Collagen Network Formation in In Vitro Models of Musculocontractural Ehlers–Danlos Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary Fibroblast Culture
2.3. Quantification of DS Chains
2.4. Preparation of Fibroblast-Embedded Collagen Gel
2.5. Transmission Electron Microscopy
2.6. Scanning Electron Microscopy
2.7. Mechanical Strength Measurement of Collagen Gel
2.8. Isolation of Decorin
2.9. Chondroitinase Digestion of Decorin
2.10. Decorin-Mediated Fibrillar Organization of Type I Collagen Gels
2.11. Statistical Analysis
3. Results
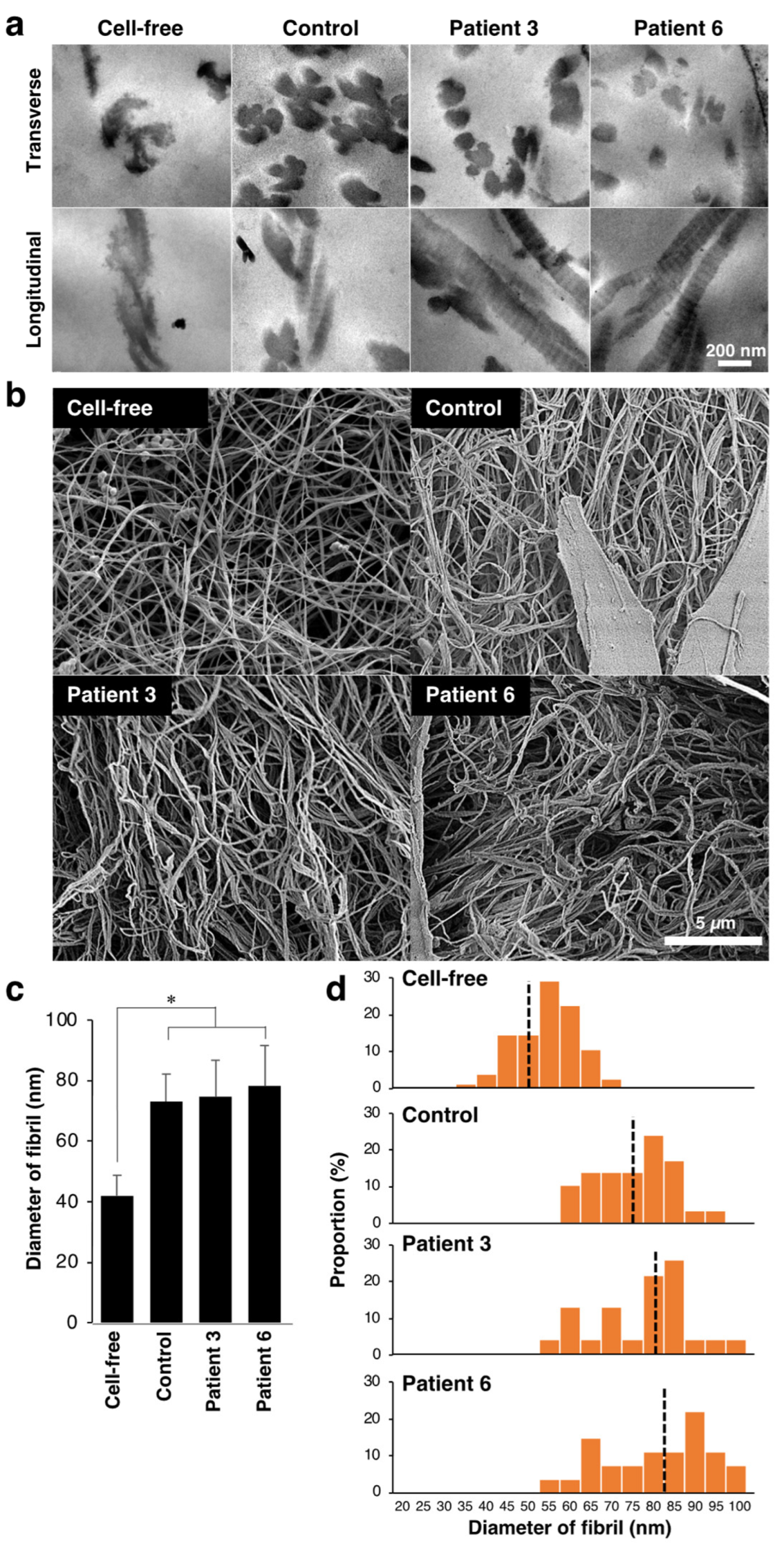
3.1. Effect of Fibroblasts from mcEDS-CHST14 Patients on Contraction, Fibrillar Organization, and Mechanics of Type I Collagen Gels
3.2. Effect of Decorin Isolated from Normal or Patient’s Fibroblasts on Type I Collagen Gel Properties
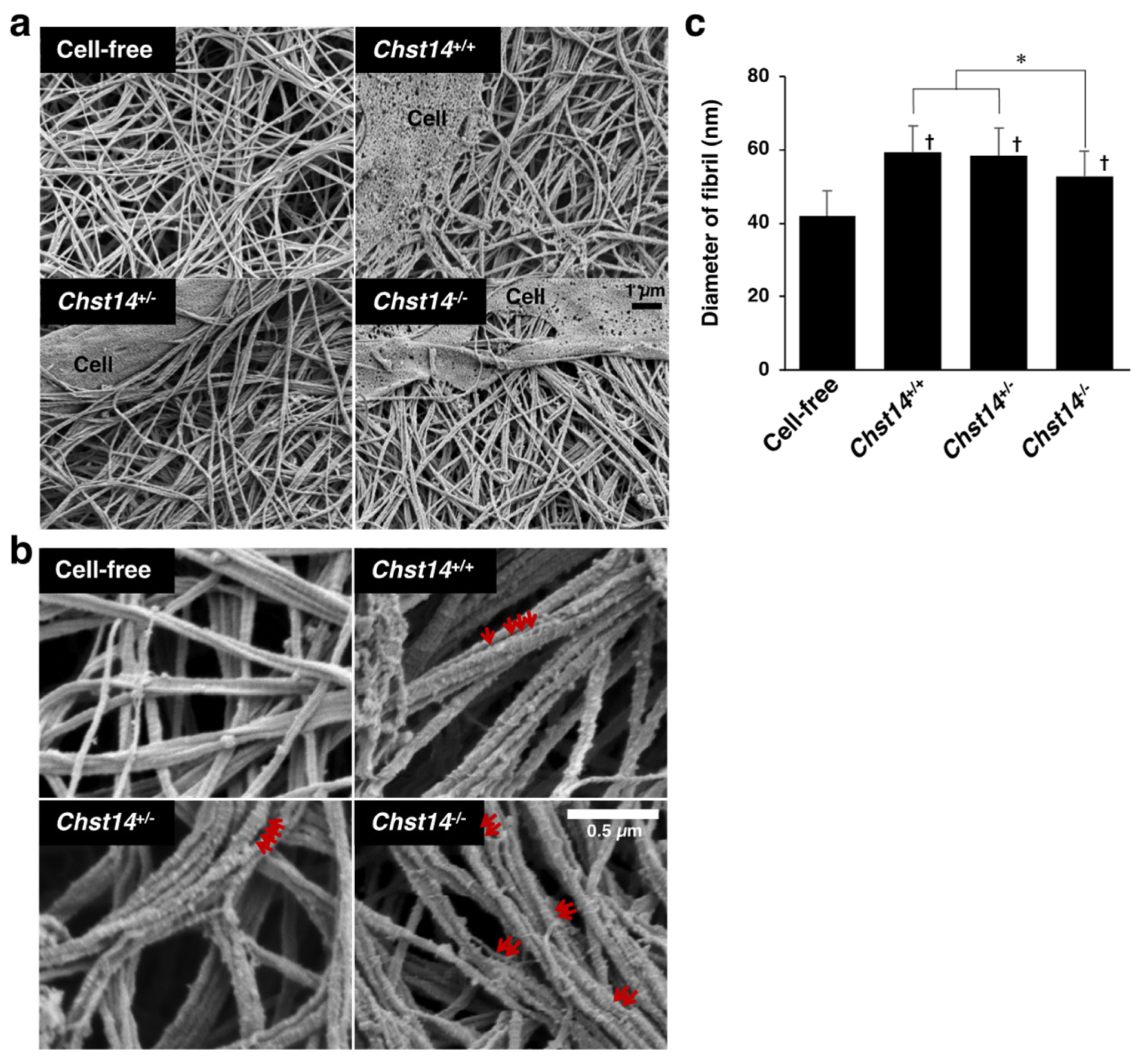
3.3. Effect of Fibroblasts and Decorin Derived from Chst14-Deficient Mice on the Fibrillar Organization of Type I Collagen Gels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinmann, B.; Royce, P.M.; Superti-Furga, A. The Ehlers-Danlos Syndrome. In Connective Tissue and Its Heritable Disorders; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 431–523. ISBN 978-0-471-22192-0. [Google Scholar]
- Brady, A.F.; Demirdas, S.; Fournel-Gigleux, S.; Ghali, N.; Giunta, C.; Kapferer-Seebacher, I.; Kosho, T.; Mendoza-Londono, R.; Pope, M.F.; Rohrbach, M.; et al. The Ehlers–Danlos syndromes, rare types. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 70–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers–Danlos syndromes. Nat. Rev. Dis. Prim. 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-R.; Bristow, J. The Ehlers-Danlos syndrome: On beyond collagens. J. Clin. Investig. 2001, 107, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Kosho, T.; Miyake, N.; Hatamochi, A.; Takahashi, J.; Kato, H.; Miyahara, T.; Igawa, Y.; Yasui, H.; Ishida, T.; Ono, K.; et al. A new Ehlers–Danlos syndrome with craniofacial characteristics, multiple congenital contractures, progressive joint and skin laxity, and multisystem fragility-related manifestations. Am. J. Med. Genet. A 2010, 152A, 1333–1346. [Google Scholar] [CrossRef]
- Miyake, N.; Kosho, T.; Mizumoto, S.; Furuichi, T.; Hatamochi, A.; Nagashima, Y.; Arai, E.; Takahashi, K.; Kawamura, R.; Wakui, K.; et al. Loss-of-function mutations of CHST14 in a new type of Ehlers-Danlos syndrome. Hum. Mutat. 2010, 31, 966–974. [Google Scholar] [CrossRef]
- Malfait, F.; Syx, D.; Vlummens, P.; Symoens, S.; Nampoothiri, S.; Hermanns-Lê, T.; Van Laer, L.; De Paepe, A. Musculocontractural Ehlers-Danlos Syndrome (former EDS type VIB) and adducted thumb clubfoot syndrome (ATCS) represent a single clinical entity caused by mutations in the dermatan-4-sulfotransferase 1 encoding CHST14 gene. Hum. Mutat. 2010, 31, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Dündar, M.; Müller, T.; Zhang, Q.; Pan, J.; Steinmann, B.; Vodopiutz, J.; Gruber, R.; Sonoda, T.; Krabichler, B.; Utermann, G.; et al. Loss of Dermatan-4-Sulfotransferase 1 Function Results in Adducted Thumb-Clubfoot Syndrome. Am. J. Hum. Genet. 2009, 85, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Minatogawa, M.; Unzaki, A.; Morisaki, H.; Syx, D.; Sonoda, T.; Janecke, A.R.; Slavotinek, A.; Voermans, N.C.; Lacassie, Y.; Mendoza-Londono, R.; et al. Clinical and molecular features of 66 patients with musculocontractural Ehlers-Danlos syndrome caused by pathogenic variants in CHST14 (mcEDS-CHST14). J. Med. Genet. 2022, 59, 865–877. [Google Scholar] [CrossRef]
- Mikami, T.; Mizumoto, S.; Kago, N.; Kitagawa, H.; Sugahara, K. Specificities of Three Distinct Human Chondroitin/Dermatan N-Acetylgalactosamine 4-O-Sulfotransferases Demonstrated Using Partially Desulfated Dermatan Sulfate as an Acceptor: IMPLICATION OF DIFFERENTIAL ROLES IN DERMATAN SULFATE BIOSYNTHESIS*. J. Biol. Chem. 2003, 278, 36115–36127. [Google Scholar] [CrossRef] [Green Version]
- Evers, M.R.; Xia, G.; Kang, H.-G.; Schachner, M.; Baenziger, J.U. Molecular Cloning and Characterization of a Dermatan-Specific N-Acetylgalactosamine 4-O-Sulfotransferase*. J. Biol. Chem. 2001, 276, 36344–36353. [Google Scholar] [CrossRef] [Green Version]
- Mizumoto, S.; Yamada, S. The Specific Role of Dermatan Sulfate as an Instructive Glycosaminoglycan in Tissue Development. Int. J. Mol. Sci. 2022, 23, 7485. [Google Scholar] [CrossRef]
- Thelin, M.A.; Bartolini, B.; Axelsson, J.; Gustafsson, R.; Tykesson, E.; Pera, E.; Oldberg, Å.; Maccarana, M.; Malmstrom, A. Biological functions of iduronic acid in chondroitin/dermatan sulfate. FEBS J. 2013, 280, 2431–2446. [Google Scholar] [CrossRef] [Green Version]
- Tykesson, E.; Hassinen, A.; Zielinska, K.; Thelin, M.A.; Frati, G.; Ellervik, U.; Westergren-Thorsson, G.; Malmström, A.; Kellokumpu, S.; Maccarana, M. Dermatan sulfate epimerase 1 and dermatan 4-O-sulfotransferase 1 form complexes that generate long epimerized 4-O-sulfated blocks. J. Biol. Chem. 2018, 293, 13725–13735. [Google Scholar] [CrossRef] [Green Version]
- Hirose, T.; Takahashi, N.; Tangkawattana, P.; Minaguchi, J.; Mizumoto, S.; Yamada, S.; Miyake, N.; Hayashi, S.; Hatamochi, A.; Nakayama, J.; et al. Structural alteration of glycosaminoglycan side chains and spatial disorganization of collagen networks in the skin of patients with mcEDS-CHST14. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 623–631. [Google Scholar] [CrossRef]
- Hirose, T.; Mizumoto, S.; Hashimoto, A.; Takahashi, Y.; Yoshizawa, T.; Nitahara-Kasahara, Y.; Takahashi, N.; Nakayama, J.; Takehana, K.; Okada, T.; et al. Systematic investigation of the skin in Chst14−/− mice: A model for skin fragility in musculocontractural Ehlers–Danlos syndrome caused by CHST14 variants (mcEDS-CHST14). Glycobiology 2021, 31, 137–150. [Google Scholar] [CrossRef]
- Syx, D.; Van Damme, T.; Symoens, S.; Maiburg, M.C.; van de Laar, I.; Morton, J.; Suri, M.; Del Campo, M.; Hausser, I.; Hermanns-Lê, T.; et al. Genetic Heterogeneity and Clinical Variability in Musculocontractural Ehlers–Danlos Syndrome Caused by Impaired Dermatan Sulfate Biosynthesis. Hum. Mutat. 2015, 36, 535–547. [Google Scholar] [CrossRef]
- Reed, C.C.; Iozzo, R.V. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002, 19, 249–255. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Tomasek, J.J.; Akiyama, S.K. Fibroblast-mediated collagen gel contraction does not require fibronectin-α 5 β 1 integrin interaction. Anat. Rec. 1992, 234, 153–160. [Google Scholar] [CrossRef]
- Nishiyama, T.; Tominaga, N.; Nakajima, K.; Hayashi, T. Quantitative Evaluation of the Factors Affecting the Process of Fibroblast-Mediated Collagen Gel Contraction by Separating the Process into Three Phases. Coll. Relat. Res. 1988, 8, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Nitahara-Kasahara, Y.; Mizumoto, S.; Inoue, Y.U.; Saka, S.; Posadas-Herrera, G.; Nakamura-Takahashi, A.; Takahashi, Y.; Hashimoto, A.; Konishi, K.; Miyata, S.; et al. A new mouse model of Ehlers-Danlos syndrome generated using CRISPR/Cas9-mediated genomic editing. Dis. Models Mech. 2021, 14, dmm048963. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Mizumoto, S.; Takahashi, Y.; Shimada, S.; Sugahara, K.; Nakayama, J.; Takeda, S.; Nomura, Y.; Nitahara-Kasahara, Y.; Okada, T.; et al. Vascular abnormalities in the placenta of Chst14−/− fetuses: Implications in the pathophysiology of perinatal lethality of the murine model and vascular lesions in human CHST14/D4ST1 deficiency. Glycobiology 2018, 28, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, Y.; Toki, S.; Ishii, Y.; Shirai, K. The Physicochemical Property of Shark Type I Collagen Gel and Membrane. J. Agric. Food Chem. 2000, 48, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Maccarana, M.; Kalamajski, S.; Kongsgaard, M.; Magnusson, S.P.; Oldberg, A.; Malmström, A. Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol. Cell. Biol. 2009, 29, 5517–5528. [Google Scholar] [CrossRef] [Green Version]
- Iozzo, R.V. The Biology of the Small Leucine-rich Proteoglycans. J. Biol. Chem. 1999, 274, 18843–18846. [Google Scholar] [CrossRef] [Green Version]
- Svensson, L.; Heineg, D.; Oldberg. Decorin-binding Sites for Collagen Type I Are Mainly Located in Leucine-rich Repeats 4-5 (∗). J. Biol. Chem. 1995, 270, 20712–20716. [Google Scholar] [CrossRef] [Green Version]
- Schönherr, E.; Hausser, H.; Beavan, L.; Kresse, H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J. Biol. Chem. 1995, 270, 8877–8883. [Google Scholar] [CrossRef] [Green Version]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Ezura, Y.; Chervoneva, I.; Robinson, P.S.; Beason, D.P.; Carine, E.T.; Soslowsky, L.J.; Iozzo, R.V.; Birk, D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006, 98, 1436–1449. [Google Scholar] [CrossRef]
- Reese, S.P.; Underwood, C.J.; Weiss, J.A. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013, 32, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.E. Proteoglycan-fibrillar collagen interactions. Biochem. J. 1988, 252, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.E. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J. Physiol. 2003, 553, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.N.; Pinali, C.; Young, R.D.; Meek, K.M.; Quantock, A.J.; Knupp, C. Structural Interactions between Collagen and Proteoglycans Are Elucidated by Three-Dimensional Electron Tomography of Bovine Cornea. Structure 2010, 18, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Nomura, Y. Structural Change in Decorin with Skin Aging. Connect. Tissue Res. 2006, 47, 249–255. [Google Scholar] [CrossRef]
- Raspanti, M.; Viola, M.; Forlino, A.; Tenni, R.; Gruppi, C.; Tira, M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Biol. 2008, 164, 134–139. [Google Scholar] [CrossRef]
- Watanabe, T.; Kametani, K.; Koyama, Y.; Suzuki, D.; Imamura, Y.; Takehana, K.; Hiramatsu, K. Ring-Mesh Model of Proteoglycan Glycosaminoglycan Chains in Tendon Based on Three-dimensional Reconstruction by Focused Ion Beam Scanning Electron Microscopy. J. Biol. Chem. 2016, 291, 23704–23708. [Google Scholar] [CrossRef] [Green Version]
- Casu, B.; Petitou, M.; Provasoli, M.; Sinaÿ, P. Conformational flexibility: A new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem. Sci. 1988, 13, 221–225. [Google Scholar] [CrossRef]
- Guidry, C.; Grinnell, F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J. Cell Sci. 1985, 79, 67–81. [Google Scholar] [CrossRef]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan Sulfate Released after Injury Is a Potent Promoter of Fibroblast Growth Factor-2 Function*. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar] [CrossRef] [Green Version]
- Trowbridge, J.M.; Rudisill, J.A.; Ron, D.; Gallo, R.L. Dermatan Sulfate Binds and Potentiates Activity of Keratinocyte Growth Factor (FGF-7)*. J. Biol. Chem. 2002, 277, 42815–42820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, M.; Deakin, J.A.; Rahmoune, H.; Fernig, D.G.; Nakamura, T.; Gallagher, J.T. Hepatocyte Growth Factor/Scatter Factor Binds with High Affinity to Dermatan Sulfate*. J. Biol. Chem. 1998, 273, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villena, J.; Brandan, E. Dermatan sulfate exerts an enhanced growth factor response on skeletal muscle satellite cell proliferation and migration. J. Cell. Physiol. 2004, 198, 169–178. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, A.; Hirose, T.; Hashimoto, K.; Mizumoto, S.; Nitahara-Kasahara, Y.; Saka, S.; Yoshizawa, T.; Okada, T.; Yamada, S.; Kosho, T.; et al. Collagen Network Formation in In Vitro Models of Musculocontractural Ehlers–Danlos Syndrome. Genes 2023, 14, 308. https://doi.org/10.3390/genes14020308
Hashimoto A, Hirose T, Hashimoto K, Mizumoto S, Nitahara-Kasahara Y, Saka S, Yoshizawa T, Okada T, Yamada S, Kosho T, et al. Collagen Network Formation in In Vitro Models of Musculocontractural Ehlers–Danlos Syndrome. Genes. 2023; 14(2):308. https://doi.org/10.3390/genes14020308
Chicago/Turabian StyleHashimoto, Ayana, Takuya Hirose, Kohei Hashimoto, Shuji Mizumoto, Yuko Nitahara-Kasahara, Shota Saka, Takahiro Yoshizawa, Takashi Okada, Shuhei Yamada, Tomoki Kosho, and et al. 2023. "Collagen Network Formation in In Vitro Models of Musculocontractural Ehlers–Danlos Syndrome" Genes 14, no. 2: 308. https://doi.org/10.3390/genes14020308




