Influence of the Osteogenomic Profile in Response to Alendronate Therapy in Postmenopausal Women with Osteoporosis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Bone Densitometry Measurements
2.3. Isolation of Genomic DNA
2.4. SNP Genotyping
2.5. Response to Treatment
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristics of Study Subjects
3.2. Allele and Genotype Frequencies and Profile Structure
3.3. SNPs and Profiles Associated with the Alendronate Treatment Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspray, T.; Hill, T. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef]
- Li, W.; Hou, S.; Yu, B.; Jin, D.; Férec, C.; Chen, J. Genetics of osteoporosis: Perspectives for personalized medicine. Pers. Med. 2010, 7, 655–668. [Google Scholar] [CrossRef]
- Mondockova, V.; Adamkovicova, M.; Lukacova, M.; Grosskopf, B.; Babosova, R.; Galbavy, D.; Martiniakova, M.; Omelka, R. The estrogen receptor 1 gene affects bone mineral density and osteoporosis treatment efficiency in Slovak postmenopausal women. BMC Med. Genet. 2018, 19, 174. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2018, 51, 258–266. [Google Scholar] [CrossRef]
- Marini, F.; Brandi, M. The future of pharmacogenetics for osteoporosis. Pharmacogenomics 2013, 14, 641–653. [Google Scholar] [CrossRef]
- Hopwood, B.; Tsykin, A.; Findlay, D.; Fazzalari, N. Gene expression profile of the bone microenvironment in human fragility fracture bone. Bone 2009, 44, 87–101. [Google Scholar] [CrossRef]
- Vega, D.; Maalouf, N.; Sakhaee, K. The Role of Receptor Activator of Nuclear Factor-κB (RANK)/RANK Ligand/Osteoprotegerin: Clinical Implications. J. Clin. Endocrinol. Metab. 2007, 92, 4514–4521. [Google Scholar] [CrossRef]
- Zupan, J.; Mencej-Bedrač, S.; Jurković-Mlakar, S.; Preželj, J.; Marc, J. Gene–gene interactions in RANK/RANKL/OPG system influence bone mineral density in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2010, 118, 102–106. [Google Scholar] [CrossRef]
- Merlotti, D.; Gennari, L.; Stolakis, K.; Nuti, R. Aromatase Activity and Bone Loss in Men. J. Osteoporos. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Lau, H.; Ho, A.; Luk, K.; Kung, A. Transforming Growth Factor β-1 Gene Polymorphisms and Bone Turnover, Bone Mineral Density and Fracture Risk in Southern Chinese Women. Calcif. Tissue Int. 2004, 74, 516–521. [Google Scholar] [CrossRef]
- Marini, F.; Brandi, M. Pharmacogenetics of osteoporosis. F1000 Biol. Rep. 2010, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Sabik, O.; Farber, C. Using GWAS to identify novel therapeutic targets for osteoporosis. Transl. Res. 2017, 181, 15–26. [Google Scholar] [CrossRef]
- Bone, H.G.; Hosking, D.; Devogelaer, J.-P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Ten Years’ Experience with Alendronate for Osteoporosis in Postmenopausal Women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef]
- Cairoli, E.; Eller-Vainicher, C.; Ulivieri, F.M.; Zhukouskaya, V.V.; Palmieri, S.; Morelli, V.; Beck-Peccoz, P.; Chiodini, I. Factors associated with bisphosphonate treatment failure in postmenopausal women with primary osteoporosis. Osteoporos. Int. 2014, 25, 1401–1410. [Google Scholar] [CrossRef]
- Sebba, A. Significance of a decline in bone mineral density while receiving oral bisphosphonate treatment. Clin. Ther. 2008, 30, 443–452. [Google Scholar] [CrossRef]
- Díez-Pérez, A.; González-Macías, J. Inadequate responders to osteoporosis treatment: Proposal for an operational definition. Osteoporos. Int. 2008, 19, 1511–1516. [Google Scholar] [CrossRef]
- Díez-Pérez, A.; Olmos, J.; Nogués, X.; Sosa, M.; Díaz-Curiel, M.; Pérez-Castrillón, J.; Pérez-Cano, R.; Muñoz-Torres, M.; Torrijos, A.; Jodar, E.; et al. Risk factors for prediction of inadequate response to antiresorptives. J. Bone Miner. Res. 2012, 27, 817–824. [Google Scholar] [CrossRef]
- Okazaki, R.; Muraoka, R.; Maehara, M.; Inoue, D. Factors associated with inadequate responses to risedronate in Japanese patients with osteoporosis. J. Bone Miner. Metab. 2018, 37, 185–197. [Google Scholar] [CrossRef]
- Abdi, S.; Bukhari, I.; Ansari, M.G.A.; BinBaz, R.A.; Mohammed, A.K.; Hussain, S.D.; Aljohani, N.; Al-Daghri, N.M. Association of Polymorphisms in RANK and RANKL Genes with Osteopenia in Arab Postmenopausal Women. Dis. Markers 2020, 2020, 1285216. [Google Scholar] [CrossRef]
- Ettinger, B.; Harris, S.T.; Kendler, D.; Kessel, B.; McClung, M.R.; Gorodeski, G.I.; Rothert, M.L.; Henderson, V.W.; Richardson, M.K.; Freedman, R.R.; et al. Management of osteoporosis in Postmenopausal women. Menopause 2010, 17, 25–54. [Google Scholar]
- Breuil, V.; Cortet, B.; Cotté, F.-E.; Arnould, B.; Dias-Barbosa, C.; Gaudin, A.-F.; Regnault, A.; De Climens, A.R.; Legrand, E. Validation of the adherence evaluation of osteoporosis treatment (ADEOS) questionnaire for osteoporotic post-menopausal women. Osteoporos. Int. 2011, 23, 445–455. [Google Scholar] [CrossRef]
- Schousboe, J.; Shepherd, J.; Bilezikian, J.; Baim, S. Executive Summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Bone Densitometry. J. Clin. Densitom. 2013, 16, 455–466.35. [Google Scholar] [CrossRef]
- Rockenbauer, E.; Børsting, C.; Stangegaard, M.; Frank-Hansen, R.; Morling, N. Successful STR and SNP typing of FTA Card samples with low amounts of DNA after DNA extraction using a Qiagen BioRobot® EZ1 Workstation. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 83–84. [Google Scholar] [CrossRef]
- Stangegaard, M.; Børsting, C.; Ferrero-Miliani, L.; Frank-Hansen, R.; Poulsen, L.; Hansen, A.; Schousboe, J.T.; Shepherd, J.A.; Bilezikian, J.P.; Baim, S. Evaluation of Four Automated Protocols for Extraction of DNA from FTA Cards. SLAS Technol. 2013, 18, 404–410. [Google Scholar] [CrossRef]
- Livak, K. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet. Anal. Biomol. Eng. 1999, 14, 143–149. [Google Scholar] [CrossRef]
- Richards, J.B. Collaborative meta-analysis: Associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann. Intern. Med. 2009, 151, 528. [Google Scholar] [CrossRef]
- Zavala-Cerna, M.G.; Moran-Moguel, M.C.; Cornejo-Toledo, J.A.; Gonzalez-Montoya, N.G.; Sanchez-Corona, J.; Salazar-Paramo, M.; Gamez-Nava, J.I. Osteoprotegerin polymorphisms in a Mexican population with rheumatoid arthritis and generalized osteoporosis: A preliminary report. J. Immunol. Res. 2015, 2015, 376197. [Google Scholar] [CrossRef]
- Magaña, J.; Gómez, R.; Cisneros, B.; Casas, L.; Valdés-Flores, M. Association of Interleukin-6 Gene Polymorphisms with Bone Mineral Density in Mexican Women. Arch. Med. Res. 2008, 39, 618–624. [Google Scholar] [CrossRef]
- Méndez, J.; Rojano-Mejía, D.; Coral-Vázquez, R.; Coronel, A.; Pedraza, J.; Casas, M.; Soriano, R.; García-García, E.; Vilchis, F.; Canto, P. Impact of genetic variants of IL-6, IL6R, LRP5, ESR1 and SP7 genes on bone mineral density in postmenopausal Mexican-Mestizo women with obesity. Gene 2013, 528, 216–220. [Google Scholar] [CrossRef]
- Nice.org.uk. 1 Recommendations|Bisphosphonates for Treating Osteoporosis|Guidance|NICE. 2022. Available online: https://www.nice.org.uk/guidance/ta464/chapter/1-Recommendations (accessed on 13 April 2022).
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Francis, R. Non-response to osteoporosis treatment. Br. Menopause Soc. J. 2004, 10, 76–80. [Google Scholar] [CrossRef]
- Watts, N.B.; Lewiecki, E.M.; Bonnick, S.L.; Laster, A.J.; Binkley, N.; Blank, R.D.; Geusens, P.P.; Miller, P.D.; Petak, S.M.; Recker, R.R.; et al. Clinical Value of Monitoring BMD in Patients Treated with Bisphosphonates for Osteoporosis. J. Bone Miner. Res. 2009, 24, 1643–1646. [Google Scholar] [CrossRef]
- Nguyen, T.; Eisman, J. Genetic profiling and individualized assessment of fracture risk. Nat. Rev. Endocrinol. 2013, 9, 153–161. [Google Scholar] [CrossRef]
- Marozik, P.; Alekna, V.; Rudenko, E.; Tamulaitiene, M.; Rudenka, A.; Mastaviciute, A.; Samokhovec, V.; Cernovas, A.; Kobets, K.; Mosse, I. Bone metabolism genes variation and response to bisphosphonate treatment in women with postmenopausal osteoporosis. PLoS ONE 2019, 14, e0221511. [Google Scholar] [CrossRef]
- Nava-Valdivia, C.; Saldaña-Cruz, A.; Corona-Sanchez, E.; Murillo-Vazquez, J.; Moran-Moguel, M.; Salazar-Paramo, M.; Perez-Guerrero, E.E.; Vazquez-Villegas, M.L.; Bonilla-Lara, D.; Rocha-Muñoz, A.D.; et al. Polymorphism rs2073618 of the TNFRSF11B (OPG) Gene and Bone Mineral Density in Mexican Women with Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 7680434. [Google Scholar] [CrossRef]
- Piedra, M.; García-Unzueta, M.T.; Berja, A.; Paule, B.; A Lavín, B.; Valero, C.; A Riancho, J.; A Amado, J. Single nucleotide polymorphisms of the OPG/RANKL system genes in primary hyperparathyroidism and their relationship with bone mineral density. BMC Med. Genet. 2011, 12, 168. [Google Scholar] [CrossRef]
- Napoli, N.; Rastelli, A.; Ma, C.; Yarramaneni, J.; Vattikutti, S.; Moskowitz, G.; Giri, T.; Mueller, C.; Kulkarny, V.; Qualls, C.; et al. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER (+) breast cancer. Bone 2013, 55, 309–314. [Google Scholar] [CrossRef]
- Gerdhem, P.; Stiger, F.; Lerner, U.H.; Lorentzon, M.; Obrant, K.; Nordström, A.; Brändström, H.; Nordström, P.; Akesson, K. Interleukin-6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75-year-old women. Osteoporos. Int. 2004, 15, 820–826. [Google Scholar] [CrossRef]
- Fajar, J.; Azharuddin, A. The association between interleukin 6 −174 G/C gene polymorphism and the risk of osteoporosis: A meta-analysis. J. Taibah Univ. Med. Sci. 2017, 12, 212–220. [Google Scholar] [CrossRef]
- Moffett, S.P.; Oakley, J.I.; A Cauley, J.; Lui, L.Y.; Ensrud, K.; Taylor, B.C.; Hillier, T.A.; Hochberg, M.C.; Li, J.; Cayabyab, S.; et al. Osteoprotegerin Lys3Asn Polymorphism and the Risk of Fracture in Older Women. J. Clin. Endocrinol. Metab. 2008, 93, 2002–2008. [Google Scholar] [CrossRef]
- Takacs, I.; Lazáry, Á.; Kósa, J.; Kiss, J.; Balla, B.; Nagy, Z.; Bácsi, K.; Speer, G.; Lakatos, P. Allelic variations of RANK/RANKL/OPG signaling system is related to bone mineral density and in vivo gene expression. Bone 2009, 44, S116. [Google Scholar] [CrossRef]
- Karaplis, A.C.; Chouha, F.; Djandji, M.; Sampalis, J.S.; Hanley, D.A. Vitamin D status and response to daily 400 IU vitamin D3 and weekly alendronate 70 mg in men and women with osteoporosis. Ann. Pharmacother. 2011, 45, 561–568. [Google Scholar] [CrossRef]
- Gennari, L. Estrogen Receptor Gene Polymorphisms and the Genetics of Osteoporosis: A HuGE Review. Am. J. Epidemiol. 2005, 161, 307–320. [Google Scholar] [CrossRef]
| Variable | OP Osteoporosis n = 82 | |
|---|---|---|
| Sex Female, n (%) | 82 (100) | |
| Smoking, n (%) | ||
| Never | 57 (69.6) | |
| Former smoker | 8 (9.7) | |
| Current | 17 (20.7) | |
| Age (years), mean ± SD | 65 ± 8.9 | |
| Body Mass Index (kg/m2), mean ± SD | 26.1 ± 3.9 | |
| Normal or low weight, n (%) | 36 (43.9) | |
| Overweight and obesity, n (%) | 46 (56.1) | |
| Menopause, n (%) | 82 (100) | |
| BMD | ||
| Base line | 12 months | |
| BMD Hip | 0.818 g/cm2 ± 0.090 | 0.850 g/cm2 ± 0.133 |
| BMD L1–L4 | 0.768 g/cm2 ± 0.075 | 0.800 g/cm2 ± 0.080 |
| Treatment | ||
| Monotherapy with alendronate | 82 (100) | |
| Results of the BMD at year | ||
| Nonresponse to alendronate | 26 (31.7) | |
| Response to alendronate | 56 (68.3) | |
| Polymorphism | Allele Frequency (%) | Genotype | Number | Genotype Frequency | HWE p Value |
|---|---|---|---|---|---|
| rs700518 | A = 18.3 C G = 81.7 T | AA AG GG | 4 22 56 | 4.9 26.8 68.2 | X2 = 0.384 p = 0.535 |
| rs9340799 | A = 58.7 G G = 41.3 A | AA AG GG | 21 46 8 | 28 61.3 10.7 | X2 = 5.253 p = 0.021 |
| rs1800795 | G = 89.6 C = 10.4 | GG GC CC | 66 15 1 | 80.5 18.3 1.2 | X2 = 0.172 p = 0.678 |
| rs724449 | C = 43.3 A T = 56.7 G | CC CT TT | 11 49 22 | 13.4 59.8 26.8 | X2 = 3.862 p = 0.0490 |
| rs3102735 | A = 77.2 T G = 22.8 C | AA AG GG | 49 27 5 | 60.5 33.3 6.2 | X2 = 0.058 p = 0.809 |
| rs2073618 | G = 74.4 C = 25.6 | GG GC CC | 43 33 4 | 53.7 41.3 5 | X2 = 0.540 p = 0.462 |
| rs1800469 | G = 61.5 T A = 38.5 C | GG GA AA | 30 36 12 | 38.5 46.1 15.4 | X2 = 0.049 p = 0.825 |
| rs9533156 | T = 92 C = 62 | TT TC CC | 22 48 7 | 28.6 62.3 9.1 | X2 = 6.743 p = 0.0094 |
| SNP | Genotype | Response | ||
|---|---|---|---|---|
| Si | No | p-Value | ||
| rs700518 | AA | 1 | 3 | 0.116 |
| AG | 14 | 8 | ||
| GG | 41 | 15 | ||
| rs9340799 | AA | 14 | 7 | 0.477 |
| AG | 32 | 17 | ||
| GG | 10 | 2 | ||
| rs1800795 | GG | 1 | 0 | 0.697 |
| GC | 11 | 4 | ||
| CC | 44 | 22 | ||
| rs724449 | CC | 6 | 5 | 0.223 |
| CT | 37 | 12 | ||
| TT | 13 | 9 | ||
| rs3102735 | AA | 3 | 2 | 0.749 |
| AG | 20 | 7 | ||
| GG | 33 | 16 | ||
| rs2073618 | GG | 2 | 2 | 0.365 |
| GC | 21 | 13 | ||
| CC | 32 | 11 | ||
| rs1800469 | GG | 17 | 13 | 0.173 |
| GA | 27 | 10 | ||
| AA | 10 | 2 | ||
| rs9533156 | TT | 4 | 4 | 0.538 |
| TC | 34 | 15 | ||
| CC | 16 | 7 | ||
| Profile | Response (%) | No Response | p-Value | OR | p-Value |
|---|---|---|---|---|---|
| G1G2C3A5C6 (1) | 30 (68.2) | 14 (31.8) | 0.981 | 1.005 | 0.98 |
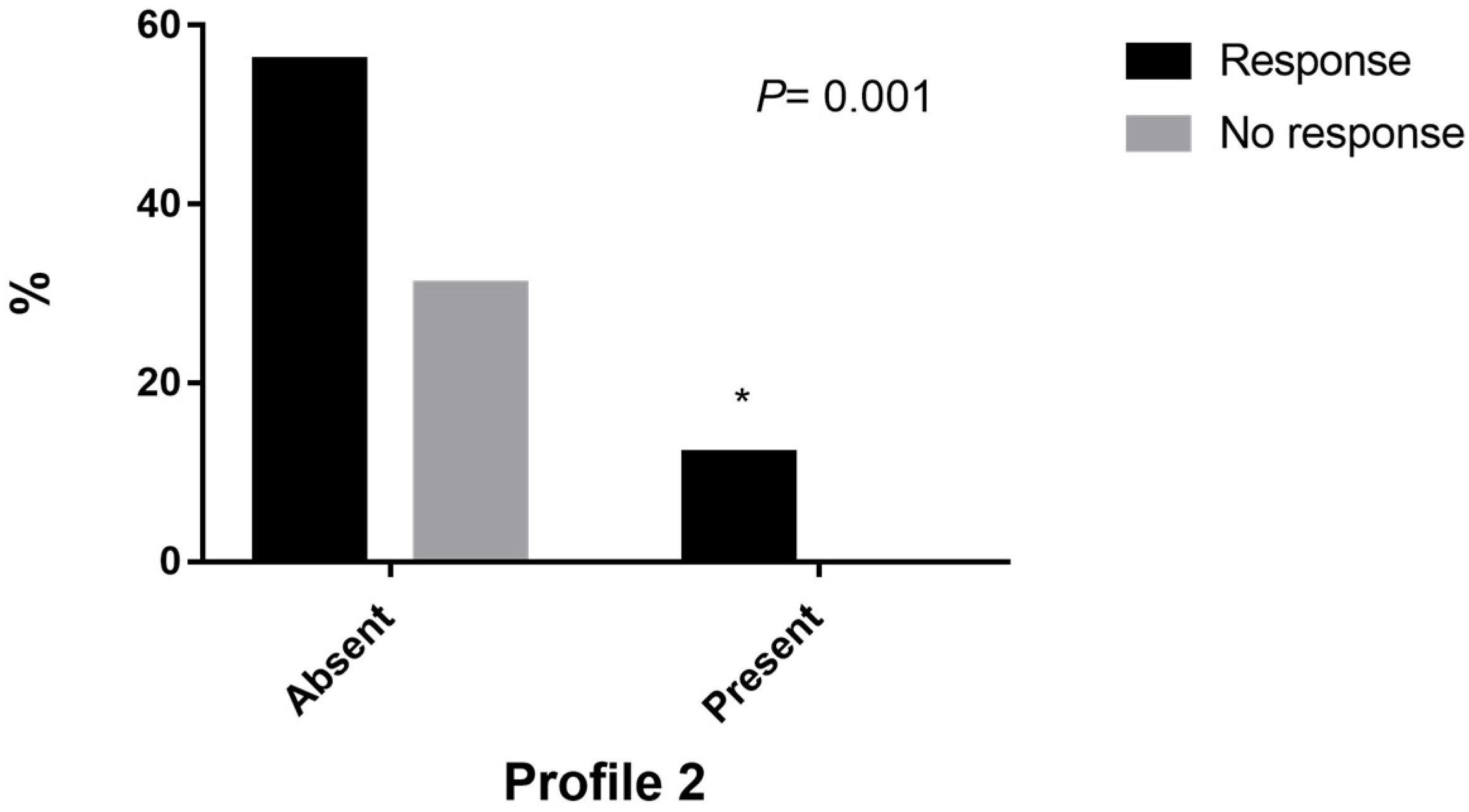
| G1C3G5C6 (2) | 10 (100) | 0 | 0.001 * | - | 0.021 |
| G1C3A6C8 (3) | 10 (66.6) | 5 (33.4) | 0.881 | 1.007 | 0.881 |
| G1C3A5G6 (4) | 6 (66.6) | 3 (33.4) | 0.912 | 1.007 | 0.912 |
| G1C3C6 (5) | 9 (60) | 6 (40) | 0.445 | 1.436 | 0.445 |
| C3A5G7 (6) | 5 (50) | 5 (50) | 0.185 | 2.154 | 0.185 |
| G1C3G5 (7) | 13 (86.7) | 2 (13.3) | 0.078 | 0.331 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villagómez Vega, A.; Gámez Nava, J.I.; Ruiz González, F.; Pérez Romero, M.; Trujillo Rangel, W.Á.; Nuño Arana, I. Influence of the Osteogenomic Profile in Response to Alendronate Therapy in Postmenopausal Women with Osteoporosis: A Retrospective Cohort Study. Genes 2023, 14, 524. https://doi.org/10.3390/genes14020524
Villagómez Vega A, Gámez Nava JI, Ruiz González F, Pérez Romero M, Trujillo Rangel WÁ, Nuño Arana I. Influence of the Osteogenomic Profile in Response to Alendronate Therapy in Postmenopausal Women with Osteoporosis: A Retrospective Cohort Study. Genes. 2023; 14(2):524. https://doi.org/10.3390/genes14020524
Chicago/Turabian StyleVillagómez Vega, Alejandra, Jorge Iván Gámez Nava, Francisco Ruiz González, Misael Pérez Romero, Walter Ángel Trujillo Rangel, and Ismael Nuño Arana. 2023. "Influence of the Osteogenomic Profile in Response to Alendronate Therapy in Postmenopausal Women with Osteoporosis: A Retrospective Cohort Study" Genes 14, no. 2: 524. https://doi.org/10.3390/genes14020524
APA StyleVillagómez Vega, A., Gámez Nava, J. I., Ruiz González, F., Pérez Romero, M., Trujillo Rangel, W. Á., & Nuño Arana, I. (2023). Influence of the Osteogenomic Profile in Response to Alendronate Therapy in Postmenopausal Women with Osteoporosis: A Retrospective Cohort Study. Genes, 14(2), 524. https://doi.org/10.3390/genes14020524






