Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Varieties
2.2. DNA Extraction
2.3. Molecular Markers Selection Criteria
2.4. Central Composite Design and Optimization
2.5. Multiplex PCR Conditions
2.6. Dynamic Range and Limit of Detection
2.7. Specificity/Selectivity
3. Results
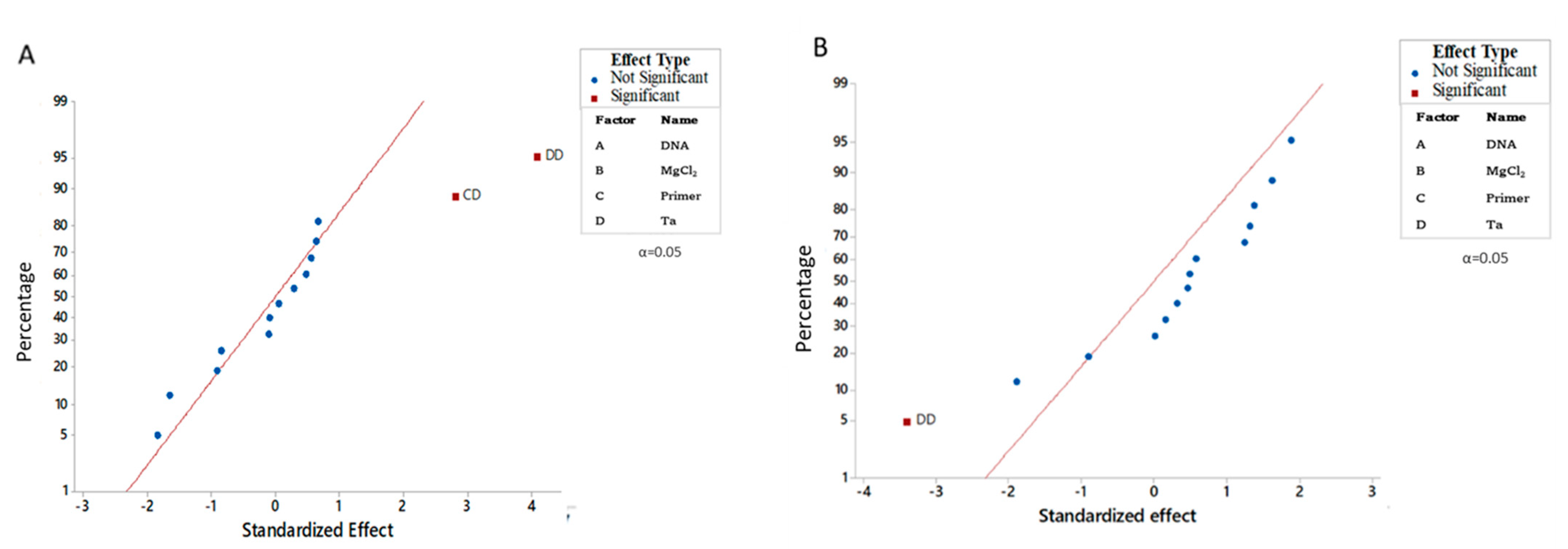
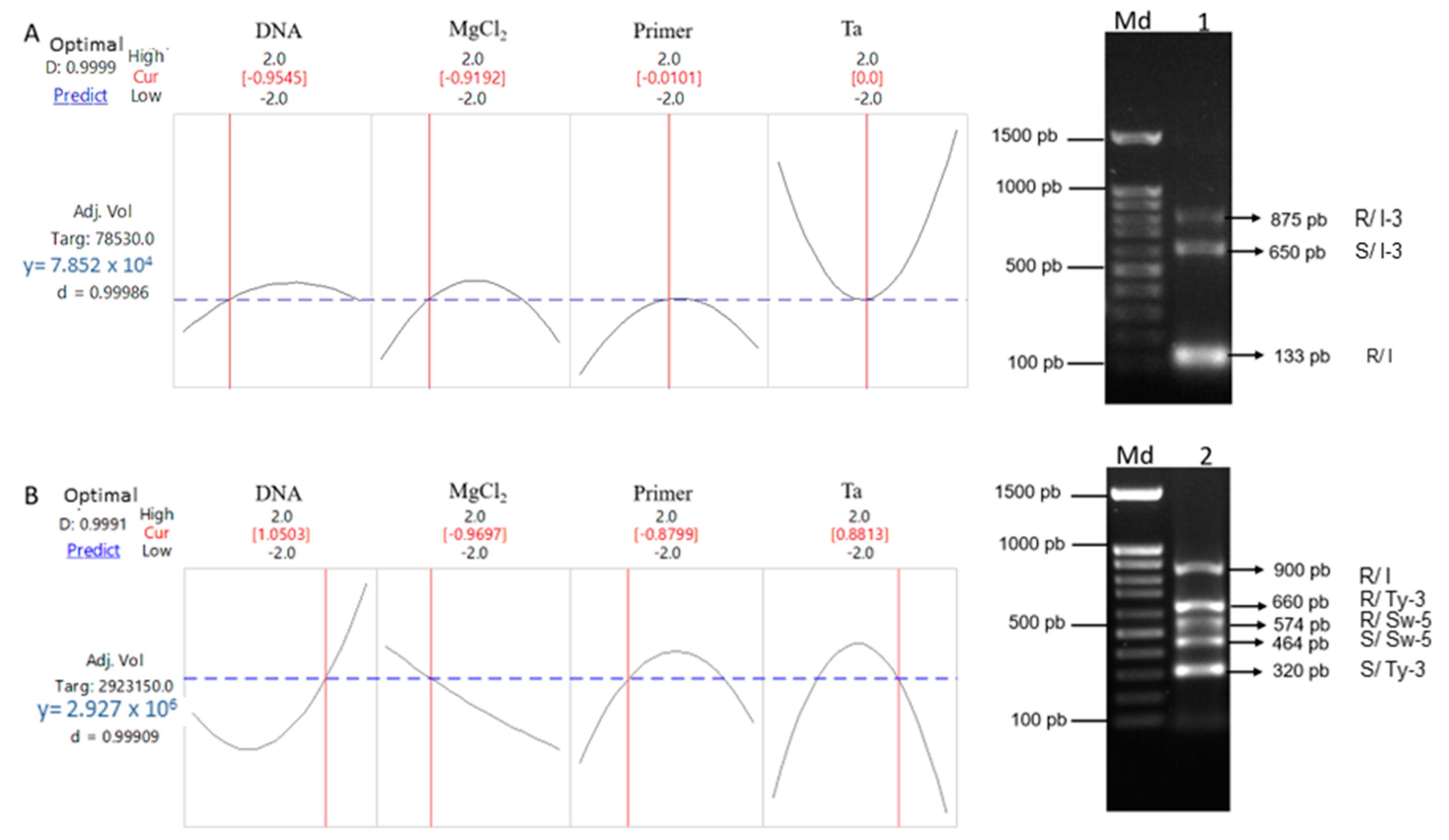
3.1. Optimization of the Multiplex PCRs Conditions
3.2. Dynamic Range, Limit of Detection and Specificity/Selectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef] [PubMed]
- SIAP. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2020/Atlas-Agroalimentario-2020 (accessed on 20 October 2022).
- Ascencio-Álvarez, A.; López-Benítez, A.; Borrego-Escalante, F.; Rodríguez-Herrera, S.; Flores-Olivas, A.; Jiménez-Díaz, F.; Gámez-Vázquez, A. Marchitez Vascular del Tomate: I. Presencia de Razas de Fusarium oxysporum f. sp. lycopersici (Sacc.) Snyder y Hansen en Culiacán, Sinaloa, México. Rev. Mex. Fitopatol. 2008, 26, 114–120. [Google Scholar]
- Al Abdallat, A.M.; Al Debei, H.S.; Asmar, H.; Misbeh, S.; Quraan, A.; Kvarnheden, A. An efficient in vitro-inoculation method for Tomato yellow leaf curl virus. Virol. J. 2010, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Melchor, O.Y.; Guzmán-Uriarte, R.; García-Estrada, R.S.; León-Félix, J.; Josefina, L.F. Geminivirus Transmitidos por Mosca Blanca (Bemisia Tabaci) en Tomate, en el Valle Agrícola de Culiacán, Sinaloa. Rev. Mex. Fitopatol. 2011, 29, 109–118. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/es/#data/QC/visualize (accessed on 20 October 2022).
- Hanson, P.; Lu, S.-F.; Wang, J.-F.; Chen, W.; Kenyon, L.; Tan, C.-W.; Tee, K.L.; Wang, Y.-Y.; Hsu, Y.-C.; Schafleitner, R.; et al. Conventional and molecular marker-assisted selection and pyramiding of genes for multiple disease resistance in tomato. Sci. Hortic. 2016, 201, 346–354. [Google Scholar] [CrossRef]
- Sánchez-Peña, P.; Oyama, K.; Núnéz-Farfán, J.; Fornoni, J.; Hernández-Verdugo, S.; Márquez-Guzmán, J.; Garzón-Tiznado, J.A. Sources of resistance to whitefly (Bemisia spp.) in wild populations of Solanum lycopersicum var. cerasiforme (Dunal) spooner G. J. Anderson et R. K. Jansen in Northwestern Mexico. Genet. Resour. Crop Evol. 2006, 53, 711–719. [Google Scholar] [CrossRef]
- Flores-Hernández, L.A.; Lobato-Ortiz, R.; García-Zavala, J.J.; Molina-Galán, J.D.; Sargerman-Jarquín, D.M.; Velasco-Alvarado, M.d.J. Parientes silvestres del tomate como fuente de germoplasma para el mejoramiento genético de la especie. Rev. Fitotec. Mex. 2017, 40, 83–91. [Google Scholar] [CrossRef]
- Arruabarrena, A.; González, A.M.; Rubio, L.; Giménez, G. Selección Asistida por Marcadores en el mejoramiento genético de tomate. Biotecnología para el sector productivo. INIA 2015, 40, 43–46. [Google Scholar]
- Velázquez-Alejos, L.P.; Aragón Martinéz M del, C.; Amelia Cornejo, R. Extracción y purificación de ADN. Herram. Mol. Apl. Ecol. Asp. Teóricos Prácticos 2010, 1, 1–26. [Google Scholar]
- Ebert, A.W.; Chou, Y.Y. The tomato collection maintained by AVRDC—The World Vegetable center: Composition, germplasm dissemination and use in breeding. Acta Hortic. 2015, 1101, 169–176. [Google Scholar] [CrossRef]
- Dias, V.D.; Oliveira, R.M.D.; Dianese, É.D.C.; Boiteux, L.S.; Cunha, M.G.D. Detecção simultânea de fatores de resistência à murcha de fusário do tomateiro por meio de PCR multiplex. Pesqui. Agropecuária Bras. 2016, 51, 925–932. [Google Scholar] [CrossRef][Green Version]
- Chen, H.-M.; Lin, C.-Y.; Yoshida, M.; Hanson, P.; Schafleitner, R. Multiplex PCR for Detection of Tomato Yellow Leaf Curl Disease and Root-Knot Nematode Resistance Genes in Tomato (Solanum lycopersicum L.). Int. J. Plant Breed. Genet. 2015, 9, 44–56. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Arens, P.; Mansilla, C.; Deinum, D.; Cavellini, L.; Moretti, A.; Rolland, S.; van der Schoot, H.; Calvache, D.; Ponz, F.; Collonnier, C.; et al. Development and evaluation of robust molecular markers linked to disease resistance in tomato for distinctness, uniformity and stability testing. Theor. Appl. Genet. 2010, 120, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Barillas, A.C.; Mejia, L.; Sánchez-Pérez, A.; Maxwell, D.P. CAPS and SCAR markers for detection of I-3 gene introgression for resistance to Fusarium oxysporium f. sp. lycopersici race 3. Rep. Tomato Genet. Coop. 2008, 58, 11–17. [Google Scholar]
- Parmar, P.P.; Bhatt, K.N.; Oza, V.P.; Patel, A.D.; Kathiria, K.B.; Subramanian, R.B. Microsatellite marker associated with Fusarium wilt resistance in tomato. World J. Agric. Sci. 2009, 5, 389–393. [Google Scholar]
- Dianese, E.C.; de Fonseca, M.E.; Kormelink, R.G.; Inoue-Nagata, A.K.; Resende, R.O.; Boiteux, L.S. Development of a locus-specific, co-dominant SCAR marker for assisted-selection of the Sw-5 (Tospovirus resistance) gene cluster in a wide range of tomato accessions. Mol. Breed. 2010, 25, 133–142. [Google Scholar] [CrossRef]
- Ji, Y.; Schuster, D.J.; Scott, J.W. Ty-3, a begomovirus resistance locus near the Tomato yellow leaf curl virus resistance locus Ty-1 on chromosome 6 of tomato. Mol. Breed. 2007, 20, 271–284. [Google Scholar] [CrossRef]
- ISO 16140: 2003; Microbiology of Food and Animal Feedinds Stuffs—Protocol for the Validación of Alternative Methods. International Organization for Standarization: Geneva, Switzerland, 2003.
- Chamberlain, J.S.; Chamberlain, J.R. Optimization of multiplex PCRs. In The polymerase Chain Reaction; Birkhäuser: Boston, MA, USA, 1994; pp. 38–46. [Google Scholar] [CrossRef]
- Bolivar, A.M.; Rojas, A.; Garcia-lugo, P. PCR y PCR-Múltiple parámetros críticos y protocolo de estandarización. Av. Biomed. 2014, 3, 25–33. [Google Scholar]
- Obradovich, J.; Jurisic, V.; Tosic, N.; Mrdjanovic, J.; Perin, B.; Pavlovic, S.; Djordjevic, N. Optimization of PCR conditions for amplification of GC-rich EGFR promoter sequence. J. Clin. Lab. Anal. 2013, 27, 487–493. [Google Scholar] [CrossRef]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for Resistance to Fusarium Wilt of Tomato: A Review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef]
- Kabaş, A.; Fidan, H.; Demirelli, M.B. Identification of new sources of resistance to resistance-breaking isolates of tomato spotted wilt virus. Saudi J. Biol. Sci. 2021, 28, 3094–3099. [Google Scholar] [CrossRef]
- Panthee, D.R.; Ibrahem, R. New molecular markers associated with the Sw-5 gene conferring resistance to tomato spotted wilt virus in tomato. J. Hortic. Sci. Biotechnol. 2013, 88, 129–134. [Google Scholar] [CrossRef]
- Nevame, A.Y.M.; Xia, L.; Nchongboh, C.G.; Hasan, M.M.; Alam, A.; Yongbo, L.; Wenting, Z.; Yafei, H.; Emon, R.M.; Ismail, M.R.; et al. Development of a new molecular marker for the resistance to tomato yellow leaf curl virus. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Nevame, A.; Xia, L.; Wenting, Z.; Nchongboh, C.G.; Wenhu, L.; Hasan, M.M.; Alam, A.; Longting, S. Validation of some disease-resistance molecular markers associated with multiple diseases in tomato for marker-assisted selection program. Scienceasia 2020, 4, 19–29. [Google Scholar] [CrossRef]
- Lafrance, R.; Villicaña, C.; Valdéz-Torres, J.B.; Martínez-Montoya, H.; Castillo-Ruiz, O.; Alemán-Castillo, S.E.; Esparza-Araiza, M.J.; León-Félix, J. Optimization of PCR-based TYLCV molecular markers by response surface methodology. Gene 2021, 785, 145606. [Google Scholar] [CrossRef]
- Tabein, S.; Behjatnia, S.A.A.; Laviano, L.; Pecchioni, N.; Accotto, G.P.; Noris, E.; Miozzi, L. Pyramiding Ty-1/Ty-3 and Ty-2 in tomato hybrids dramatically inhibits symptom expression and accumulation of tomato yellow leaf curl disease inducing viruses. Arch. Phytopathol. Plant Prot. 2017, 50, 213–227. [Google Scholar] [CrossRef]
- Mahfouze, S.A.; Mahfouze, H.A. A Comparison between CAPS and SCAR Markers in the Detection of Resistance Genes in some Tomato Genotypes against Tomato Yellow Leaf Curl Virus and Whitefly. Jordan J. Biol. Sci. 2019, 12, 123–133. [Google Scholar]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Dogan, Y.; Comertpay, G.; Yıldız, M.; Hatipoglu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N.; et al. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]


| Companies | Hybrids | Declared Resistance by Suppliers | Purposes |
|---|---|---|---|
| Hatear | Álvaro | HR: Fol (1,2); Vd; ToMV IR: TSWV; TYLCV; Mi; Mj; ToTV | Validation (protocol 1) |
| Hazera | Vanessa | HR: Fol (1,2,3) TSWV; Pst; ToANV IR: TYLCV | Optimization (protocol 2) and validation (protocols 1 and 2) |
| Seminis | SV8579TE | HR: Fol (0,2); Va; Vd; ToMV IR TYLCV; Mi | Validation (protocol 1) |
| Seminis | SV3543TE | R: Fol (1,2,3); Tmv; TYLCV: ToTV; TYTV | Validation (protocol 1) |
| Seminis | SVTE8444 | IR: Fol (1,2,3); TYLCV; Ma; Mi; Mj; Vd | Optimization (protocol 1) and validation (protocols 1 and 2) |
| Sakata | Valerio | IR: Fol (1,2,3); TYLCV; ToMV; Vd R: Aal | Validation (protocol 1 and 2) |
| No information | DRD600 | Fol (1) | Validation (protocol 1) |
| No information | D-74 | HR: Fol (1,3); TYLCV | Optimization (protocol 2) and validation (protocols 1 and 2) |
| Victory Seeds | Bonny Best | SC: Fol (1, 3); TYLCV | Validation (protocols 1 and 2) |
| Eden Seeds | Walter | IR: Fol (1) (IR) | Validation (protocols 1) |
| Genes | Markers | Types | Chr. | Primer sequences | Ta. (°C) | Amplicons (bp) | References | |
|---|---|---|---|---|---|---|---|---|
| Forward | Reverse | |||||||
| I | At-2 | SCAR | 11 | cgaatctgtatattacatccgtcgt | ggtgaataccgatcatagtcgag | 54 | 130 R | Arens et al. [16] |
| I-3 | P7-43 | SCAR | 7 | cacgggatatgttrttgataagcatg | gtctttaccacaggaactttatcacc | 53 | 875 R/650 S | Barillas et al. [17] |
| I | SSR-67 | SSR | 11 | gcacgagaccaagcagatta | gggcctttcctccagtagac | -- | 900 R | Parmar et al. [18] |
| Sw5 | SW-5 | SCAR | 9 | aattaggttcttgaagcccatct | ttccgcatcagccaatagtgt | 54 | 575 R/464 S | Dianese et al. [19] |
| Ty-3 | P6-25 | SCAR | 6 | ggtagtggaaatgatgctgctc | gctctgcctattgtcccatatataacc | 53 | 660 R/320 S | Ji et al. [20] |
| Variables | Axial (−2) | Factorial (−1) | Central (0) | Factorial (1) | Axial (2) |
|---|---|---|---|---|---|
| Ta. (°C) | 50 | 52 | 54 | 56 | 58 |
| DNA (ng) | 10 | 30 | 50 | 70 | 90 |
| MgCl2 (mM) | 1.5 | 2 | 2.5 | 3 | 3.5 |
| Primer (µM) | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 |
| Source | DF | AT-2; P7-43 | SSR-67; SW5; P6-25 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Seq SS | % Cont. | F | p | Seq SS | % Cont. | F | p | ||
| Model | 15 | 53557084617 | 73.21 | 2.55 | 0.044 * | 6.297 × 1013 | 73.62 | 2.60 | 0.041 * |
| Blocks | 1 | 857170949 | 1.17 | 0.61 | 0.447 | 1.463 × 1013 | 17.10 | 9.07 | 0.009 * |
| Linear | 4 | 1463078506 | 2.00 | 0.26 | 0.898 | 7.391 × 1012 | 8.64 | 1.15 | 0.375 |
| A | 1 | 612566451 | 0.84 | 0.44 | 0.519 | 4.198 × 1012 | 4.91 | 2.60 | 0.129 |
| B | 1 | 111438211 | 0.15 | 0.08 | 0.782 | 22124160 | 0.00 | 0.00 | 0.997 |
| C | 1 | 428883822 | 0.59 | 0.31 | 0.589 | 3.785 × 1011 | 0.44 | 0.23 | 0.635 |
| D | 1 | 310190021 | 0.42 | 0.22 | 0.645 | 2.815 × 1012 | 3.29 | 1.75 | 0.208 |
| Square | 4 | 38452271841 | 52.56 | 6.87 | 0.003 * | 3.314 × 1013 | 38.74 | 5.14 | 0.009 * |
| A×A | 1 | 1242705553 | 1.70 | 0.73 | 0.406 | 1.013 × 1013 | 11.84 | 3.51 | 0.082 |
| B×B | 1 | 6742079470 | 9.22 | 3.43 | 0.085 | 1.109 × 1012 | 1.30 | 0.02 | 0.881 |
| C×C | 1 | 7173212681 | 9.81 | 2.75 | 0.120 | 3.261 × 1012 | 3.81 | 3.59 | 0.079 |
| D×D | 1 | 23294274137 | 31.84 | 16.64 | 0.001 * | 1.864 × 1013 | 21.79 | 11.56 | 0.004 * |
| 2-way int. | 6 | 12784563320 | 17.48 | 1.52 | 0.242 | 7.816 × 1012 | 9.14 | 0.81 | 0.580 |
| A×B | 1 | 11760784 | 0.02 | 0.01 | 0.928 | 1.331 × 1012 | 1.56 | 0.83 | 0.379 |
| A×C | 1 | 18012102 | 0.02 | 0.01 | 0.911 | 3.289 × 1011 | 0.38 | 0.20 | 0.658 |
| A×D | 1 | 1186384543 | 1.62 | 0.85 | 0.373 | 1.528 × 1011 | 0.18 | 0.09 | 0.763 |
| B×C | 1 | 2876642 | 0.00 | 0.00 | 0.964 | 3.006 × 1012 | 3.51 | 1.86 | 0.194 |
| B×D | 1 | 553783263 | 0.76 | 0.40 | 0.539 | 5.399 × 1011 | 0.63 | 0.33 | 0.572 |
| C×D | 1 | 11011745986 | 15.05 | 7.87 | 0.014 * | 2.458 × 1012 | 2.87 | 1.52 | 0.237 |
| Error | 14 | 19595818329 | 26.79 | 2.257 × 1013 | 26.38 | ||||
| Lack of fit | 10 | 11800089736 | 16.13 | 0.61 | 0.763 | 1.771 × 1013 | 20.70 | 1.46 | 0.382 |
| Pure error | 4 | 7795728593 | 10.66 | 4.858 × 1012 | 5.68 | ||||
| Total | 29 | 73152902946 | 100.00 | 8.554 × 1013 | 100.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafrance, R.; Valdez-Torres, J.B.; Villicaña, C.; García-Estrada, R.S.; Esparza-Araiza, M.J.; León-Félix, J. Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation. Genes 2023, 14, 337. https://doi.org/10.3390/genes14020337
Lafrance R, Valdez-Torres JB, Villicaña C, García-Estrada RS, Esparza-Araiza MJ, León-Félix J. Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation. Genes. 2023; 14(2):337. https://doi.org/10.3390/genes14020337
Chicago/Turabian StyleLafrance, Richecarde, José Benigno Valdez-Torres, Claudia Villicaña, Raymundo Saúl García-Estrada, Mayra Janeth Esparza-Araiza, and Josefina León-Félix. 2023. "Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation" Genes 14, no. 2: 337. https://doi.org/10.3390/genes14020337
APA StyleLafrance, R., Valdez-Torres, J. B., Villicaña, C., García-Estrada, R. S., Esparza-Araiza, M. J., & León-Félix, J. (2023). Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation. Genes, 14(2), 337. https://doi.org/10.3390/genes14020337







