Pharmacogenetic Analysis of the MIR146A rs2910164 and MIR155 rs767649 Polymorphisms and Response to Anti-TNF Treatment in Patients with Crohn’s Disease and Psoriasis
Abstract
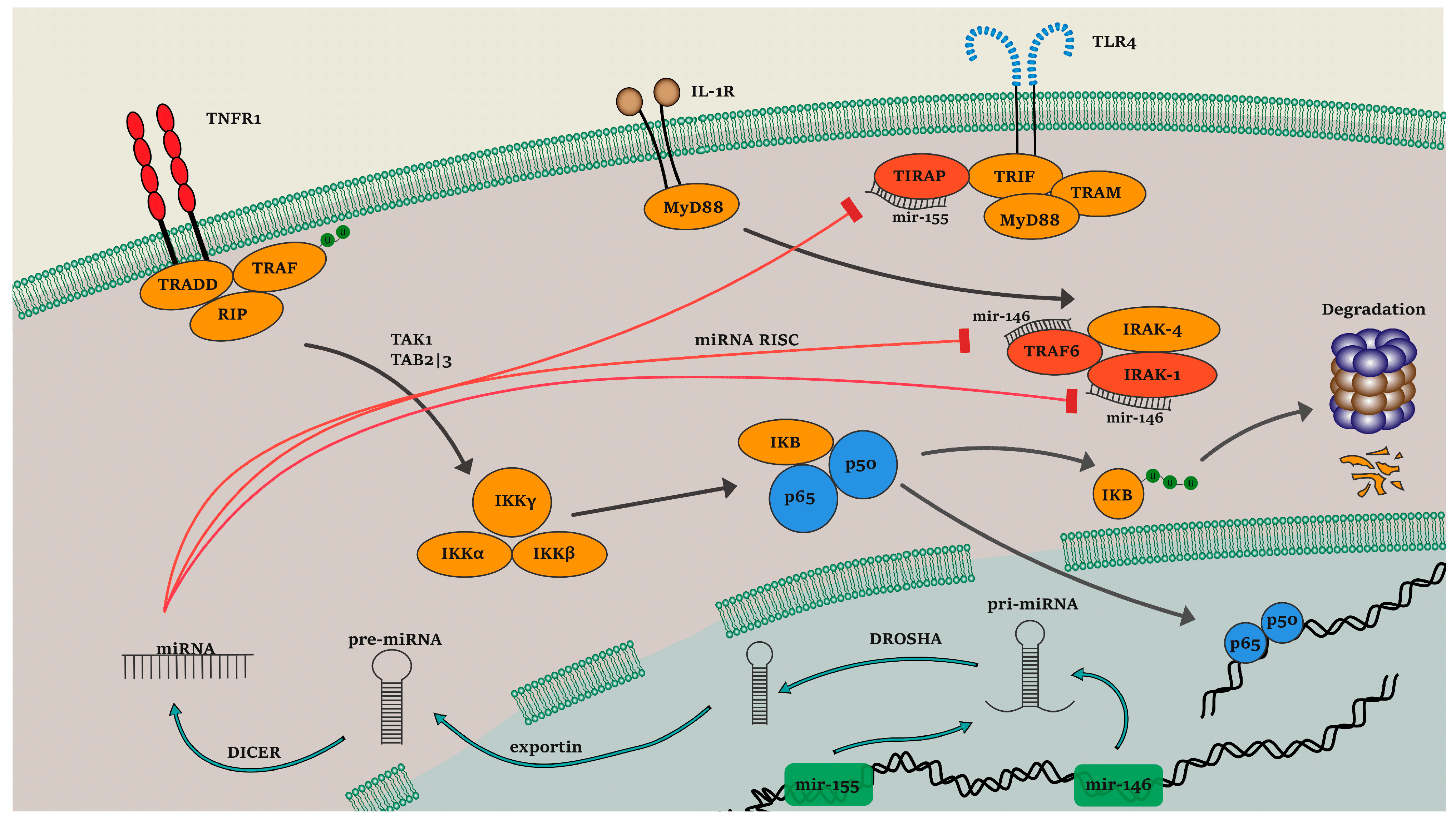
:1. Introduction
2. Materials and Methods
2.1. Clinical Assessment of the Included Patients
2.2. Genotyping
2.3. TFBS Analysis on MIR155 rs767649
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Included Patients
3.2. Genotyping Results
3.3. Motif Analysis Uncovers the Functional Role of MIR155 rs767649
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayden, M.S.; Ghosh, S. Regulation of NF-ΚB by TNF Family Cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Wang, M.-H.; Picco, M.F. Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 449–461. [Google Scholar] [CrossRef]
- Antonatos, C.; Grafanaki, K.; Asmenoudi, P.; Xiropotamos, P.; Nani, P.; Georgakilas, G.K.; Georgiou, S.; Vasilopoulos, Y. Contribution of the Environment, Epigenetic Mechanisms and Non-Coding RNAs in Psoriasis. Biomedicines 2022, 10, 1934. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s Disease Inflammation and Recurrence. Biol. Direct. 2020, 15, 23. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Chima, M.; Lebwohl, M. TNF Inhibitors for Psoriasis. Semin. Cutan. Med. Surg. 2018, 37, 134–142. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Mylonas, A.; Conrad, C. Psoriasis: Classical vs. Paradoxical. The Yin-Yang of TNF and Type I Interferon. Front. Immunol. 2018, 9, 2746. [Google Scholar] [CrossRef]
- Masouri, S.; Stefanaki, I.; Ntritsos, G.; Kypreou, K.P.; Drakaki, E.; Evangelou, E.; Nicolaidou, E.; Stratigos, A.J.; Antoniou, C. A Pharmacogenetic Study of Psoriasis Risk Variants in a Greek Population and Prediction of Responses to Anti-TNF-α and Anti-IL-12/23 Agents. Mol. Diagn. Ther. 2016, 20, 221–225. [Google Scholar] [CrossRef]
- Pierik, M.; Rutgeerts, P.; Vlietinck, R.; Vermeire, S. Pharmacogenetics in Inflammatory Bowel Disease. World J. Gastroenterol. WJG 2006, 12, 3657. [Google Scholar]
- Mohagheghi Darehranj, S.; Alatab, S.; Vahedi, H.; Sadeghi, A.; Sima, A.; Malekzadeh, M.; Anoshiravani, A.; Fakheri, H.; Ebrahimi Daryani, N.; Mousavi, A.; et al. Efficacy of Anti-TNF Therapy for the Treatment of Patients with Moderate-to-Severe Inflammatory Bowel Disease; a First Iranian Report. Middle East J. Dig. Dis. 2019, 12, 12–18. [Google Scholar] [CrossRef]
- Yamauchi, P.S.; Bissonnette, R.; Teixeira, H.D.; Valdecantos, W.C. Systematic Review of Efficacy of Anti–Tumor Necrosis Factor (TNF) Therapy in Patients with Psoriasis Previously Treated with a Different Anti–TNF Agent. J. Am. Acad. Dermatol. 2016, 75, 612–618.e6. [Google Scholar] [CrossRef]
- Hornschuh, M.; Wirthgen, E.; Wolfien, M.; Singh, K.P.; Wolkenhauer, O.; Däbritz, J. The Role of Epigenetic Modifications for the Pathogenesis of Crohn’s Disease. Clin. Epigenet. 2021, 13, 108. [Google Scholar] [CrossRef]
- Brooks, W.H.; Le Dantec, C.; Pers, J.-O.; Youinou, P.; Renaudineau, Y. Epigenetics and Autoimmunity. J. Autoimmun. 2010, 34, J207–J219. [Google Scholar] [CrossRef]
- Stanczyk, J.; Pedrioli, D.M.L.; Brentano, F.; Sanchez-Pernaute, O.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; Kyburz, D. Altered Expression of MicroRNA in Synovial Fibroblasts and Synovial Tissue in Rheumatoid Arthritis. Arthritis Rheum. 2008, 58, 1001–1009. [Google Scholar] [CrossRef]
- Nakasa, T.; Miyaki, S.; Okubo, A.; Hashimoto, M.; Nishida, K.; Ochi, M.; Asahara, H. Expression of MicroRNA-146 in Rheumatoid Arthritis Synovial Tissue. Arthritis Rheum. 2008, 58, 1284–1292. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA Profiling of Multiple Sclerosis Lesions Identifies Modulators of the Regulatory Protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef]
- Li, Y.; Du, C.; Wang, W.; Ma, G.; Cui, L.; Zhou, H.; Tao, H.; Yao, L.; Zhao, B.; Li, K. Genetic Association of MiR-146a with Multiple Sclerosis Susceptibility in the Chinese Population. Cell. Physiol. Biochem. 2015, 35, 281–291. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.S.; Lee, K.H.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Smith, L.; Koyanagi, A.; Jacob, L.; Li, H.; et al. Roles of MicroRNAs in Inflammatory Bowel Disease. Int. J. Biol. Sci. 2021, 17, 2112–2123. [Google Scholar] [CrossRef]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. MiR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef]
- El-Komy, M.; Amin, I.; El-Hawary, M.S.; Saadi, D.; Shaker, O. Upregulation of the MiRNA-155, MiRNA-210, and MiRNA-20b in Psoriasis Patients and Their Relation to IL-17. Int. J. Immunopathol. Pharmacol. 2020, 34, 205873842093374. [Google Scholar] [CrossRef]
- Wang, S.; Cao, X.; Ding, B.; Chen, J.; Cui, M.; Xu, Y.; Lu, X.; Zhang, Z.; He, A.; Jin, H. The Rs767649 Polymorphism in the Promoter of MiR-155 Contributes to the Decreased Risk for Cervical Cancer in a Chinese Population. Gene 2016, 595, 109–114. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Wei, Q.-K.; Yuan, Y.; Zhou, F.; Ge, Y.-Y.; Yang, J.-R.; Su, H.; Zhuang, S.-M. A Functional Polymorphism in the MiR-146a Gene Is Associated with the Risk for Hepatocellular Carcinoma. Carcinogenesis 2008, 29, 2126–2131. [Google Scholar] [CrossRef]
- Zou, D.; Yin, J.; Ye, Z.; Zeng, Q.; Tian, C.; Wang, Y.; Chen, Q.; Chen, R. Association between the MiR-146a Rs2910164 Polymorphism and Childhood Acute Lymphoblastic Leukemia Susceptibility in an Asian Population. Front. Genet. 2020, 11, 886. [Google Scholar] [CrossRef]
- Papaconstantinou, I.; Kapizioni, C.; Legaki, E.; Xourgia, E.; Karamanolis, G.; Gklavas, A.; Gazouli, M. Association of MiR-146 Rs2910164, MiR-196a Rs11614913, MiR-221 Rs113054794 and MiR-224 Rs188519172 Polymorphisms with Anti-TNF Treatment Response in a Greek Population with Crohn’s Disease. WJGPT 2017, 8, 193–200. [Google Scholar] [CrossRef]
- Stavrou, E.F.; Chatzopoulou, F.; Antonatos, C.; Pappa, P.; Makridou, E.; Oikonomou, K.; Kapsoritakis, A.; Potamianos, P.S.; Karmiris, K.; Tzathas, C.; et al. Pharmacogenetic Analysis of Canonical versus Noncanonical Pathway of NF-KB in Crohn’s Disease Patients under Anti-Tumor Necrosis Factor-α Treatment. Pharm. Genom. 2022, 32, 235–241. [Google Scholar] [CrossRef]
- Dezfuli, N.K.; Adcock, I.M.; Alipoor, S.D.; Seyfi, S.; Salimi, B.; Mafi Golchin, M.; Dalil Roofchayee, N.; Varhram, M.; Mortaz, E. The MiR-146a SNP Rs2910164 and MiR-155 SNP Rs767649 Are Risk Factors for Non-Small Cell Lung Cancer in the Iranian Population. Can. Respir. J. 2020, 2020, 8179415. [Google Scholar] [CrossRef]
- Akkız, H.; Bayram, S.; Bekar, A.; Akgöllü, E.; Üsküdar, O.; Sandıkçı, M. No Association of Pre-MicroRNA-146a Rs2910164 Polymorphism and Risk of Hepatocellular Carcinoma Development in Turkish Population: A Case-Control Study. Gene 2011, 486, 104–109. [Google Scholar] [CrossRef]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Berhanu Lemma, R.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Pérez, N.; et al. JASPAR 2022: The 9th Release of the Open-Access Database of Transcription Factor Binding Profiles. Nucleic Acids Res. 2022, 50, D165–D173. [Google Scholar] [CrossRef]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for Occurrences of a given Motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Associations between Functional Polymorphisms in the NFκB Signaling Pathway and Response to Anti-TNF Treatment in Danish Patients with Inflammatory Bowel Disease. Pharm. J. 2014, 14, 526–534. [Google Scholar] [CrossRef]
- Ovejero-Benito, M.C.; Muñoz-Aceituno, E.; Sabador, D.; Almoguera, B.; Prieto-Pérez, R.; Hakonarson, H.; Coto-Segura, P.; Carretero, G.; Reolid, A.; Llamas-Velasco, M.; et al. Genome-wide Association Analysis of Psoriasis Patients Treated with Anti-TNF Drugs. Exp. Dermatol. 2020, 29, 1225–1232. [Google Scholar] [CrossRef]
- Lauro, R.; Mannino, F.; Irrera, N.; Squadrito, F.; Altavilla, D.; Squadrito, G.; Pallio, G.; Bitto, A. Pharmacogenetics of Biological Agents Used in Inflammatory Bowel Disease: A Systematic Review. Biomedicines 2021, 9, 1748. [Google Scholar] [CrossRef]
- Caputo, V.; Strafella, C.; Cosio, T.; Lanna, C.; Campione, E.; Novelli, G.; Giardina, E.; Cascella, R. Pharmacogenomics: An Update on Biologics and Small-Molecule Drugs in the Treatment of Psoriasis. Genes 2021, 12, 1398. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, S.; Wu, X.; Pu, X.; Kang, X. Association of Rs2910164 Polymorphism in MiR-146a Gene with Psoriasis Susceptibility: A Meta-Analysis. Medicine 2019, 98, e14401. [Google Scholar] [CrossRef]
- Ran, D.; Cai, M.; Zhang, X. Genetics of Psoriasis: A Basis for Precision Medicine. Precis. Clin. Med. 2019, 2, 120–130. [Google Scholar] [CrossRef]
- Nowak, J.K.; Adams, A.T.; Kalla, R.; Lindstrøm, J.C.; Vatn, S.; Bergemalm, D.; Keita, Å.V.; Gomollón, F.; Jahnsen, J.; Vatn, M.H.; et al. Characterisation of the Circulating Transcriptomic Landscape in Inflammatory Bowel Disease Provides Evidence for Dysregulation of Multiple Transcription Factors Including NFE2, SPI1, CEBPB, and IRF2. J. Crohn’s Colitis 2022, 16, 1255–1268. [Google Scholar] [CrossRef]
- Polina, E.R.; Oliveira, F.M.; Sbruzzi, R.C.; Crispim, D.; Canani, L.H.; Santos, K.G. Gene Polymorphism and Plasma Levels of MiR-155 in Diabetic Retinopathy. Endocr. Connect. 2019, 8, 1591–1599. [Google Scholar] [CrossRef]
- Xie, K.; Ma, H.; Liang, C.; Wang, C.; Qin, N.; Shen, W.; Gu, Y.; Yan, C.; Zhang, K.; Dai, N.; et al. A Functional Variant in MiR-155 Regulation Region Contributes to Lung Cancer Risk and Survival. Oncotarget 2015, 6, 42781–42792. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, S.E.; Shaker, O.G.; Abdelwahed, M.Y.; Ahmed, N.A.; Abdelhameed, H.G.; Bosilah, A.H.; Mohammed, S.R. Association of MicroRNA-155 Rs767649 Polymorphism with Susceptibility to Preeclampsia. Int. J. Mol. Cell. Med. 2019, 8. [Google Scholar] [CrossRef]
- Mohammed, S.R.; Shaker, O.G.; Mohammed, A.A.; Fouad, N.A.; Hussein, H.A.; Ahmed, N.A.; Ahmed, O.M.; Ali, D.Y.; Mohamed, M.M.; Ibrahim, A.A. Impact of MiR-155 (Rs767649 A>T) and MiR-146a (Rs57095329 A>G) Polymorphisms in System Lupus Erythematosus Susceptibility in an Egyptian Cohort. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1425–1435. [Google Scholar]
- Dikeakos, P.; Theodoropoulos, G.; Rizos, S.; Tzanakis, N.; Zografos, G.; Gazouli, M. Association of the MiR-146aC>G, MiR-149T>C, and MiR-196a2T>C Polymorphisms with Gastric Cancer Risk and Survival in the Greek Population. Mol. Biol. Rep. 2014, 41, 1075–1080. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Mourmoura, E.; Balis, C.; Tsezou, A. Impact of MiR-SNP Rs2910164 on MiR-146a Expression in Osteoarthritic Chondrocytes. Adv. Med. Sci. 2020, 65, 78–85. [Google Scholar] [CrossRef]
- Han, Y.M.; Koh, J.; Kim, J.W.; Lee, C.; Koh, S.-J.; Kim, B.; Lee, K.L.; Im, J.P.; Kim, J.S. NF-Kappa B Activation Correlates with Disease Phenotype in Crohn’s Disease. PLoS ONE 2017, 12, e0182071. [Google Scholar] [CrossRef] [Green Version]
| Primer Sequence | Tm | Restriction Enzyme | |
|---|---|---|---|
| MIR155 rs767649 | |||
| Forward primer | 5′-CCTGTATGACAAGGTTGTGTTTG-3′ | 58.9 °C | Tsp45I |
| Reverse primer | 5′-GCTGGCATACTATTCTACCCATAA-3′ | 59.3 °C | |
| MIR146A rs2910164 | |||
| Forward primer | 5′-CATGGGTTGTGTCAGTGTCAGAGCT-3′ | 64.6 °C | SacI |
| Reverse primer | 5′-TGCCTTCTGTCTCCAGTCTTCCAA-3′ | 62.7 °C | |
| Variable | Psoriasis (n = 100) | Crohn’s Disease (n = 103) |
|---|---|---|
| Age in years, mean ± S.D. | 45.12 ± 11.23 | 43 ± 15.33 |
| Disease duration in years, mean ± S.D. | 15.11 ± 10.35 | 6.54 ± 3.1 |
| Age of onset in years, mean ± S.D. | 38.25 ± 10.75 | 39 ± 11.25 |
| Clinical index at baseline, mean ± S.D. | 28.85 ± 9.65 | 171.14 ± 114.17 |
| Clinical index after the duration of the therapy, mean ± S.D. | 4.27 ± 4.52 | 48.57 ± 47.82 |
| Responders, percentage | 68 (68%) | 71 (68.9%) |
| Nonresponders, percentage | 32 (32%) | 32 (31.1%) |
| Anti-TNF therapy (R/NR) | ||
| Etanercept | 37 (24/13) | - |
| Infliximab | 53 (41/12) | 84 (56/28) |
| Adalimumab | 10 (3/7) | 19 (15/4) |
| Gene, Variant | Statistical Test | R | NR | OR | 95% CI | P | PC |
|---|---|---|---|---|---|---|---|
| MIR146A rs2910164 | Genotypic [GG/GC/CC] | 68/3/0 | 29/3/0 | - | - | 0.507 | >0.99 |
| Cochran–Armitage [G/C] | 139/3 | 61/3 | 2.278 | 0.447–11.611 | 0.321 | >0.99 | |
| Dominant [(GG + GC)/CC] | 71/0 | 32/0 | 2.2 | 0.042–113.332 | 0.695 | >0.99 | |
| Recessive [GG/(GC + CC)] | 68/3 | 29/3 | 2.344 | 0.446–12.310 | 0.313 | >0.99 | |
| MIR155 rs767649 | Genotypic [TT/TA/AA] | 29/32/10 | 13/18/1 | - | - | 0.217 | 0.870 |
| Cochran–Armitage [T/A] | 90/52 | 44/20 | 0.786 | 0.419–1.476 | 0.454 | >0.99 | |
| Dominant [(TT + TA)/AA] | 61/10 | 31/1 | 0.196 | 0.024–1.607 | 0.129 | 0.517 | |
| Recessive [TT/(TA + AA)] | 29/42 | 13/19 | 1.009 | 0.431–2.359 | 0.983 | >0.99 |
| Gene, Variant | Statistical Test | R | NR | OR | 95% CI | P | PC |
|---|---|---|---|---|---|---|---|
| MIR146A rs2910164 | Genotypic [GG/GC/CC] | 65/3/0 | 27/4/1 | - | - | 0.107 | 0.430 |
| Cochran–Armitage [G/C] | 133/3 | 58/6 | 4.586 | 1.108–18.970 | 0.035 | 0.142 | |
| Dominant [(GG + GC)/CC] | 68/0 | 31/1 | 6.523 | 0.258–164.649 | 0.254 | >0.99 | |
| Recessive [GG/(GC + CC)] | 65/3 | 27/5 | 4.012 | 0.895–17.983 | 0.069 | 0.278 | |
| MIR155 rs767649 | Genotypic [TT/TA/AA] | 30/37/1 | 26/6/0 | - | - | 0.002 | 0.008 |
| Cochran–Armitage [T/A] | 97/39 | 58/6 | 0.257 | 0.102–0.644 | 0.003 | 0.012 | |
| Dominant [(TT + TA)/AA] | 67/1 | 32/0 | 0.692 | 0.027–17.464 | 0.823 | >0.99 | |
| Recessive [TT/(TA + AA)] | 30/38 | 26/6 | 0.182 | 0.066–0.499 | 0.001 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nani, P.; Ladopoulou, M.; Papaioannou, E.H.; Papagianni, E.D.; Antonatos, C.; Xiropotamos, P.; Kapsoritakis, A.; Potamianos, P.S.; Karmiris, K.; Tzathas, C.; et al. Pharmacogenetic Analysis of the MIR146A rs2910164 and MIR155 rs767649 Polymorphisms and Response to Anti-TNF Treatment in Patients with Crohn’s Disease and Psoriasis. Genes 2023, 14, 445. https://doi.org/10.3390/genes14020445
Nani P, Ladopoulou M, Papaioannou EH, Papagianni ED, Antonatos C, Xiropotamos P, Kapsoritakis A, Potamianos PS, Karmiris K, Tzathas C, et al. Pharmacogenetic Analysis of the MIR146A rs2910164 and MIR155 rs767649 Polymorphisms and Response to Anti-TNF Treatment in Patients with Crohn’s Disease and Psoriasis. Genes. 2023; 14(2):445. https://doi.org/10.3390/genes14020445
Chicago/Turabian StyleNani, Paraskevi, Melpomeni Ladopoulou, Evgenia H. Papaioannou, Evangelia D. Papagianni, Charalabos Antonatos, Panagiotis Xiropotamos, Andreas Kapsoritakis, Petros S. Potamianos, Konstantinos Karmiris, Charalambos Tzathas, and et al. 2023. "Pharmacogenetic Analysis of the MIR146A rs2910164 and MIR155 rs767649 Polymorphisms and Response to Anti-TNF Treatment in Patients with Crohn’s Disease and Psoriasis" Genes 14, no. 2: 445. https://doi.org/10.3390/genes14020445






