Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum
Abstract
1. Introduction
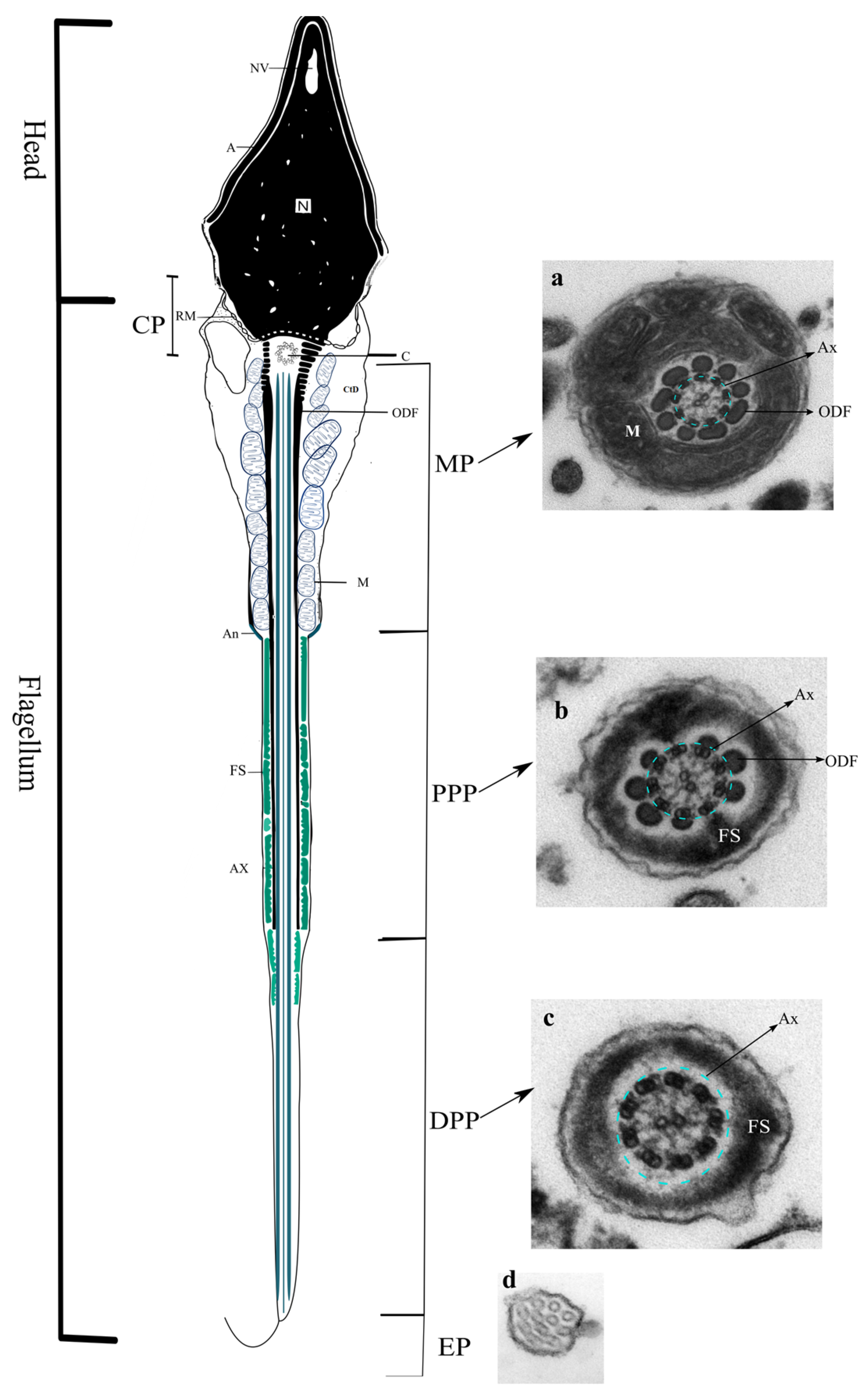
2. Sperm Flagellum
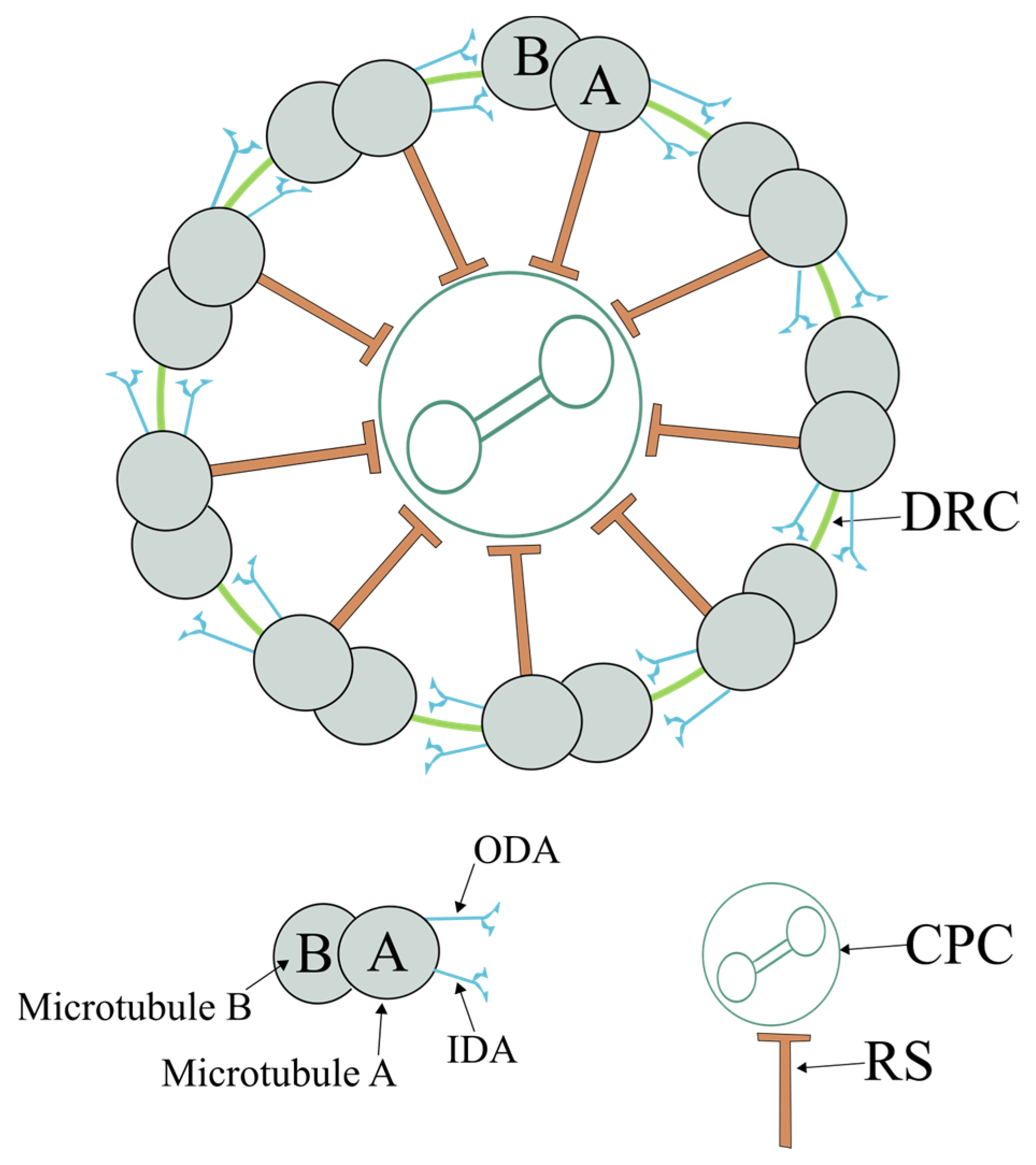
2.1. Axoneme
2.2. Connecting Piece
2.3. Midpiece
2.4. Principal Piece
2.5. Endpiece
3. Motility Mechanisms
4. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive techniques |
| CPC | Central pair complex |
| CBF | Ciliary beat frequency |
| CP | Connecting piece |
| cAMP | Cyclic adenosine monophosphate |
| DC | Distal centriole |
| DA | Dynein arms |
| DNAAFs | Dynein axonemal assembly factors |
| DRC | Dynein regulatory complex |
| DFS | Dysplasia of FS |
| EP | Endpiece |
| FS | Fibrous sheath |
| iPSC | Induced pluripotent stem cells |
| IDA | Inner dynein arms |
| ICSI | Intracytoplasmic sperm injection |
| IFT | Intraflagellar transport |
| IMT | Intramanchette transport |
| MP | Midpiece |
| ODF | Outer dense fibers |
| ODA | Outer dynein arms |
| PP | Principal piece |
| PKA | Protein kinase A |
| AKAP | Protein kinase A-anchoring protein |
| PC | Proximal centriole |
| RS | Radial spokes |
| ROS | Reactive oxygen species |
| RSP | RS proteins |
| SEPT | Septins |
| NHEs | Sodium–hydrogen exchangers |
| sAC | Soluble adenylyl cyclase |
| SDH | Succinate dehydrogenase |
| TAILS | Tail axoneme intra-lumenal spiral |
References
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.H.; Blanco, A.M. Asthenozoospermia: Analysis of a large population. Syst. Biol. Reprod. Med. 2003, 49, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-G.; Chen, W.-K.; Fei, Q.-J.; Liu, Y.-L.; Liu, X.-D.; Huang, H.; Shang, X.-J. Analysis of semen quality of 38 905 infertile male patients during 2008-2016 in Wenzhou, China. Asian J. Androl. 2021, 23, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. How Do Sperm Get to the Egg? Bioengineering Expertise Needed! Exp. Mech. 2009, 50, 1267–1274. [Google Scholar] [CrossRef]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sa, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Aliakbari, F.; Eshghifar, N.; Mirfakhraie, R.; Pourghorban, P.; Azizi, F. Coding and Non-Coding RNAs, as Male Fertility and Infertility Biomarkers. Int. J. Fertil. Steril. 2021, 15, 158–166. [Google Scholar] [CrossRef]
- Cannarella, R.; Barbagallo, F.; Crafa, A.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. Seminal Plasma Transcriptome and Proteome: Towards a Molecular Approach in the Diagnosis of Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 7308–7321. [Google Scholar] [CrossRef]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silber, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef]
- La, H.; Yoo, H.; Lee, E.J.; Thang, N.X.; Choi, H.J.; Oh, J.; Park, J.H.; Hong, K. Insights from the Applications of Single-Cell Transcriptomic Analysis in Germ Cell Development and Reproductive Medicine. Int. J. Mol. Sci 2021, 22, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders. Front. Cell Dev. Biol. 2021, 9, 634536–634553. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Zheng, X.; Manske, G.L.; Vargo, A.; Shami, A.N.; Li, J.Z.; Hammoud, S.S. Decoding the Spermatogenesis Program: New Insights from Transcriptomic Analyses. Annu. Rev. Genet. 2022, 56, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e1658. [Google Scholar] [CrossRef]
- Neuhaus, N.; Yoon, J.; Terwort, N.; Kliesch, S.; Seggewiss, J.; Huge, A.; Voss, R.; Schlatt, S.; Grindberg, R.V.; Scholer, H.R. Single-cell gene expression analysis reveals diversity among human spermatogonia. Mol. Hum. Reprod. 2017, 23, 79–90. [Google Scholar] [CrossRef]
- Soraggi, S.; Riera, M.; Rajpert-De Meyts, E.; Schierup, M.H.; Almstrup, K. Evaluating genetic causes of azoospermia: What can we learn from a complex cellular structure and single-cell transcriptomics of the human testis? Hum. Genet. 2021, 140, 183–201. [Google Scholar] [CrossRef]
- Asadpour, R.; Mofidi Chelan, E. Using microRNAs as molecular biomarkers for the evaluation of male infertility. Andrologia 2022, 54, e14298. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Y.L.; Yu, L.; Sharma, S.; Fei, Z.L.; Drevet, J.R. Epididymal small non-coding RNA studies: Progress over the past decade. Andrology 2019, 7, 681–689. [Google Scholar] [CrossRef] [PubMed]
- de Mateo, S.; Sassone-Corsi, P. Regulation of spermatogenesis by small non-coding RNAs: Role of the germ granule. Semin. Cell Dev. Biol. 2014, 29, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiafini, M.A.; Sarafidou, T.; Mamuris, Z. The Role of Long Noncoding RNAs on Male Infertility: A Systematic Review and In Silico Analysis. Biology 2022, 11, 1510–1542. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.D.; Evrard, B.; Darde, T.A.; Le Béguec, C.; Le Bras, Y.; Bensalah, K.; Lavoué, S.; Jost, B.; Primig, M.; Dejucq-Rainsford, N.; et al. RNA profiling of human testicular cells identifies syntenic lncRNAs associated with spermatogenesis. Hum. Reprod 2019, 34, 1278–1290. [Google Scholar] [CrossRef]
- Walker, W.H. Regulation of mammalian spermatogenesis by miRNAs. Semin. Cell Dev. Biol. 2022, 121, 24–31. [Google Scholar] [CrossRef]
- Fenner, A. Male factor infertility: Piwi, Hiwi, Miwi: Essential genes for effective spermiogenesis. Nat. Rev. Urol. 2017, 14, 451. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, M.; Zhang, J.; Feng, Y.; Xie, Z.; Liu, S.; Zhu, D.; Luo, Y. The gene regulatory role of non-coding RNAs in non-obstructive azoospermia. Front. Endocrinol. 2022, 13, 959487. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P.; Manandhar, G. Mammalian spermatogenesis and sperm structure: Anatomical and compartmental analysis. In The Sperm Cell-Production, Maturation, Fertilization, Regeneration; De Jonge, C.J., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–31. [Google Scholar]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2015, 4, e979623. [Google Scholar] [CrossRef]
- Taschner, M.; Lorentzen, E. The Intraflagellar Transport Machinery. Cold Spring Harb. Perspect. Biol. 2016, 8, a028092. [Google Scholar] [CrossRef]
- Gonçalves, J.; Pelletier, L. The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol. Cells 2017, 40, 243–253. [Google Scholar] [CrossRef]
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012, 13, 608–618. [Google Scholar] [CrossRef]
- Lechtreck, K.F. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem. Sci. 2015, 40, 765–778. [Google Scholar] [CrossRef]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825. [Google Scholar] [CrossRef] [PubMed]
- San Agustin, J.T.; Pazour, G.J.; Witman, G.B. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol. Biol. Cell 2015, 26, 4358–4372. [Google Scholar] [CrossRef] [PubMed]
- Kierszenbaum, A.L.; Gil, M.; Rivkin, E.; Tres, L.L. Ran, a GTP-binding protein involved in nucleocytoplasmic transport and microtubule nucleation, relocates from the manchette to the centrosome region during rat spermiogenesis. Mol. Reprod. Dev. 2002, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, J.-P.; Kann, M.-L. The cytoskeleton of mammalian spermatozoa. Biol. Cell 1994, 81, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Kierszenbaum, A.L. Intramanchette transport (IMT): Managing the making of the spermatid head, centrosome, and tail. Mol. Reprod. Dev. 2002, 63, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.S.; Sironen, A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016, 151, R43–R54. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L.; Rivkin, E.; Tres, L.L. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis 2011, 1, 221–230. [Google Scholar] [CrossRef]
- Ma, Q.; Cao, C.; Zhuang, C.; Luo, X.; Li, X.; Wan, H.; Ye, J.; Chen, F.; Cui, L.; Zhang, Y.; et al. AXDND1, a novel testis-enriched gene, is required for spermiogenesis and male fertility. Cell Death Discov. 2021, 7, 348. [Google Scholar] [CrossRef]
- Pereira, R.; Oliveira, M.E.; Santos, R.; Oliveira, E.; Barbosa, T.; Santos, T.; Gonçalves, P.; Ferraz, L.; Pinto, S.; Barros, A.; et al. Characterization of CCDC103 expression profiles: Further insights in primary ciliary dyskinesia and in human reproduction. J. Assist. Reprod. Genet. 2019, 36, 1683–1700. [Google Scholar] [CrossRef]
- Nicastro, D.; Schwartz, C.; Pierson, J.; Gaudette, R.; Porter, M.E.; McIntosh, J.R. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 2006, 313, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Stoyanova, M.; Rademacher, G.; Dutcher, S.K.; Brown, A.; Zhang, R. Structure of the Decorated Ciliary Doublet Microtubule. Cell 2019, 179, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Whittaker, M.; Milligan, R.A.; Downing, K.H. High-Resolution Model of the Microtubule. Cell 1999, 96, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sakakibara, H.; Oiwa, K. Force-Generating Mechanism of Axonemal Dynein in Solo and Ensemble. Int. J. Mol. Sci. 2020, 21, 2843–2860. [Google Scholar] [CrossRef] [PubMed]
- Heuser, T.; Raytchev, M.; Krell, J.; Porter, M.E.; Nicastro, D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 2009, 187, 921–933. [Google Scholar] [CrossRef]
- King, S.M. The dynein microtubule motor. Biochim. Biophys. Acta 2000, 1496, 60–75. [Google Scholar] [CrossRef]
- King, S.M. Axonemal Dynein Arms. Cold Spring Harb. Perspect. Biol. 2016, 8, a028100. [Google Scholar] [CrossRef]
- Roberts, A.J.; Malkova, B.; Walker, M.L.; Sakakibara, H.; Numata, N.; Kon, T.; Ohkura, R.; Edwards, T.A.; Knight, P.J.; Sutoh, K.; et al. ATP-driven remodeling of the linker domain in the dynein motor. Structure 2012, 20, 1670–1680. [Google Scholar] [CrossRef]
- Roberts, A.J.; Numata, N.; Walker, M.L.; Kato, Y.S.; Malkova, B.; Kon, T.; Ohkura, R.; Arisaka, F.; Knight, P.J.; Sutoh, K.; et al. AAA+ Ring and Linker Swing Mechanism in the Dynein Motor. Cell 2009, 136, 485–495. [Google Scholar] [CrossRef]
- King, S.M. Regulatory mechanics of outer-arm dynein motors. In Dyneins, 2nd ed; King, S.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2018; pp. 250–269. [Google Scholar]
- King, S.M. Composition and assembly of axonemal dyneins. In Dyneins, 2nd ed.; King, S.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2018; pp. 162–201. [Google Scholar]
- Hwang, J.; Hunter, E.L.; Sale, W.S.; Wirschell, M. Control of axonemal inner dynein arms. In Dyneins, 2nd ed.; King, S.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2018; pp. 270–297. [Google Scholar]
- Desai, P.B.; Dean, A.B.; Mitchell, D.R. Cytoplasmic preassembly and trafficking of axonemal dyneins. In Dyneins, 2nd ed.; King, S.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2018; pp. 140–161. [Google Scholar]
- King, S.M.; Patel-King, R.S. The oligomeric outer dynein arm assembly factor CCDC103 is tightly integrated within the ciliary axoneme and exhibits periodic binding to microtubules. J. Biol. Chem. 2015, 290, 7388–7401. [Google Scholar] [CrossRef]
- Knowles, M.R.; Leigh, M.W.; Ostrowski, L.E.; Huang, L.; Carson, J.L.; Hazucha, M.J.; Yin, W.; Berg, J.S.; Davis, S.D.; Dell, S.D. Exome Sequencing Identifies Mutations in CCDC114 as a Cause of Primary Ciliary Dyskinesia. Am. J. Hum. Genet. 2013, 92, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hjeij, R.; Lindstrand, A.; Francis, R.; Zariwala, M.A.; Liu, X.; Li, Y.; Damerla, R.; Dougherty, G.W.; Abouhamed, M.; Olbrich, H.; et al. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am. J. Hum. Genet. 2013, 93, 357–367. [Google Scholar] [CrossRef]
- Wallmeier, J.; Shiratori, H.; Dougherty, G.W.; Edelbusch, C.; Hjeij, R.; Loges, N.T.; Menchen, T.; Olbrich, H.; Pennekamp, P.; Raidt, J.; et al. TTC25 Deficiency Results in Defects of the Outer Dynein Arm Docking Machinery and Primary Ciliary Dyskinesia with Left-Right Body Asymmetry Randomization. Am. J. Hum. Genet. 2016, 99, 460–469. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Schmidts, M.; Loges, N.T.; Freshour, J.; Dritsoula, A.; Hirst, R.A.; O’Callaghan, C.; Blau, H.; Al Dabbagh, M.; Olbrich, H.; et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat. Genet. 2012, 44, 381–389. [Google Scholar] [CrossRef]
- Horani, A.; Druley, T.E.; Zariwala, M.A.; Patel, A.C.; Levinson, B.T.; Van Arendonk, L.G.; Thornton, K.C.; Giacalone, J.C.; Albee, A.J.; Wilson, K.S.; et al. Whole-Exome Capture and Sequencing Identifies HEATR2 Mutation as a Cause of Primary Ciliary Dyskinesia. Am. J. Hum. Genet. 2012, 91, 685–693. [Google Scholar] [CrossRef]
- Loges, N.T.; Olbrich, H.; Becker-Heck, A.; Häffner, K.; Heer, A.; Reinhard, C.; Schmidts, M.; Kispert, A.; Zariwala, M.a.; Leigh, M.W.; et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 2009, 85, 883–889. [Google Scholar] [CrossRef]
- Bower, R.; Tritschler, D.; VanderWaal, K.; Perrone, C.A.; Mueller, J.; Fox, L.; Sale, W.S.; Porter, M.E. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol. Biol. Cell 2013, 24, 1134–1152. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tritschler, D.; Song, K.; Barber, C.F.; Cobb, J.S.; Porter, M.E.; Nicastro, D. Building Blocks of the Nexin-Dynein Regulatory Complex in Chlamydomonas Flagella. J. Biol. Chem. 2011, 286, 29175–29191. [Google Scholar] [CrossRef] [PubMed]
- Awata, J.; Song, K.; Lin, J.; King, S.M.; Sanderson, M.J.; Nicastro, D.; Witman, G.B. DRC3 connects the N-DRC to dynein g to regulate flagellar waveform. Mol. Biol. Cell 2015, 26, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Wirschell, M.; Olbrich, H.; Werner, C.; Tritschler, D.; Bower, R.; Sale, W.S.; Loges, N.T.; Pennekamp, P.; Lindberg, S.; Stenram, U.; et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat. Genet. 2013, 45, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.C.; O’Toole, E.; Perrone, C.A.; Giddings, T.; Porter, M.E. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J. Cell Biol. 1994, 127, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Piperno, G.; Mead, K.; LeDizet, M.; Moscatelli, A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J. Cell Biol. 1994, 125, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Piperno, G.; Mead, K.; Shestak, W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 1992, 118, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E. Ciliary and flagellar motility and the nexin-dynein regulatory complex. In Dyneins, 2nd ed.; King, S.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2018; pp. 298–335. [Google Scholar]
- Gui, M.; Ma, M.; Sze-Tu, E.; Wang, X.; Koh, F.; Zhong, E.D.; Berger, B.; Davis, J.H.; Dutcher, S.K.; Zhang, R.; et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 2021, 28, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Grossman-Haham, I.; Coudray, N.; Yu, Z.; Wang, F.; Zhang, N.; Bhabha, G.; Vale, R.D. Structure of the radial spoke head and insights into its role in mechanoregulation of ciliary beating. Nat. Struct. Mol. Biol. 2021, 28, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Diener, D.R.; Yang, C.; Kohno, T.; Pazour, G.J.; Dienes, J.M.; Agrin, N.S.; King, S.M.; Sale, W.S.; Kamiya, R.; et al. Radial spoke proteins of Chlamydomonas flagella. J. Cell Sci. 2006, 119, 1165–1174. [Google Scholar] [CrossRef]
- Gaillard, A.R.; Diener, D.R.; Rosenbaum, J.L.; Sale, W.S. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP). J. Cell Biol. 2001, 153, 443–448. [Google Scholar] [CrossRef]
- Gupta, A.; Diener, D.R.; Sivadas, P.; Rosenbaum, J.L.; Yang, P. The versatile molecular complex component LC8 promotes several distinct steps of flagellar assembly. J. Cell Biol. 2012, 198, 115–126. [Google Scholar] [CrossRef]
- Jivan, A.; Earnest, S.; Juang, Y.-C.; Cobb, M.H. Radial Spoke Protein 3 Is a Mammalian Protein Kinase A-anchoring Protein That Binds ERK1/2. J. Biol. Chem. 2009, 284, 29437–29445. [Google Scholar] [CrossRef]
- Michel, J.J.C.; Scott, J.D. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 235–257. [Google Scholar] [CrossRef]
- Luconi, M.; Cantini, G.; Baldi, E.; Forti, G. Role of a-kinase anchoring proteins (AKAPs) in reproduction. Front. Biosci. 2011, 16, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.G.; Lygren, B.; Dokurno, P.; Hoshi, N.; McConnachie, G.; Tasken, K.; Carlson, C.R.; Scott, J.D.; Barford, D. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol. Cell 2006, 24, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E.; Sale, W.S. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 2000, 151, F37–F42. [Google Scholar] [CrossRef]
- Diener, D.R.; Yang, P.; Geimer, S.; Cole, D.G.; Sale, W.S.; Rosenbaum, J.L. Sequential assembly of flagellar radial spokes. Cytoskeleton 2011, 68, 389–400. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Yang, P. Radial Spokes-A Snapshot of the Motility Regulation, Assembly, and Evolution of Cilia and Flagella. Cold Spring Harb. Perspect. Biol. 2017, 9, a028126–a028139. [Google Scholar] [CrossRef]
- Smith, E.F.; Lefebvre, P.A. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil. Cytoskeleton 1997, 38, 1–8. [Google Scholar] [CrossRef]
- Teves, M.E.; Nagarkatti-Gude, D.R.; Zhang, Z.; Strauss, J.F. Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes. Cytoskeleton 2016, 73, 3–22. [Google Scholar] [CrossRef]
- McKenzie, C.W.; Lee, L. Genetic interaction between central pair apparatus genes CFAP221, CFAP54, and SPEF2 in mouse models of primary ciliary dyskinesia. Sci. Rep. 2020, 10, 12337. [Google Scholar] [CrossRef]
- Lechtreck, K.F.; Gould, T.J.; Witman, G.B. Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia 2013, 2, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Ariyoshi, T.; Noga, A.; Kamiya, R.; Hirono, M. Space-Dependent Formation of Central Pair Microtubules and Their Interactions with Radial Spokes. PLoS ONE 2014, 9, e110513. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, Y.I.; Yao, E.; Lin, C.; Yang, J.-H.; Wilson, C.W.; Gacayan, R.; Chuang, P.-T. Fused (Stk36) is a ciliary protein required for central pair assembly and motile cilia orientation in the mammalian oviduct. Dev. Dyn. 2013, 242, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Oliveira, J.; Ferraz, L.; Barros, A.; Santos, R.; Sousa, M. Mutation analysis in patients with total sperm immotility. J. Assist. Reprod. Genet. 2015, 32, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Oliveira, J.; Sousa, M. A molecular approach to sperm immotility in humans: A review. Med. Reprod. Y Embriol. Clínica 2014, 1, 15–25. [Google Scholar] [CrossRef]
- Sousa, M.; Oliveira, E.; Alves, A.; Gouveia, M.; Figueiredo, H.; Ferraz, L.; Barros, A.; Sa, R. Ultrastructural analysis of five patients with total sperm immotility. Zygote 2015, 23, 900–907. [Google Scholar] [CrossRef]
- Grudzinskas, J.G.; Yovich, J.L. Sperm structure and function. In Gametes—The Spermatozoon; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Woolley, D.M.; Fawcett, D.W. The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anat. Rec. 1973, 177, 289–301. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Mazur, M.; Fishman, E.L.; Sindhwani, P. The Role of Sperm Centrioles in Human Reproduction—The Known and the Unknown. Front. Cell Dev. Biol. 2019, 7, 188–203. [Google Scholar] [CrossRef]
- Fawcett, D.W.; Phillips, D.M. The fine structure and development of the neck region of the mammalian spermatozoon. Anat. Rec. 1969, 165, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Garanina, A.S.; Alieva, I.B.; Bragina, E.E.; Blanchard, E.; Arbeille, B.; Guerif, F.; Uzbekova, S.; Uzbekov, R.E. The Centriolar Adjunct(-)Appearance and Disassembly in Spermiogenesis and the Potential Impact on Fertility. Cells 2019, 8, 180–191. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Lesich, K.A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210. [Google Scholar] [CrossRef]
- Mortimer, D. The functional anatomy of the human spermatozoon: Relating ultrastructure and function. Mol. Hum. Reprod. 2018, 24, 567–592. [Google Scholar] [CrossRef] [PubMed]
- Ounjai, P.; Kim, K.D.; Lishko, P.V.; Downing, K.H. Three-Dimensional Structure of the Bovine Sperm Connecting Piece Revealed by Electron Cryotomography. Biol. Reprod. 2012, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Stratton, C.J.; Bao, J.; Zheng, H.; Bhetwal, B.P.; Yanagimachi, R.; Yan, W. Spata6 is required for normal assembly of the sperm connecting piece and tight head–tail conjunction. Proc. Natl. Acad. Sci. USA 2015, 112, E430–E439. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Dai, S.; Shen, G.; Yang, Y.; Shen, Y. Identification of nonfunctional SPATA20 causing acephalic spermatozoa syndrome in humans. Clin. Genet. 2022, 1–10. [Google Scholar] [CrossRef]
- Oko, R. Comparative analysis of proteins from the fibrous sheath and outer dense fibers of rat spermatozoa. Biol. Reprod. 1988, 39, 169–182. [Google Scholar] [CrossRef]
- Fawcett, D.W. A comparative view of sperm ultrastructure. Biol. Reprod. 1970, 2, 90–127. [Google Scholar] [CrossRef]
- Baltz, J.M.; Williams, P.O.; Cone, R.A. Dense fibers protect mammalian sperm against damage. Biol. Reprod. 1990, 43, 485–491. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Ping, P.; Wang, G.; Yuan, X.; Sun, F. Outer dense fibers stabilize the axoneme to maintain sperm motility. J. Cell. Mol. Med. 2018, 22, 1755–1768. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rawe, V.Y. The making of abnormal spermatozoa: Cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010, 341, 349–357. [Google Scholar] [CrossRef]
- Brohmann, H.; Pinnecke, S.; Hoyer-Fender, S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J. Biol. Chem. 1997, 272, 10327–10332. [Google Scholar] [CrossRef]
- Shao, X.; Tarnasky, H.A.; Schalles, U.; Oko, R.; van der Hoorn, F.A. Interactional cloning of the 84-kDa major outer dense fiber protein Odf84. Leucine zippers mediate associations of Odf84 and Odf27. J. Biol. Chem. 1997, 272, 6105–6113. [Google Scholar] [CrossRef] [PubMed]
- Schalles, U.; Shao, X.; van der Hoorn, F.A.; Oko, R. Developmental expression of the 84-kDa ODF sperm protein: Localization to both the cortex and medulla of outer dense fibers and to the connecting piece. Dev. Biol. 1998, 199, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Diekman, A.B.; Olson, G.; Goldberg, E. Expression of the human antigen SPAG2 in the testis and localization to the outer dense fibers in spermatozoa. Mol. Reprod. Dev. 1998, 50, 284–293. [Google Scholar] [CrossRef]
- Shao, X.; Murthy, S.; Demetrick, D.J.; Van der Hoorn, F.A. Human outer dense fiber gene, ODF2, localizes to chromosome 9q34. Cytogenet. Genome Res. 1999, 83, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Fuzesi, L.; Hoyer-Fender, S. Outer dense fibre proteins from human sperm tail: Molecular cloning and expression analyses of two cDNA transcripts encoding proteins of approximately 70 kDa. Mol. Hum. Reprod. 1999, 5, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Tarnasky, H.A.; Lee, J.P.; Oko, R.; van der Hoorn, F.A. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev. Biol. 1999, 211, 109–123. [Google Scholar] [CrossRef]
- Shao, X.; Xue, J.; van der Hoorn, F.A. Testicular protein Spag5 has similarity to mitotic spindle protein Deepest and binds outer dense fiber protein Odf1. Mol. Reprod. Dev. 2001, 59, 410–416. [Google Scholar] [CrossRef]
- Fontaine, J.M.; Rest, J.S.; Welsh, M.J.; Benndorf, R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones 2003, 8, 62–69. [Google Scholar] [CrossRef]
- Zarsky, H.A.; Tarnasky, H.A.; Cheng, M.; van der Hoorn, F.A. Novel RING finger protein OIP1 binds to conserved amino acid repeats in sperm tail protein ODF1. Biol. Reprod. 2003, 68, 543–552. [Google Scholar] [CrossRef]
- Donkor, F.F.; Monnich, M.; Czirr, E.; Hollemann, T.; Hoyer-Fender, S. Outer dense fibre protein 2 (ODF2) is a self-interacting centrosomal protein with affinity for microtubules. J. Cell Sci. 2004, 117, 4643–4651. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Ghafouri-Fard, S.; Modarressi, M.H. Expression of splice variants of cancer-testis genes ODF3 and ODF4 in the testis of a prostate cancer patient. Genet. Mol. Res. 2012, 11, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Meinhardt, A.; Zhang, B.; Grzmil, P.; Adham, I.M.; Hoyer-Fender, S. The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol. Cell. Biol. 2012, 32, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, L.; Schneider, E.K.; Scott, C.; DeKretser, D.; Muller, C.H.; Hondermarck, H.; Velkov, T.; Baker, M.A. Deficiency in Outer Dense Fiber 1 Is a Marker and Potential Driver of Idiopathic Male Infertility. Mol. Cell. Proteomics 2016, 15, 3685–3693. [Google Scholar] [CrossRef]
- Tarnasky, H.; Cheng, M.; Ou, Y.; Thundathil, J.C.; Oko, R.; van der Hoorn, F.A. Gene trap mutation of murine Outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev. Biol. 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Ratinaud, M.H.; Cordelli, E.; Spanò, M.; Julien, R. Mouse testis cell sorting according to DNA and mitochondrial changes during spermatogenesis. Cytometry 1995, 19, 304–312. [Google Scholar] [CrossRef]
- Otani, H.; Tanaka, O.; Kasai, K.-I.; Yoshioka, T. Development of mitochondrial helical sheath in the middle piece of the mouse spermatid tail: Regular dispositions and synchronized changes. Anat. Rec. 1988, 222, 26–33. [Google Scholar] [CrossRef]
- Hawthorne, S.K.; Goodarzi, G.; Bagarova, J.; Gallant, K.E.; Busanelli, R.R.; Olend, W.J.; Kleene, K.C. Comparative genomics of the sperm mitochondria-associated cysteine-rich protein gene. Genomics 2006, 87, 382–391. [Google Scholar] [CrossRef]
- Hirata, S.; Hoshi, K.; Shoda, T.; Mabuchi, T. Spermatozoon and mitochondrial DNA. Reprod. Med. Biol. 2002, 1, 41–47. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Ford, W.C.L. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 2006, 12, 269–274. [Google Scholar] [CrossRef]
- du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Losano, J.D.A.; Angrimani, D.S.R.; Ferreira Leite, R.; Simões da Silva, B.D.C.; Barnabe, V.H.; Nichi, M. Spermatic mitochondria: Role in oxidative homeostasis, sperm function and possible tools for their assessment. Zygote 2018, 26, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Ford, W. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial functionality modifies human sperm acrosin activity, acrosome reaction capability and chromatin integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Ren, M.; Xu, Y.; Erdjument-Bromage, H.; Donelian, A.; Phoon, C.K.L.; Terada, N.; Strathdee, D.; Neubert, T.A.; Schlame, M. Extramitochondrial cardiolipin suggests a novel function of mitochondria in spermatogenesis. J. Cell Biol. 2019, 218, 1491–1502. [Google Scholar] [CrossRef]
- Demain, L.A.; Conway, G.S.; Newman, W.G. Genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017, 91, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Kao, S.-H.; Chao, H.-T.; Wei, Y.-H. Mitochondrial Deoxyribonucleic Acid 4977-bp Deletion is Associated with Diminished Fertility and Motility of Human Sperm1. Biol. Reprod. 1995, 52, 729–736. [Google Scholar] [CrossRef]
- Ni, F.; Zhou, Y.; Zhang, W.X.; Wang, X.M.; Song, X.M.; Jiang, H. Mitochondrial variations in the MT-ND4 and MT-TL1 genes are associated with male infertility. Syst. Biol. Reprod. Med. 2017, 63, 2–6. [Google Scholar] [CrossRef]
- Woodhouse, R.M.; Frolows, N.; Wang, G.; Hawdon, A.; Wong, E.H.K.; Dansereau, L.C.; Su, Y.; Adair, L.D.; New, E.J.; Philp, A.M.; et al. Mitochondrial succinate dehydrogenase function is essential for sperm motility and male fertility. iScience 2022, 25, 105573–105603. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Yeung, C.H.; Fetic, S.; Sobhani, A.; Nieschlag, E. Cytoplasmic droplets are normal structures of human sperm but are not well preserved by routine procedures for assessing sperm morphology. Hum. Reprod. 2004, 19, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Agarwal, A.; van der Linde, M.; du Plessis, S.S. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod. Biol. Endocrinol. 2012, 10, 92. [Google Scholar] [CrossRef]
- Cooper, T.G. The epididymis, cytoplasmic droplets and male fertility. Asian J. Androl. 2011, 13, 130–138. [Google Scholar] [CrossRef]
- Holstein, A.F.; Roosen-Runge, E.C. Spermatozoa. In Atlas of Human Spermatogenesis; Grosse: Berlin, Germany, 1981. [Google Scholar]
- Toure, A.; Rode, B.; Hunnicutt, G.R.; Escalier, D.; Gacon, G. Septins at the annulus of mammalian sperm. Biol. Chem. 2011, 392, 799–803. [Google Scholar] [CrossRef]
- Kwitny, S.; Klaus, A.V.; Hunnicutt, G.R. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol. Reprod. 2010, 82, 669–678. [Google Scholar] [CrossRef]
- Kuo, P.L.; Chiang, H.S.; Wang, Y.Y.; Kuo, Y.C.; Chen, M.F.; Yu, I.S.; Teng, Y.N.; Lin, S.W.; Lin, Y.H. SEPT12-microtubule complexes are required for sperm head and tail formation. Int. J. Mol. Sci. 2013, 14, 22102–22116. [Google Scholar] [CrossRef]
- Lin, Y.H.; Kuo, Y.C.; Chiang, H.S.; Kuo, P.L. The role of the septin family in spermiogenesis. Spermatogenesis 2011, 1, 298–302. [Google Scholar] [CrossRef]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-C.A.; Lin, Y.-H.; Kuo, Y.-C.; Shen, C.-J.; Pan, H.-A.; Kuo, P.-L. The expression pattern of SEPT7 correlates with sperm morphology. J. Assist. Reprod. Genet. 2010, 27, 299–307. [Google Scholar] [CrossRef]
- Kissel, H.; Georgescu, M.-M.; Larisch, S.; Manova, K.; Hunnicutt, G.R.; Steller, H. The Sept4 Septin Locus Is Required for Sperm Terminal Differentiation in Mice. Dev. Cell 2005, 8, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lin, Y.-M.; Wang, Y.-Y.; Yu, I.; Lin, Y.-W.; Wang, Y.-H.; Wu, C.-M.; Pan, H.-A.; Chao, S.-C.; Yen, P.H. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009, 174, 1857–1868. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Shen, Y.R.; Chen, H.I.; Lin, Y.H.; Wang, Y.Y.; Chen, Y.R.; Wang, C.Y.; Kuo, P.L. SEPT12 orchestrates the formation of mammalian sperm annulus by organizing core octameric complexes with other SEPT proteins. J. Cell Sci. 2015, 128, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Steels, J.D.; Estey, M.P.; Froese, C.D.; Reynaud, D.; Pace-Asciak, C.; Trimble, W.S. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil. Cytoskelet. 2007, 64, 794–807. [Google Scholar] [CrossRef]
- Hosseinifar, H.; Shafipour, M.; Modarresi, T.; Azad, M.; Sadighi Gilani, M.A.; Shahhosseini, M.; Sabbaghian, M. Relationship between absence of annulus and asthenozoospermia in Iranian men. J. Assist. Reprod. Genet. 2014, 31, 1681–1685. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, Y.H.; Chen, H.I.; Wang, Y.Y.; Chiou, Y.W.; Lin, H.H.; Pan, H.A.; Wu, C.M.; Su, S.M.; Hsu, C.C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef]
- Lhuillier, P.; Rode, B.; Escalier, D.; Lorès, P.; Dirami, T.; Bienvenu, T.; Gacon, G.; Dulioust, E.; Touré, a. Absence of annulus in human asthenozoospermia: Case report. Hum. Reprod. 2009, 24, 1296–1303. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, Y.Y.; Chen, H.I.; Kuo, Y.C.; Chiou, Y.W.; Lin, H.H.; Wu, C.M.; Hsu, C.C.; Chiang, H.S.; Kuo, P.L. SEPTIN12 genetic variants confer susceptibility to teratozoospermia. PLoS ONE 2012, 7, e34011. [Google Scholar] [CrossRef]
- Shen, Y.R.; Wang, H.Y.; Kuo, Y.C.; Shih, S.C.; Hsu, C.H.; Chen, Y.R.; Wu, S.R.; Wang, C.Y.; Kuo, P.L. SEPT12 phosphorylation results in loss of the septin ring/sperm annulus, defective sperm motility and poor male fertility. PLoS Genet. 2017, 13, e1006631. [Google Scholar] [CrossRef]
- Sugino, Y.; Ichioka, K.; Soda, T.; Ihara, M.; Kinoshita, M.; Ogawa, O.; Nishiyama, H. Septins as diagnostic markers for a subset of human asthenozoospermia. J. Urol. 2008, 180, 2706–2709. [Google Scholar] [CrossRef]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.a. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef]
- Johnson, L.R.; Foster, J.A.; Haig-Ladewig, L.; Vanscoy, H.; Rubin, C.S.; Moss, S.B.; Gerton, G.L. Assembly of AKAP82, a Protein Kinase A Anchor Protein, into the Fibrous Sheath of Mouse Sperm. Dev. Biol. 1997, 192, 340–350. [Google Scholar] [CrossRef]
- Li, Y.-F.; He, W.; Kim, Y.-H.; Mandal, A.; Digilio, L.; Klotz, K.; Flickinger, C.J.; Herr, J.C. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod. Biol. Endocrinol. 2010, 8, 101. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, H.; Pask, A.J.; O’Brien, D.A.; Shaw, G.; Renfree, M.B. A-kinase anchoring protein 4 has a conserved role in mammalian spermatogenesis. Reproduction 2009, 137, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, T.; Huang, Q.; Zhang, S.; Li, W.; Zhang, L.; Hess, R.A.; Pazour, G.J.; Zhang, Z. Intraflagellar transport protein 74 is essential for spermatogenesis and male fertility in mice. Biol. Reprod. 2019, 101, 188–199. [Google Scholar] [CrossRef]
- Kinukawa, M.; Oda, S.; Shirakura, Y.; Okabe, M.; Ohmuro, J.; Baba, S.A.; Nagata, M.; Aoki, F. Roles of cAMP in regulating microtubule sliding and flagellar bending in demembranated hamster spermatozoa. FEBS Lett. 2006, 580, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Almog, T.; Lazar, S.; Reiss, N.; Etkovitz, N.; Milch, E.; Rahamim, N.; Dobkin-Bekman, M.; Rotem, R.; Kalina, M.; Ramon, J.; et al. Identification of Extracellular Signal-regulated Kinase 1/2 and p38 MAPK as Regulators of Human Sperm Motility and Acrosome Reaction and as Predictors of Poor Spermatozoan Quality. J. Biol. Chem. 2008, 283, 14479–14489. [Google Scholar] [CrossRef] [PubMed]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef]
- Olmedo, S.B.; Nodar, F.; Chillik, C.; Chemes, H.E. Successful intracytoplasmic sperm injection with spermatozoa from a patient with dysplasia of the fibrous sheath and chronic respiratory disease. Hum. Reprod. 1997, 12, 1497–1499. [Google Scholar] [CrossRef]
- Chemes, H.E.; Olmedo, S.B.; Carrere, C.; Oses, R.; Carizza, C.; Leisner, M.; Blaquier, J. Ultrastructural pathology of the sperm flagellum: Association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum. Reprod. 1998, 13, 2521–2526. [Google Scholar] [CrossRef]
- Rawe, V.Y.; Galaverna, G.D.; Acosta, A.A.; Olmedo, S.B.; Chemes, H.E. Incidence of tail structure distortions associated with dysplasia of the fibrous sheath in human spermatozoa. Hum. Reprod. 2001, 16, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Rawe, V.Y.; Olmedo, S.B.; Benmusa, A.; Shiigi, S.M.; Chemes, H.E.; Sutovsky, P. Sperm ubiquitination in patients with dysplasia of the fibrous sheath. Hum. Reprod. 2002, 17, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Collodel, G.; Estenoz, M.; Manca, D.; Moretti, E.; Piomboni, P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum. Reprod. 2005, 20, 2790–2794. [Google Scholar] [CrossRef]
- Escalier, D.; Albert, M. New fibrous sheath anomaly in spermatozoa of men with consanguinity. Fertil. Steril. 2006, 86, 219.e211–219.e219. [Google Scholar] [CrossRef]
- Croft, J.T.; Zabeo, D.; Subramanian, R.; Höög, J.L. Composition, structure and function of the eukaryotic flagellum distal tip. Essays Biochem. 2018, 62, 815–828. [Google Scholar] [CrossRef]
- Zabeo, D.; Croft, J.T.; Höög, J.L. Axonemal doublet microtubules can split into two complete singlets in human sperm flagellum tips. FEBS Lett. 2019, 593, 892–902. [Google Scholar] [CrossRef]
- Zabeo, D.; Heumann, J.M.; Schwartz, C.L.; Suzuki-Shinjo, A.; Morgan, G.; Widlund, P.O.; Höög, J.L. A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep. 2018, 8, 2727. [Google Scholar] [CrossRef]
- Neal, C.V.; Hall-McNair, A.L.; Kirkman-Brown, J.; Smith, D.J.; Gallagher, M.T. Doing more with less: The flagellar end piece enhances the propulsive effectiveness of human spermatozoa. Phys. Rev. Fluids 2020, 5, 073101. [Google Scholar] [CrossRef]
- Kinukawa, M.; Ohmuro, J.; Baba, S.A.; Murashige, S.; Okuno, M.; Nagata, M.; Aoki, F. Analysis of Flagellar Bending in Hamster Spermatozoa: Characterization of an Effective Stroke. Biol. Reprod. 2005, 73, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Shingyoji, C. Effects of Imposed Bending on Microtubule Sliding in Sperm Flagella. Curr. Biol. 2004, 14, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Han, L.; Wang, Y.; Chai, P.; Kuo, Y.-w.; Yang, R.; Hu, F.; Yang, Y.; Howard, J.; Zhang, K. Structures of outer-arm dynein array on microtubule doublet reveal a motor coordination mechanism. Nat. Struct. Mol. Biol. 2021, 28, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Omoto, C.K.; Gibbons, I.R.; Kamiya, R.; Shingyoji, C.; Takahashi, K.; Witman, G.B. Rotation of the central pair microtubules in eukaryotic flagella. Mol. Biol. Cell 1999, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Loreng, T.D.; Smith, E.F. The Central Apparatus of Cilia and Eukaryotic Flagella. Cold Spring Harb. Perspect. Biol. 2017, 9, a028118–a028132. [Google Scholar] [CrossRef]
- Smith, E.F.; Yang, P. The radial spokes and central apparatus: Mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton 2004, 57, 8–17. [Google Scholar] [CrossRef]
- Smith, E.F. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell 2002, 13, 3303–3313. [Google Scholar] [CrossRef]
- DiPetrillo, C.G.; Smith, E.F. Pcdp1 is a central apparatus protein that binds Ca(2+)-calmodulin and regulates ciliary motility. J. Cell Biol. 2010, 189, 601–612. [Google Scholar] [CrossRef]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax 2003, 58, 333–338. [Google Scholar] [CrossRef]
- Chilvers, M.A.; O’Callaghan, C. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: Comparison with the photomultiplier and photodiode methods. Thorax 2000, 55, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Ferretti, F.; Masini, M.; Gualtieri, G.; Moretti, E. Influence of age on sperm characteristics evaluated by light and electron microscopies. Sci. Rep. 2021, 11, 4989. [Google Scholar] [CrossRef]
- Eskenazi, B.; Wyrobek, A.J.; Sloter, E.; Kidd, S.A.; Moore, L.; Young, S.; Moore, D. The association of age and semen quality in healthy men. Hum. Reprod. 2003, 18, 447–454. [Google Scholar] [CrossRef]
- Gadêlha, H.; Hernández-Herrera, P.; Montoya, F.; Darszon, A.; Corkidi, G. Human sperm uses asymmetric and anisotropic flagellar controls to regulate swimming symmetry and cell steering. Sci. Adv. 2020, 6, eaba5168. [Google Scholar] [CrossRef]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Stephens, D.T.; Trautman, K.; Smith, G.D.; Khatra, B.; da Cruz e Silva, E.F.; Greengard, P. Sperm Motility Development in the Epididymis is Associated with Decreased Glycogen Synthase Kinase-3 and Protein Phosphatase 1 Activity1. Biol. Reprod. 1996, 54, 709–718. [Google Scholar] [CrossRef]
- Ahmadi, H.; Csabai, T.; Gorgey, E.; Rashidiani, S.; Parhizkar, F.; Aghebati-Maleki, L. Composition and effects of seminal plasma in the female reproductive tracts on implantation of human embryos. Biomed. Pharmacother. 2022, 151, 113065. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Aljabali, S.M. Men with oligozoospermia had lower level of seminal plasma pyridoxine compared to normozoospermic men. Heliyon 2022, 8, e11983. [Google Scholar] [CrossRef] [PubMed]
- Banjoko, S.O.; Adeseolu, F.O. Seminal Plasma pH, Inorganic Phosphate, Total and Ionized Calcium Concentrations In The Assessment of Human Spermatozoa Function. J. Clin. Diagn. Res. 2013, 7, 2483–2486. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Parrilla, I.; Alminana, C.; del Olmo, D.; Roca, J.; Martinez, E.A.; Vazquez, J.M. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod. Domest. Anim. 2012, 47 (Suppl. 3), 12–21. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Jodar, M. Sperm and seminal plasma RNAs: What roles do they play beyond fertilization? Reproduction 2019, 158, R113–R123. [Google Scholar] [CrossRef]
- Karabulut, S.; Korkmaz, S.; Güneş, E.; Kabil, E.; Keskin, İ.; Usta, M.; Omurtag, G.Z. Seminal trace elements and their relationship with sperm parameters. Andrologia 2022, 54, e14610. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908.e2. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022–7049. [Google Scholar] [CrossRef]
- Mahdavinezhad, F.; Gilani, M.A.S.; Gharaei, R.; Ashrafnezhad, Z.; Valipour, J.; Nashtaei, M.S.; Amidi, F. Protective roles of seminal plasma exosomes and microvesicles during human sperm cryopreservation. Reprod. Biomed. Online 2022, 45, 341–353. [Google Scholar] [CrossRef]
- Mitra, A.; Richardson, R.T.; O’Rand, M.G. Analysis of Recombinant Human Semenogelin as an Inhibitor of Human Sperm Motility. Biol. Reprod. 2010, 82, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Druart, X. Sperm Interaction with the Female Reproductive Tract. Reprod. Domest. Anim. 2012, 47, 348–352. [Google Scholar] [CrossRef]
- Jokiniemi, A.; Magris, M.; Ritari, J.; Kuusipalo, L.; Lundgren, T.; Partanen, J.; Kekäläinen, J. Post-copulatory genetic matchmaking: HLA-dependent effects of cervical mucus on human sperm function. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201682. [Google Scholar] [CrossRef]
- Wang, H.; McGoldrick, L.L.; Chung, J.-J. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 2021, 18, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.S.; Avenarius, M.R.; Fellous, M.; Zhang, Y.; Meyer, N.C.; Auer, J.; Serres, C.; Kahrizi, K.; Najmabadi, H.; Beckmann, J.S.; et al. Genetic male infertility and mutation of CATSPER ion channels. Eur. J. Hum. Genet. 2010, 18, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Avenarius, M.R.; Hildebrand, M.S.; Zhang, Y.; Meyer, N.C.; Smith, L.L.; Kahrizi, K.; Najmabadi, H.; Smith, R.J. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 2009, 84, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Avidan, N.; Tamary, H.; Dgany, O.; Cattan, D.; Pariente, A.; Thulliez, M.; Borot, N.; Moati, L.; Barthelme, A.; Shalmon, L.; et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 2003, 11, 497–502. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Wiesehoefer, C.; Shah, N.B.; Reetz, E.; Hwang, J.Y.; Huang, X.; Wang, T.-e.; Lishko, P.V.; Davies, K.M.; et al. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat. Commun. 2022, 13, 3439. [Google Scholar] [CrossRef]
- Yang, F.; Gracia Gervasi, M.; Orta, G.; Tourzani, D.A.; De la Vega-Beltran, J.L.; Ruthel, G.; Darszon, A.; Visconti, P.E.; Wang, P.J. C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella. Development 2022, 149, dev199988. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wu, H.; Zhang, H.; Zhang, H.; Mao, J.; Liu, D.; Zhao, L.; Lin, H.; Tang, W.; et al. Sodium-Hydrogen-Exchanger expression in human sperm and its relationship with semen parameters. J. Assist. Reprod Genet. 2017, 34, 795–801. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.C.; Zuo, W.L.; Lu, C.L.; Chen, M.; Zhang, X.S.; Li, Y.C.; Cai, H.; Zhou, W.L.; Hu, Z.Y.; et al. A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Front. Biosci. 2010, 2, 566–581. [Google Scholar] [CrossRef]
- Wertheimer, E.V.; Salicioni, A.M.; Liu, W.; Trevino, C.L.; Chavez, J.; Hernández-González, E.O.; Darszon, A.; Visconti, P.E. Chloride Is Essential for Capacitation and for the Capacitation-associated Increase in Tyrosine Phosphorylation. J. Biol. Chem. 2008, 283, 35539–35550. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Xu, W.M.; Chen, Z.H.; Ni, Y.; Yuan, Y.Y.; Zhou, S.C.; Zhou, W.W.; Tsang, L.L.; Chung, Y.W.; Höglund, P.; et al. Cl− Is Required for HCO3− Entry Necessary for Sperm Capacitation in Guinea Pig: Involvement of a Cl−/HCO3− Exchanger (SLC26A3) and CFTR1. Biol. Reprod. 2009, 80, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Shi, Q.X.; Chen, W.Y.; Zhou, C.X.; Ni, Y.; Rowlands, D.K.; Yi Liu, G.; Zhu, H.; Ma, Z.G.; Wang, X.F.; et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl. Acad. Sci. USA 2007, 104, 9816–9821. [Google Scholar] [CrossRef]
- Rode, B.; Dirami, T.; Bakouh, N.; Rizk-Rabin, M.; Norez, C.; Lhuillier, P.; Lorès, P.; Jollivet, M.; Melin, P.; Zvetkova, I.; et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: A potential role during sperm capacitation. Hum. Mol. Genet. 2012, 21, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Puga Molina, L.C.; Pinto, N.A.; Torres, N.I.; González-Cota, A.L.; Luque, G.M.; Balestrini, P.A.; Romarowski, A.; Krapf, D.; Santi, C.M.; Treviño, C.L.; et al. CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC. J. Biol. Chem. 2018, 293, 9924–9936. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.-A.S.; Smith, J.F.; Lishko, P.V. Slo1 is the principal potassium channel of human spermatozoa. eLife 2013, 2, e01009. [Google Scholar] [CrossRef]
- Brenker, C.; Zhou, Y.; Müller, A.; Echeverry, F.A.; Trötschel, C.; Poetsch, A.; Xia, X.-M.; Bönigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 2014, 3, e01438. [Google Scholar] [CrossRef]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, F.; Ramos-Espiritu, L.S.; Buck, J.; Levin, L.R.; Manfredi, G. cAMP and Mitochondria. Physiology 2013, 28, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; Verheyen, G.; Raick, D.; Camus, M.; Devroey, P.; Tournaye, H. Absolute asthenozoospermia and ICSI: What are the options? Hum. Reprod. Update 2011, 17, 684–692. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Shen, L.; Zheng, A.; Meng, Q.; Li, H.; Yang, S. Clinical detection, diagnosis and treatment of morphological abnormalities of sperm flagella: A review of literature. Front. Genet. 2022, 13, 1034951–1034967. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]

| Infertility Phenotype | Genes |
|---|---|
| Asthenospermia | AKAP3, AKAP4, AXDND1, CATSPER1, CATSPER2, CATSPER3, CATSPER4, CCDC103, CCDC40, CFAP43, CFAP44, CFAP70, COPS7A, CRHR1, CUL3, DEFB126, DNAAF1, DNAAF6, DNAH6, DNAH11, DNAH17, DNAH5, DNAH8, DNAH9, DNAI1, DNAJB13, DNHD1, DRC1, HIP1, HTX11, INSL6 IQCG, IQUB, KLHL7, KRT34, LRRC6, MT-C03, NEDD4, NSUN7, QRICH2, RSPH3, RSPH6A, SEPTIN4, SLC26A8, SPAG17, SPATA33, TEKT2, ZMYND10 |
| Multiple morphological anomalies of the flagella (MMAF) | BRWD1, CCDC34, CCDC39, CEP135, CFAP251, CFAP58, CFAP61, CFAP69, CFAP74, DNAH1, DNAH10, DNAH17, DNAH2, DNAH5, DNAH6, DNAH7, DNAH8, DZIP1, DZP1, FSIP2, MAATS1, ODF2, QRICH2, SPAG6, SPATA16, SPEF2, TTC21A, TTC29, WDR19, WDR66 |
| Nonobstructive azoospermia | AR, ABLIM1, AHRR, ART3, ATM, AZFa, AZFb, AZFc, BCL2, BPDY2, BPY2, CCDC34, CDC42BPA, CDY2A, DAZ1, DBX3Y, DMC1, DMRT1, DNMT3B, EPSTI1, ETV5, FANCM, GNAO1, HLA-DRA, HSF2, HSFY1, KLHL10, M1AP, MCM8, MEIOB, MLH3, MSMB, MTHFR, NANOS1, NPAS2, NR5A1, PACRG, PIWIL2, PNLDC1, PYGO2, RBMX, RBYMIAI, REC8, SIRPG, SOHLH1, SOX5, SPINK2, SRSF6, STAG3, STX2, SYCE1, SYCE1L, SYCP3, TAF4B, TDRD9, TEX11, TEX14, TEX15, USP9Y, WT1, XRCC2, ZMYND15 |
| Obstructive azoospermia | ADGRG2, CFTR |
| Oligozoospermia | AXDND1, DAZ1, DAZ2, DICER1, DNMT1, EPHX2, GSTM1, GSTT1, KIT, KITLG, NR0B1, NR5A1, OR2W3, PARP1, PIWIL3, PIWIL4, PLK4, PON1, PON2, PRM1, PSAT1, SIRPA, SOX6, USP8, ZMYND15 |
| Teratozoospermia | AURKC, BSCL2, CCIN, CCDC90B, CCDC91, DPY19L2, SPATA20, SPA17, CYP1A1, FBXO43, PPP2R3C, SEPTIN12, ZPBP, DPY19L2, PICK1, SPATA16, SEPTIN4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.; Sousa, M. Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum. Genes 2023, 14, 383. https://doi.org/10.3390/genes14020383
Pereira R, Sousa M. Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum. Genes. 2023; 14(2):383. https://doi.org/10.3390/genes14020383
Chicago/Turabian StylePereira, Rute, and Mário Sousa. 2023. "Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum" Genes 14, no. 2: 383. https://doi.org/10.3390/genes14020383
APA StylePereira, R., & Sousa, M. (2023). Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum. Genes, 14(2), 383. https://doi.org/10.3390/genes14020383






