 2015, 148, 310–317. [Google Scholar] [CrossRef] [Green Version]
- McClure, J.J.; Palanisamy, V. Muscle-Specific FXR1 Isoforms in Squamous Cell Cancer. Trends Cancer 2019, 5, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Datta, C.; Truesdell, S.S.; Wu, K.Q.; Bukhari, S.I.A.; Ngue, H.; Buchanan, B.; Le Tonqueze, O.; Lee, S.; Kollu, S.; Granovetter, M.A.; et al. Ribosome changes reprogram translation for chemosurvival in G0 leukemic cells. Sci. Adv. 2022, 8, eabo1304. [Google Scholar] [CrossRef]
- Malik, A.M.; Barmada, S.J. Matrin 3 in neuromuscular disease: Physiology and pathophysiology. JCI Insight 2021, 6, e143948. [Google Scholar] [CrossRef]
- Salem, A.; Wilson, C.J.; Rutledge, B.S.; Dilliott, A.; Farhan, S.; Choy, W.Y.; Duennwald, M.L. Matrin3: Disorder and ALS Pathogenesis. Front. Mol. Biosci. 2022, 8, 794646. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, F.; Li, N.; Yuan, X.; Zhang, L.; Zhao, S.; Jin, L.; Qiu, Y. The long non-coding RNA MEG3 inhibits oral squamous cell carcinoma progression via GATA3. FEBS Open Bio 2023, 13, 195–208. [Google Scholar] [CrossRef]
- Yan, X.; Jia, H.; Zhao, J. LncRNA MEG3 attenuates the malignancy of retinoblastoma cells through inactivating PI3K /Akt/mTOR signaling pathway. Exp. Eye Res. 2022, 226, 109340. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Lye, Y.S.; Chen, Y.R. TAR DNA-binding protein 43 oligomers in physiology and pathology. IUBMB Life 2022, 74, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Brown, A.L.; Belmont, J.W.; Scheurer, M.E.; Arroyo, V.M.; Foster, K.L.; Kern, K.D.; Hudson, M.M.; Leisenring, W.M.; Okcu, M.F.; et al. Genetic variation in the body mass index of adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Cancer 2021, 127, 310–318. [Google Scholar] [CrossRef]
- Rajakumar, S.; Jamespaulraj, S.; Shah, Y.; Kejamurthy, P.; Jaganathan, M.K.; Mahalingam, G.; Devi, K.T.R. Long non-coding RNAs: An overview on miRNA sponging and its co-regulation in lung cancer. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
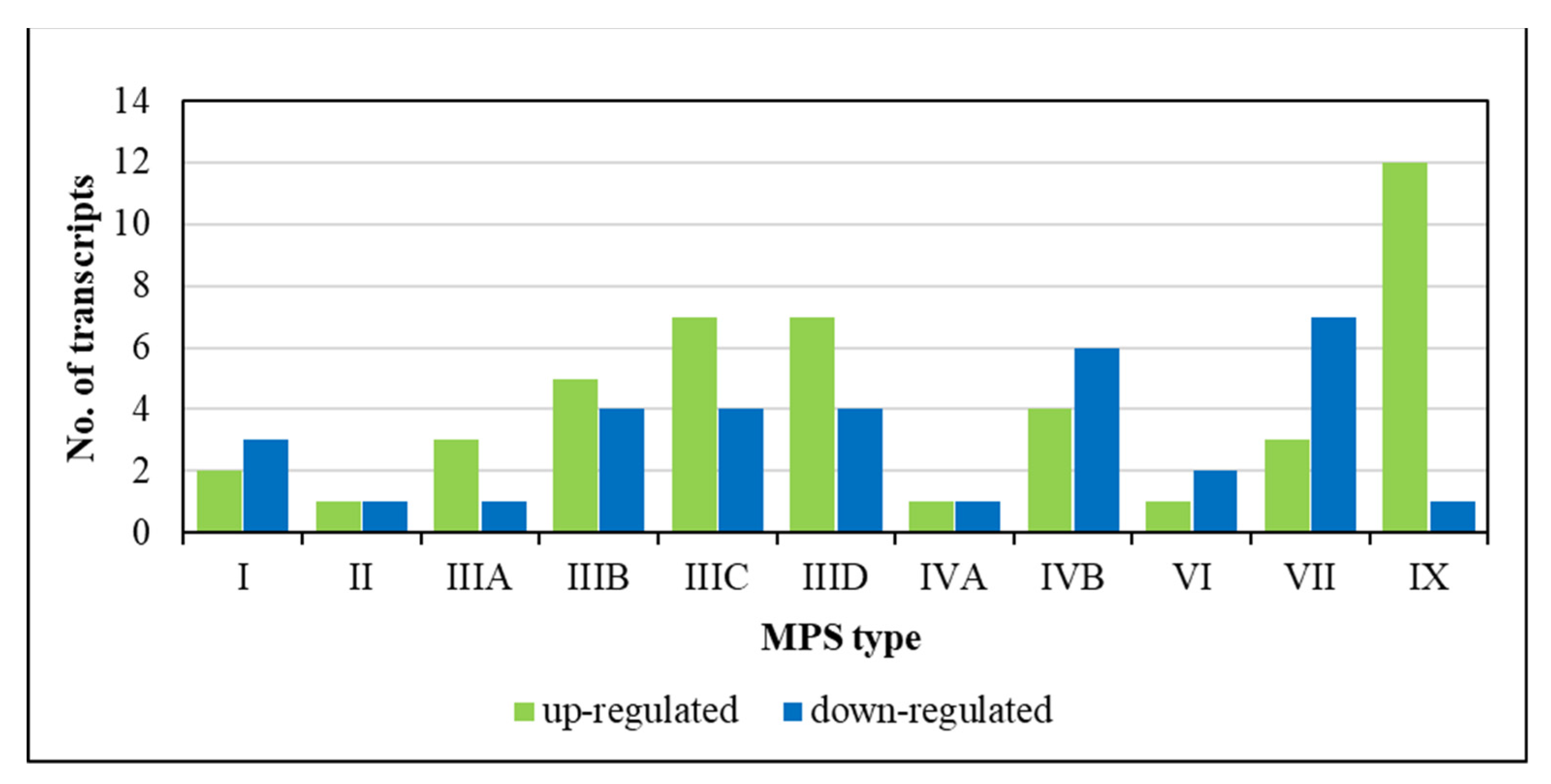
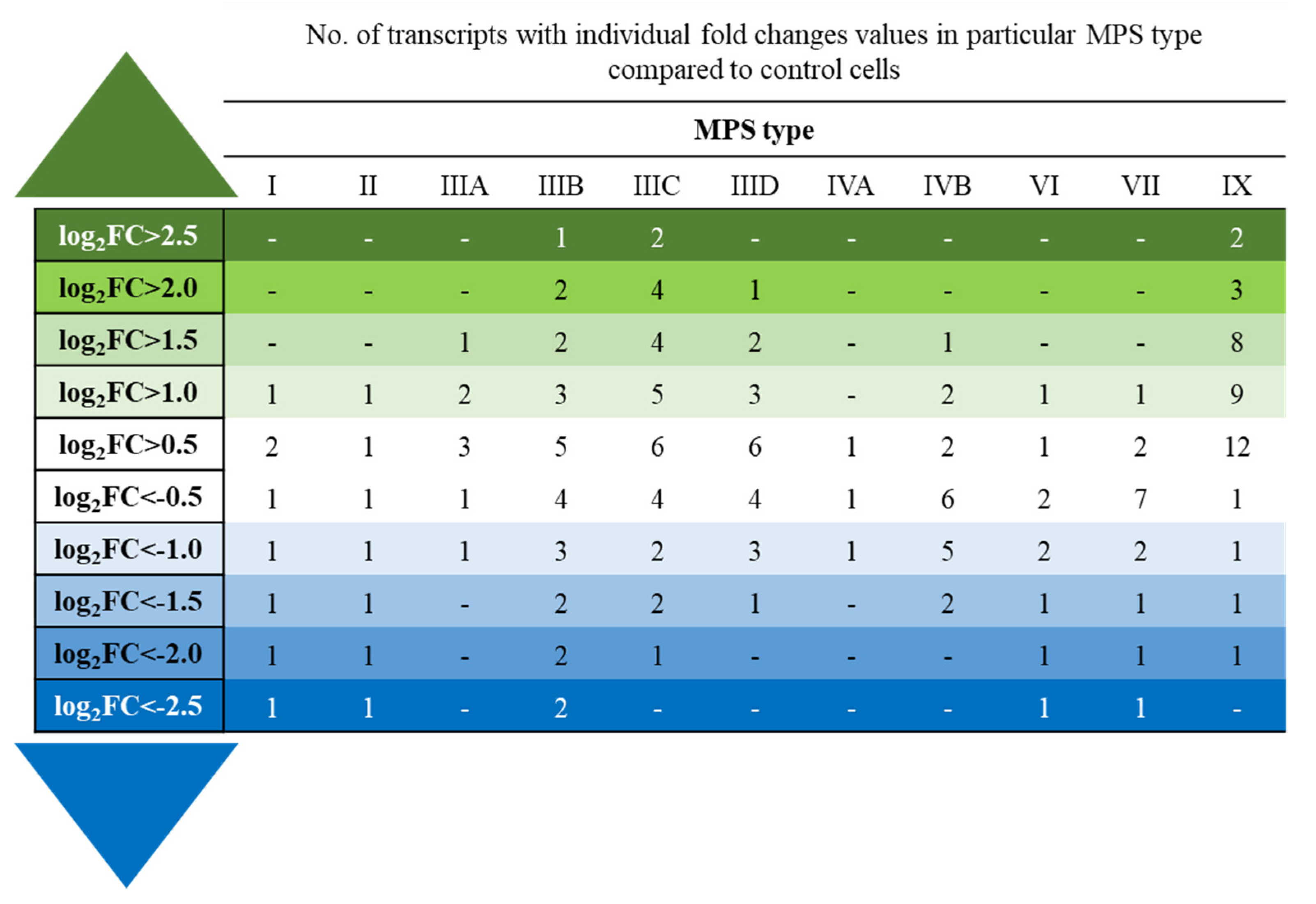

| MPS Type | Coriell Institute Catalogue Number | Sex of Patient | Age (Number of Years at the Time of Sample Collection) | Mutated Gene | Mutation(s) |
|---|---|---|---|---|---|
| MPS I | GM00798 | F | 1 | IDUA | p.Trp402Ter/p.Trp402Ter |
| MPS II | GM13203 | M | 3 | IDS | p.His70ProfsTer29/- |
| MPS IIIA | GM00879 | F | 3 | SGSH | p.Glu447Lys/p.Arg245His |
| MPS IIIB | GM00156 | M | 7 | NAGLU | p.Arg626Ter/p.Arg626Ter |
| MPS IIIC | GM05157 | M | 8 | HGSNAT | p.Gly262Arg/p.Arg509Asp |
| MPS IIID | GM05093 | M | 7 | GNS | p.Arg355Ter/p.Arg355Ter |
| MPS IVA | GM00593 | F | 7 | GALNS | p.Arg386Cys/p.Phe285Ter |
| MPS IVB | GM03251 | F | 4 | GLB1 | p.Trp273Leu/p.Trp509Cys |
| MPS VI | GM03722 | F | 3 | ARSB | Not determined |
| MPS VII | GM00121 | M | 3 | GUSB | p.Trp627Cys/p.Arg356Ter |
| MPS IX | GM17494 | F | 14 | HYAL1 | Not determined |
| Transcript | log2FC of Selected Transcripts’ Levels in at Least 4 MPS Types vs. HDFa Line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIB | IIIC | IIID | IVA | IVB | VI | VII | IX | |
| GAS5 | 0.83 | 1.16 | 1.26 | 0.93 | 2.98 | 0.11 | 0.36 | 0.69 | 1.35 | −0.02 | 0.96 |
| ILF3-DT | 0.63 | 0.55 | 0.33 | −0.34 | 0.79 | 0.68 | 0.77 | 0.31 | 0.81 | 0.01 | 0.82 |
| SNHG8 | 0.76 | −0.11 | 1.68 | −0.62 | 1.06 | 1.25 | 0.33 | −1.29 | −0.46 | −0.58 | 1.54 |
| LINC00667 | 1.10 | 0.73 | 0.73 | 0.80 | −0.08 | −0.40 | −0.49 | 1.10 | −0.10 | −1.14 | 1.23 |
| LINC02381 | −0.10 | −0.57 | −0.10 | −1.39 | −2.14 | −1.58 | −1.48 | −1.39 | −1.06 | 0.16 | −1.27 |
| Transcript | log2FC > 2.5 or <−2.5 of Selected Transcripts’ Levels in Particular MPS Type vs. HDFa Line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIB | IIIC | IIID | IVA | IVB | VI | VII | IX | |
| SNHG5 | ↓ | − | − | ↓ | − | − | − | − | − | ↓ | − |
| LINC01705 | ↓ | ↓ | − | − | − | − | − | − | ↓ | − | − |
| LINC00856 | − | − | − | ↓ | − | − | − | − | − | − | − |
| CYTOR | − | − | − | − | − | − | − | − | − | − | ↓ |
| MEG3 (t.1) | − | − | − | ↑ | ↑ | − | − | − | − | − | − |
| MEG3 (t.2) | − | − | − | − | − | − | − | − | − | − | ↑ |
| MEG3 (t.3) | − | − | − | − | − | − | − | − | − | − | ↑ |
| GAS5 | − | − | − | − | ↑ | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyske, Z.; Gaffke, L.; Pierzynowska, K.; Węgrzyn, G. Expression of Long Noncoding RNAs in Fibroblasts from Mucopolysaccharidosis Patients. Genes 2023, 14, 271. https://doi.org/10.3390/genes14020271
Cyske Z, Gaffke L, Pierzynowska K, Węgrzyn G. Expression of Long Noncoding RNAs in Fibroblasts from Mucopolysaccharidosis Patients. Genes. 2023; 14(2):271. https://doi.org/10.3390/genes14020271
Chicago/Turabian StyleCyske, Zuzanna, Lidia Gaffke, Karolina Pierzynowska, and Grzegorz Węgrzyn. 2023. "Expression of Long Noncoding RNAs in Fibroblasts from Mucopolysaccharidosis Patients" Genes 14, no. 2: 271. https://doi.org/10.3390/genes14020271







