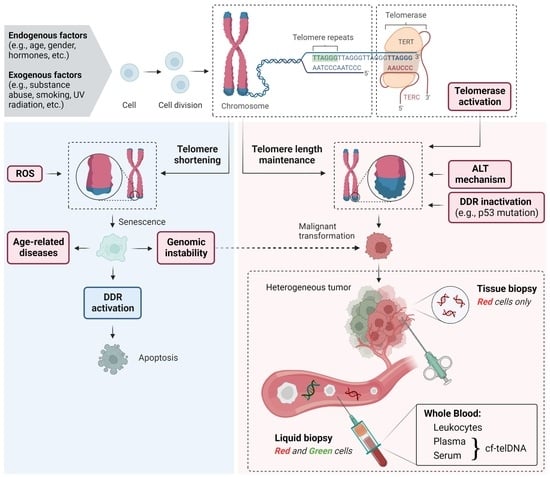
Telomere Length Changes in Cancer: Insights on Carcinogenesis and Potential for Non-Invasive Diagnostic Strategies
Abstract
:1. Introduction
2. Telomere Structure and Homeostasis
3. Telomeres in Health and Disease
3.1. Role of Telomeres in Non-Cancer Diseases
3.2. Role of Telomeres in Cancer
4. Applications of Telomeric DNA in Cancer Assessment
4.1. Tissue/Cell-Derived DNA-Based Approaches
4.2. Cell-Free DNA-Based Approaches
5. Methodological Aspects of Telomere Length Measurement
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinkova, A.; Brynychova, I.; Svacina, A.; Jirkovska, M.; Korabecna, M. Cell-Free DNA from Human Plasma and Serum Differs in Content of Telomeric Sequences and Its Ability to Promote Immune Response. Sci. Rep. 2017, 7, 2591. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Three Decades of Progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Trybek, T.; Kowalik, A.; Góźdź, S.; Kowalska, A. Telomeres and Telomerase in Oncogenesis (Review). Oncol. Lett. 2020, 20, 1015–1027. [Google Scholar] [CrossRef]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Characteristics and Applications. Eur. J. Hum. Genet. 2018, 26, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Szilágyi, M.; Pös, O.; Márton, É.; Buglyó, G.; Soltész, B.; Keserű, J.; Penyige, A.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Main Characteristics and Clinical Application. Int. J. Mol. Sci. 2020, 21, 6827. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of Telomeres and Telomerase in Cancer, and Advances in Telomerase-Targeted Therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doksani, Y.; Wu, J.Y.; de Lange, T.; Zhuang, X. Super-Resolution Fluorescence Imaging of Telomeres Reveals TRF2-Dependent T-Loop Formation. Cell 2013, 155, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommerup, H.; Dousmanis, A.; de Lange, T. Unusual Chromatin in Human Telomeres. Mol. Cell. Biol. 1994, 14, 5777–5785. [Google Scholar] [PubMed] [Green Version]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The Shortest Telomere, not Average Telomere Length, Is Critical for Cell Viability and Chromosome Stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres Shorten during Ageing of Human Fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does Oxidative Stress Shorten Telomeres in vivo? A Review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Zglinicki, T. Oxidative Stress Shortens Telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Fouquerel, E.; Lormand, J.; Bose, A.; Lee, H.-T.; Kim, G.S.; Li, J.; Sobol, R.W.; Freudenthal, B.D.; Myong, S.; Opresko, P.L. Oxidative Guanine Base Damage Regulates Human Telomerase Activity. Nat. Struct. Mol. Biol. 2016, 23, 1092–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef] [Green Version]
- di Fagagna, F.D.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA Damage Checkpoint Response in Telomere-Initiated Senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Muraki, K.; Nyhan, K.; Han, L.; Murnane, J.P. Mechanisms of Telomere Loss and Their Consequences for Chromosome Instability. Front. Oncol. 2012, 2, 135. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- de Lange, T. How Telomeres Solve the End-Protection Problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekaert, S.; Derradji, H.; Baatout, S. Telomere Biology in Mammalian Germ Cells and during Development. Dev. Biol. 2004, 274, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase Activity in Human Germline and Embryonic Tissues and Cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and Telomerase in Stem Cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [Green Version]
- De Vitis, M.; Berardinelli, F.; Sgura, A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devereux, T.R.; Horikawa, I.; Anna, C.H.; Annab, L.A.; Afshari, C.A.; Barrett, J.C. DNA Methylation Analysis of the Promoter Region of the Human Telomerase Reverse Transcriptase (hTERT) Gene. Cancer Res. 1999, 59, 6087–6090. [Google Scholar]
- Shin, K.-H.; Kang, M.K.; Dicterow, E.; Park, N.-H. Hypermethylation of the hTERT Promoter Inhibits the Expression of Telomerase Activity in Normal Oral Fibroblasts and Senescent Normal Oral Keratinocytes. Br. J. Cancer 2003, 89, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinn, R.L.; Pruitt, K.; Eguchi, S.; Baylin, S.B.; Herman, J.G. hTERT Is Expressed in Cancer Cell Lines despite Promoter DNA Methylation by Preservation of Unmethylated DNA and Active Chromatin around the Transcription Start Site. Cancer Res. 2007, 67, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Napier, C.E.; Vryer, R.; Dimitriadis, E.; Manuelpillai, U.; Sharkey, A.; Craig, J.M.; Reddel, R.R.; Saffery, R. DNA Methylation Mediated up-Regulation of TERRA Non-Coding RNA Is Coincident with Elongated Telomeres in the Human Placenta. Mol. Hum. Reprod. 2016, 22, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Slatter, T.L.; Tan, X.; Yuen, Y.C.; Gunningham, S.; Ma, S.S.; Daly, E.; Packer, S.; Devenish, C.; Royds, J.A.; Hung, N.A. The Alternative Lengthening of Telomeres Pathway May Operate in Non-Neoplastic Human Cells. J. Pathol. 2012, 226, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pedram, M.; Sprung, C.N.; Gao, Q.; Lo, A.W.I.; Reynolds, G.E.; Murnane, J.P. Telomere Position Effect and Silencing of Transgenes near Telomeres in the Mouse. Mol. Cell. Biol. 2006, 26, 1865–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere Position Effect: Regulation of Gene Expression with Progressive Telomere Shortening over Long Distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippe, K.; Luke, B. TERRA and the State of the Telomere. Nat. Struct. Mol. Biol. 2015, 22, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, L.; Lu, S. Role of TERRA in the Regulation of Telomere Length. Int. J. Biol. Sci. 2015, 11, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Stout, G.J.; Blasco, M.A. Telomere Length and Telomerase Activity Impact the UV Sensitivity Syndrome Xeroderma Pigmentosum C. Cancer Res. 2013, 73, 1844–1854. [Google Scholar] [CrossRef] [Green Version]
- Gorenjak, V.; Akbar, S.; Stathopoulou, M.G.; Visvikis-Siest, S. The Future of Telomere Length in Personalized Medicine. Front. Biosci. 2018, 23, 1628–1654. [Google Scholar]
- Bakaysa, S.L.; Mucci, L.A.; Slagboom, P.E.; Boomsma, D.I.; McClearn, G.E.; Johansson, B.; Pedersen, N.L. Telomere Length Predicts Survival Independent of Genetic Influences. Aging Cell 2007, 6, 769–774. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Blackburn, E.H.; Shannon, K.M. The Rate of Telomere Sequence Loss in Human Leukocytes Varies with Age. Proc. Natl. Acad. Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef] [Green Version]
- Dalgård, C.; Benetos, A.; Verhulst, S.; Labat, C.; Kark, J.D.; Christensen, K.; Kimura, M.; Kyvik, K.O.; Aviv, A. Leukocyte Telomere Length Dynamics in Women and Men: Menopause vs Age Effects. Int. J. Epidemiol. 2015, 44, 1688–1695. [Google Scholar] [CrossRef] [Green Version]
- Fair, B.; Mellon, S.H.; Epel, E.S.; Lin, J.; Révész, D.; Verhoeven, J.E.; Penninx, B.W.; Reus, V.I.; Rosser, R.; Hough, C.M.; et al. Telomere Length Is Inversely Correlated with Urinary Stress Hormone Levels in Healthy Controls but not in Un-Medicated Depressed Individuals-Preliminary Findings. J. Psychosom. Res. 2017, 99, 177–180. [Google Scholar] [CrossRef]
- Coburn, S.B.; Graubard, B.I.; Trabert, B.; McGlynn, K.A.; Cook, M.B. Associations between Circulating Sex Steroid Hormones and Leukocyte Telomere Length in Men in the National Health and Nutrition Examination Survey. Andrology 2018, 6, 542–546. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated Telomere Shortening in Response to Life Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [Green Version]
- Vidacek, N.Š.; Nanic, L.; Ravlic, S.; Sopta, M.; Geric, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.W.; Fung, T.T.; McEvoy, C.T.; Lin, J.; Epel, E.S. Diet Quality Indices and Leukocyte Telomere Length Among Healthy US Adults: Data from the National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2018, 187, 2192–2201. [Google Scholar] [CrossRef] [Green Version]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [Green Version]
- Welendorf, C.; Nicoletti, C.F.; Pinhel, M.A.d.S.; Noronha, N.Y.; de Paula, B.M.F.; Nonino, C.B. Obesity, Weight Loss, and Influence on Telomere Length: New Insights for Personalized Nutrition. Nutrition 2019, 66, 115–121. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Hayes, M.G.; Kuzawa, C.W. Delayed Paternal Age of Reproduction in Humans Is Associated with Longer Telomeres across Two Generations of Descendants. Proc. Natl. Acad. Sci. USA 2012, 109, 10251–10256. [Google Scholar] [CrossRef] [Green Version]
- Alexeeff, S.E.; Schaefer, C.A.; Kvale, M.N.; Shan, J.; Blackburn, E.H.; Risch, N.; Ranatunga, D.K.; Jorgenson, E.; Hoffmann, T.J.; Sakoda, L.C.; et al. Telomere Length and Socioeconomic Status at Neighborhood and Individual Levels among 80,000 Adults in the Genetic Epidemiology Research on Adult Health and Aging Cohort. Environ. Epidemiol. 2019, 3, e049. [Google Scholar] [CrossRef]
- Yamaki, N.; Matsushita, S.; Hara, S.; Yokoyama, A.; Hishimoto, A.; Higuchi, S. Telomere Shortening in Alcohol Dependence: Roles of Alcohol and Acetaldehyde. J. Psychiatr. Res. 2019, 109, 27–32. [Google Scholar] [CrossRef]
- Barragán, R.; Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; St-Onge, M.-P.; Portolés, O.; Corella, D. Effect of Physical Activity, Smoking, and Sleep on Telomere Length: A Systematic Review of Observational and Intervention Studies. J. Clin. Med. Res. 2021, 11, 76. [Google Scholar] [CrossRef]
- Miri, M.; Nazarzadeh, M.; Alahabadi, A.; Ehrampoush, M.H.; Rad, A.; Lotfi, M.H.; Sheikhha, M.H.; Sakhvidi, M.J.Z.; Nawrot, T.S.; Dadvand, P. Air Pollution and Telomere Length in Adults: A Systematic Review and Meta-Analysis of Observational Studies. Environ. Pollut. 2019, 244, 636–647. [Google Scholar] [CrossRef]
- Vieri, M.; Bruemmendorf, T.H.; Beier, F. Treatment of Telomeropathies. Best Pract. Res. Clin. Haematol. 2021, 34, 101282. [Google Scholar] [CrossRef]
- Nelson, N.D.; Bertuch, A.A. Dyskeratosis Congenita as a Disorder of Telomere Maintenance. Mutat. Res. 2012, 730, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.K.; Singh, P.; Al-Saeed, F.A.; Ahmed, A.E.; Kumar, S.; Kumar, A.; Dev, K.; Dohare, R. Unravelling the Role of Telomere Shortening with Ageing and Their Potential Association with Diabetes, Cancer, and Related Lifestyle Factors. Tissue Cell 2022, 79, 101925. [Google Scholar] [CrossRef]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Lin, Y.; Huang, F.; Ji, H.; Li, Y.; Lin, S.; Chen, X.; Duan, S. Differences in Leukocyte Telomere Length between Coronary Heart Disease and Normal Population: A Multipopulation Meta-Analysis. BioMed Res. Int. 2019, 2019, 5046867. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Xia, Q.; Xia, Q.; Wang, B.; Yang, C.; Liang, J.; Liu, X. Potential Roles of Telomeres and Telomerase in Neurodegenerative Diseases. Int. J. Biol. Macromol. 2020, 163, 1060–1078. [Google Scholar] [CrossRef]
- Yu, H.-J.; Koh, S.-H. Is Telomere Length Shortening a Risk Factor for Neurodegenerative Disorders? Dement. Neurocogn. Disord. 2022, 21, 83–92. [Google Scholar] [CrossRef]
- Machiela, E.; Southwell, A.L. Biological Aging and the Cellular Pathogenesis of Huntington’s Disease. J. Huntingt. Dis. 2020, 9, 115–128. [Google Scholar] [CrossRef]
- Scarabino, D.; Veneziano, L.; Peconi, M.; Frontali, M.; Mantuano, E.; Corbo, R.M. Leukocyte Telomere Shortening in Huntington’s Disease. J. Neurol. Sci. 2019, 396, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Fragkiadaki, P.; Nikitovic, D.; Kalliantasi, K.; Sarandi, E.; Thanasoula, M.; Stivaktakis, P.D.; Nepka, C.; Spandidos, D.A.; Tosounidis, T.; Tsatsakis, A. Telomere Length and Telomerase Activity in Osteoporosis and Osteoarthritis. Exp. Ther. Med. 2020, 19, 1626–1632. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Gobbini, E.; Trovesi, C.; Cassani, C.; Longhese, M.P. Telomere Uncapping at the Crossroad between Cell Cycle Arrest and Carcinogenesis. Mol. Cell. Oncol. 2014, 1, e29901. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.P.; Codd, V. Genetic Determinants of Telomere Length and Cancer Risk. Curr. Opin. Genet. Dev. 2020, 60, 63–68. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Li, L.; Zhou, Y.; Wang, C.; Hou, S. The Association between Telomere Length and Cancer Prognosis: Evidence from a Meta-Analysis. PLoS ONE 2015, 10, e0133174. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Zhu, X.; Han, W.; Xue, W.; Zou, Y.; Xie, C.; Du, J.; Jin, G. The Association between Telomere Length and Cancer Risk in Population Studies. Sci. Rep. 2016, 6, 22243. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Qu, K.; Pang, Q.; Wang, Z.; Zhou, Y.; Liu, C. Association between Telomere Length and Survival in Cancer Patients: A Meta-Analysis and Review of Literature. Front. Med. 2016, 10, 191–203. [Google Scholar] [CrossRef]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the Promoter of the Telomerase Gene Contribute to Tumorigenesis by a Two-Step Mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [Green Version]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived from Cells with Low Rates of Self-Renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoi, T.; Yoshida, M.; Kitamura, Y.; Yoshino, T.; Kawachi, A.; Shimomura, A.; Noguchi, E.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. TERT Promoter Hotspot Mutations in Breast Cancer. Breast Cancer 2018, 25, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Cárcano, F.M.; Vidal, D.O.; van Helvoort Lengert, A.; Neto, C.S.; Queiroz, L.; Marques, H.; Baltazar, F.; da Silva Martinelli, C.M.; Soares, P.; da Silva, E.C.A.; et al. Hotspot TERT Promoter Mutations Are Rare Events in Testicular Germ Cell Tumors. Tumour Biol. 2016, 37, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Bielski, C.M.; Rinne, M.L.; Hahn, W.C.; Sellers, W.R.; Stegmeier, F.; Garraway, L.A.; Kryukov, G.V. TERT Promoter Mutations and Monoallelic Activation of TERT in Cancer. Oncogenesis 2015, 4, e176. [Google Scholar] [CrossRef] [Green Version]
- Mosrati, M.A.; Willander, K.; Falk, I.J.; Hermanson, M.; Höglund, M.; Stockelberg, D.; Wei, Y.; Lotfi, K.; Söderkvist, P. Association between TERT Promoter Polymorphisms and Acute Myeloid Leukemia Risk and Prognosis. Oncotarget 2015, 6, 25109–25120. [Google Scholar] [CrossRef]
- Lam, G.; Xian, R.R.; Li, Y.; Burns, K.H.; Beemon, K.L. Lack of TERT Promoter Mutations in Human B-Cell Non-Hodgkin Lymphoma. Genes 2016, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A., Jr.; et al. TERT Promoter Mutations Occur Early in Urothelial Neoplasia and Are Biomarkers of Early Disease and Disease Recurrence in Urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef] [Green Version]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High Frequency of Telomerase Reverse-Transcriptase Promoter Somatic Mutations in Hepatocellular Carcinoma and Preneoplastic Lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [Green Version]
- Dratwa, M.; Wysoczanska, B.; Turlej, E.; Anisiewicz, A.; Maciejewska, M.; Wietrzyk, J.; Bogunia-Kubik, K. Heterogeneity of Telomerase Reverse Transcriptase Mutation and Expression, Telomerase Activity and Telomere Length across Human Cancer Cell Lines Cultured in Vitro. Exp. Cell Res. 2020, 396, 112298. [Google Scholar] [CrossRef]
- Panero, J.; Alves-Paiva, R.M.; Roisman, A.; Santana-Lemos, B.A.; Falcão, R.P.; Oliveira, G.; Martins, D.; Stanganelli, C.; Slavutsky, I.; Calado, R.T. Acquired TERT Promoter Mutations Stimulate TERT Transcription in Mantle Cell Lymphoma. Am. J. Hematol. 2016, 91, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejowski, J.; de Lange, T. Telomeres in Cancer: Tumour Suppression and Genome Instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT Promoter and FGFR3 and Telomere Length in Bladder Cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Altibi, A.M.A.; Duong, U.N.P.; Ngo, H.T.T.; Pham, T.Q.; Chan, A.K.-Y.; Park, C.-K.; Fung, K.-M.; Hassell, L. TERT Promoter Mutation and Its Interaction with IDH Mutations in Glioma: Combined TERT Promoter and IDH Mutations Stratifies Lower-Grade Glioma into Distinct Survival Subgroups-A Meta-Analysis of Aggregate Data. Crit. Rev. Oncol. Hematol. 2017, 120, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Yan, D.; Han, J.; Zhu, L. TERT Gene rs2736100 and rs2736098 Polymorphisms Are Associated with Increased Cancer Risk: A Meta-Analysis. Biochem. Genet. 2022, 60, 241–266. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Dong, Y.; Chang, J.; Wu, Y.; Chang, S.; Che, G. Cumulative Evidence for Relationships Between Multiple Variants in the TERT and CLPTM1L Region and Risk of Cancer and Non-Cancer Disease. Front. Oncol. 2022, 12, 946039. [Google Scholar] [CrossRef]
- Lawlor, R.T.; Veronese, N.; Pea, A.; Nottegar, A.; Smith, L.; Pilati, C.; Demurtas, J.; Fassan, M.; Cheng, L.; Luchini, C. Alternative Lengthening of Telomeres (ALT) Influences Survival in Soft Tissue Sarcomas: A Systematic Review with Meta-Analysis. BMC Cancer 2019, 19, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Z.; Wei, S.; Liu, Z.; Pooley, K.A.; Dunning, A.M.; Svenson, U.; Roos, G.; Hosgood, H.D., 3rd; Shen, M.; et al. Shortened Telomere Length Is Associated with Increased Risk of Cancer: A Meta-Analysis. PLoS ONE 2011, 6, e20466. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, Y.; Zhou, X.; Huang, S.; Zhao, H.; Zeng, P. Assessing the Relationship Between Leukocyte Telomere Length and Cancer Risk/Mortality in UK Biobank and TCGA Datasets with the Genetic Risk Score and Mendelian Randomization Approaches. Front. Genet. 2020, 11, 583106. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-Free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Alix-Panabières, C. Liquid Biopsy and Minimal Residual Disease—Latest Advances and Implications for Cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Circulating Tumour Cells in Cancer Patients: Challenges and Perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Gao, Q.; Zeng, Q.; Wang, Z.; Li, C.; Xu, Y.; Cui, P.; Zhu, X.; Lu, H.; Wang, G.; Cai, S.; et al. Circulating Cell-Free DNA for Cancer Early Detection. Innovation 2022, 3, 100259. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.T.; Chin, Y.M.; Nakamura, Y.; Low, S.-K. Clonal Hematopoiesis in Liquid Biopsy: From Biological Noise to Valuable Clinical Implications. Cancers 2020, 12, 2277. [Google Scholar] [CrossRef]
- Leal, A.; van Grieken, N.C.T.; Palsgrove, D.N.; Phallen, J.; Medina, J.E.; Hruban, C.; Broeckaert, M.A.M.; Anagnostou, V.; Adleff, V.; Bruhm, D.C.; et al. White Blood Cell and Cell-Free DNA Analyses for Detection of Residual Disease in Gastric Cancer. Nat. Commun. 2020, 11, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.; Baldassano, S.N.; Loh, P.-L.; Kording, K.; Litt, B.; Issadore, D. Machine Learning to Detect Signatures of Disease in Liquid Biopsies—A User’s Guide. Lab A Chip 2018, 18, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere Measurement by Quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.V.; Schneider, K.M.; Teumer, A.; Rudolph, K.L.; Hartmann, D.; Rader, D.J.; Strnad, P. Association of Telomere Length with Risk of Disease and Mortality. JAMA Intern. Med. 2022, 182, 291. [Google Scholar] [CrossRef]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of Telomere Length across Human Tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Zhu, W.; Liu, T.; Xie, S.-H.; Zhong, L.-X.; Cai, Y.-Y.; Li, X.-N.; Liang, M.; Chen, W.; et al. The Association of Telomere Length in Peripheral Blood Cells with Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Lansdorp, P.M. Telomeres, Aging, and Cancer: The Big Picture. Blood 2022, 139, 813–821. [Google Scholar] [CrossRef]
- Carroll, J.E.; Olmstead, R.; Haque, R.; Irwin, M.R. Accelerated Mononuclear Cell Telomere Attrition in Breast Cancer Survivors with Depression History: A 2-Year Longitudinal Cohort Study. Cancer 2022, 128, 3109–3119. [Google Scholar] [CrossRef]
- Schratz, K.E.; Haley, L.; Danoff, S.K.; Blackford, A.L.; DeZern, A.E.; Gocke, C.D.; Duffield, A.S.; Armanios, M. Cancer Spectrum and Outcomes in the Mendelian Short Telomere Syndromes. Blood 2020, 135, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Jebaraj, B.M.C.; Stilgenbauer, S. Telomere Dysfunction in Chronic Lymphocytic Leukemia. Front. Oncol. 2020, 10, 612665. [Google Scholar] [CrossRef]
- Vodenkova, S.; Kroupa, M.; Polivkova, Z.; Musak, L.; Ambrus, M.; Schneiderova, M.; Kozevnikovova, R.; Vodickova, L.; Rachakonda, S.; Hemminki, K.; et al. Chromosomal Damage and Telomere Length in Peripheral Blood Lymphocytes of Cancer Patients. Oncol. Rep. 2020, 44, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Díez-González, L.; Ocaña, A.; Šeruga, B.; Amir, E.; Templeton, A.J. Prognostic Role of Telomere Length in Malignancies: A Meta-Analysis and Meta-Regression. Exp. Mol. Pathol. 2017, 102, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Son, N.; Cui, Y.; Xi, W. Association Between Telomere Length and Skin Cancer and Aging: A Mendelian Randomization Analysis. Front. Genet. 2022, 13, 931785. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Telomere Length and Breast Cancer Prognosis: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2017, 26, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Gallicchio, L.; Gadalla, S.M.; Murphy, J.D.; Simonds, N.I. The Effect of Cancer Treatments on Telomere Length: A Systematic Review of the Literature. J. Natl. Cancer Inst. 2018, 110, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, Z. Telomere Length as a Prognostic Factor for Overall Survival in Colorectal Cancer Patients. Cell. Physiol. Biochem. 2016, 38, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Kachuri, L.; Latifovic, L.; Liu, G.; Hung, R.J. Systematic Review of Genetic Variation in Chromosome 5p15.33 and Telomere Length as Predictive and Prognostic Biomarkers for Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1537–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccherini, M.; Gentiluomo, M.; Fornili, M.; Lucenteforte, E.; Baglietto, L.; Campa, D. Association between Telomere Length and Mitochondrial Copy Number and Cancer Risk in Humans: A Meta-Analysis on More than 300,000 Individuals. Crit. Rev. Oncol. Hematol. 2021, 167, 103510. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Luchini, C.; Demurtas, J.; Soysal, P.; Stubbs, B.; Hamer, M.; Nottegar, A.; Lawlor, R.T.; Lopez-Sanchez, G.F.; Firth, J.; et al. Telomere Length and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. Ageing Res. Rev. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Llorca-Cardeñosa, M.J.; Peña-Chilet, M.; Mayor, M.; Gomez-Fernandez, C.; Casado, B.; Martin-Gonzalez, M.; Carretero, G.; Lluch, A.; Martinez-Cadenas, C.; Ibarrola-Villava, M.; et al. Long Telomere Length and a TERT-CLPTM1 Locus Polymorphism Association with Melanoma Risk. Eur. J. Cancer 2014, 50, 3168–3177. [Google Scholar] [CrossRef]
- Hu, R.; Hua, X.-G.; Jiang, Q.-C. Associations of Telomere Length in Risk and Recurrence of Prostate Cancer: A Meta-Analysis. Andrologia 2019, 51, e13304. [Google Scholar] [CrossRef]
- Naing, C.; Aung, K.; Lai, P.K.; Mak, J.W. Association between Telomere Length and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. BMC Cancer 2017, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Caini, S.; Raimondi, S.; Johansson, H.; De Giorgi, V.; Zanna, I.; Palli, D.; Gandini, S. Telomere Length and the Risk of Cutaneous Melanoma and Non-Melanoma Skin Cancer: A Review of the Literature and Meta-Analysis. J. Dermatol. Sci. 2015, 80, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Yunesian, M.; Nabizadeh, R.; Mehdipour, P.; Aghaie, A. Is Leukocyte Telomere Length Related with Lung Cancer Risk?: A Meta-Analysis. Iran. Biomed. J. 2017, 21, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Buglyó, G.; Styk, J.; Pös, O.; Csók, Á.; Repiska, V.; Soltész, B.; Szemes, T.; Nagy, B. Liquid Biopsy as a Source of Nucleic Acid Biomarkers in the Diagnosis and Management of Lynch Syndrome. Int. J. Mol. Sci. 2022, 23, 4284. [Google Scholar] [CrossRef]
- Styk, J.; Buglyó, G.; Pös, O.; Csók, Á.; Soltész, B.; Lukasz, P.; Repiská, V.; Nagy, B.; Szemes, T. Extracellular Nucleic Acids in the Diagnosis and Progression of Colorectal Cancer. Cancers 2022, 14, 3712. [Google Scholar] [CrossRef]
- Styk, J.; Pös, Z.; Pös, O.; Radvanszky, J.; Turnova, E.H.; Buglyó, G.; Klimova, D.; Budis, J.; Repiska, V.; Nagy, B.; et al. Microsatellite Instability Assessment Is Instrumental for Predictive, Preventive and Personalised Medicine: Status Quo and Outlook. EPMA J. 2023, 14, 143–165. [Google Scholar] [CrossRef]
- Pös, Z.; Pös, O.; Styk, J.; Mocova, A.; Strieskova, L.; Budis, J.; Kadasi, L.; Radvanszky, J.; Szemes, T. Technical and Methodological Aspects of Cell-Free Nucleic Acids Analyzes. Int. J. Mol. Sci. 2020, 21, 8634. [Google Scholar] [CrossRef] [PubMed]
- Idei, T.; Sakamoto, H.; Yamamoto, T. Terminal Restriction Fragments of Telomere Are Detectable in Plasma and Their Length Correlates with Clinical Status of Ovarian Cancer Patients. J. Int. Med. Res. 2002, 30, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Zhang, L.; Ma, J.-L.; Zhou, T.; Li, Z.-X.; Liu, W.-D.; Li, W.-Q.; Deng, D.-J.; You, W.-C.; et al. Telomere Length of Circulating Cell-Free DNA and Gastric Cancer in a Chinese Population at High-Risk. Front. Oncol. 2019, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Benati, M.; Montagnana, M.; Danese, E.; Mazzon, M.; Paviati, E.; Garzon, S.; Laganà, A.S.; Casarin, J.; Giudici, S.; Raffaelli, R.; et al. Aberrant Telomere Length in Circulating Cell-Free DNA as Possible Blood Biomarker with High Diagnostic Performance in Endometrial Cancer. Pathol. Oncol. Res. 2020, 26, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wan, S.; Hann, H.-W.; Myers, R.E.; Hann, R.S.; Au, J.; Chen, B.; Xing, J.; Yang, H. Relative Telomere Length: A Novel Non-Invasive Biomarker for the Risk of Non-Cirrhotic Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Infection. Eur. J. Cancer 2012, 48, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Hann, H.-W.; Ye, Z.; Hann, R.S.; Lai, Y.; Wang, C.; Li, L.; Myers, R.E.; Li, B.; Xing, J.; et al. Prospective and Longitudinal Evaluations of Telomere Length of Circulating DNA as a Risk Predictor of Hepatocellular Carcinoma in HBV Patients. Carcinogenesis 2017, 38, 439–446. [Google Scholar] [CrossRef]
- Urfali, M.; Silan, F.; Urfali, F.; Gurgen, A.; Ozdemir, O. The Comparison of Telomere Length in Cancer Patients: Plasma, Whole Blood and Tumor Tissue. Med. Sci. 2021, 10, 1117–1121. [Google Scholar] [CrossRef]
- Dey, S.; Marino, N.; Bishop, K.; Dahlgren, P.N.; Shendre, A.; Storniolo, A.M.; He, C.; Tanaka, H. A Plasma Telomeric Cell-Free DNA Level in Unaffected Women with BRCA1 Or/and BRCA2 Mutations: A Pilot Study. Oncotarget 2018, 9, 4214–4222. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Tanaka, H. Aberrant Reduction of Telomere Repetitive Sequences in Plasma Cell-Free DNA for Early Breast Cancer Detection. Oncotarget 2015, 6, 29795–29807. [Google Scholar] [CrossRef] [Green Version]
- North, J.P. Cell-Free Telomere DNA as a Biomarker for Treatment Response and Tumor Burden in Glioblastoma. Diploma Thesis, University of Mississippi, Oxford, MS, USA, 2018. [Google Scholar]
- Zheng, Z.; Lian, S.; Lu, C.; Li, F.; Yu, X.; Ai, L.; Wu, B.; Wei, K.; Zhou, W.; Xie, Y.; et al. Early Detection and Disease Monitoring of Hepatocellular Carcinoma Using Circulating Telomere DNA. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Wan, S.; Hann, H.-W.; Myers, R.E.; Fu, X.; Hann, R.S.; Kim, S.H.; Tang, H.; Xing, J.; Yang, H. Telomere Length in Circulating Serum DNA as a Novel Non-Invasive Biomarker for Cirrhosis: A Nested Case-Control Analysis. Liver Int. 2012, 32, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Petrara, M.R.; Giunco, S.; Serraino, D.; Dolcetti, R.; De Rossi, A. Post-Transplant Lymphoproliferative Disorders: From Epidemiology to Pathogenesis-Driven Treatment. Cancer Lett. 2015, 369, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Terrin, L.; Rampazzo, E.; Pucciarelli, S.; Agostini, M.; Bertorelle, R.; Esposito, G.; DelBianco, P.; Nitti, D.; De Rossi, A. Relationship between Tumor and Plasma Levels of hTERT mRNA in Patients with Colorectal Cancer: Implications for Monitoring of Neoplastic Disease. Clin. Cancer Res. 2008, 14, 7444–7451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tani, N.; Ichikawa, D.; Ikoma, D.; Tomita, H.; Sai, S.; Ikoma, H.; Okamoto, K.; Ochiai, T.; Ueda, Y.; Otsuji, E.; et al. Circulating Cell-Free mRNA in Plasma as a Tumor Marker for Patients with Primary and Recurrent Gastric Cancer. Anticancer Res. 2007, 27, 1207–1212. [Google Scholar]
- Giunco, S.; Rampazzo, E.; Celeghin, A.; Petrara, M.R.; De Rossi, A. Telomere and Telomerase in Carcinogenesis: Their Role as Prognostic Biomarkers. Curr. Pathobiol. Rep. 2015, 3, 315–328. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, J.; Sun, P.; Shang, J. Circulating Cell-Free Human Telomerase Reverse Transcriptase mRNA in Plasma and Its Potential Diagnostic and Prognostic Value for Gastric Cancer. Int. J. Clin. Oncol. 2013, 18, 478–486. [Google Scholar] [CrossRef]
- March-Villalba, J.A.; Martínez-Jabaloyas, J.M.; Herrero, M.J.; Santamaria, J.; Aliño, S.F.; Dasí, F. Cell-Free Circulating Plasma hTERT mRNA Is a Useful Marker for Prostate Cancer Diagnosis and Is Associated with Poor Prognosis Tumor Characteristics. PLoS ONE 2012, 7, e43470. [Google Scholar] [CrossRef] [Green Version]
- Miura, N.; Nakamura, H.; Sato, R.; Tsukamoto, T.; Harada, T.; Takahashi, S.; Adachi, Y.; Shomori, K.; Sano, A.; Kishimoto, Y.; et al. Clinical Usefulness of Serum Telomerase Reverse Transcriptase (hTERT) mRNA and Epidermal Growth Factor Receptor (EGFR) mRNA as a Novel Tumor Marker for Lung Cancer. Cancer Sci. 2006, 97, 1366–1373. [Google Scholar] [CrossRef]
- Rampazzo, E.; Del Bianco, P.; Bertorelle, R.; Boso, C.; Perin, A.; Spiro, G.; Bergamo, F.; Belluco, C.; Buonadonna, A.; Palazzari, E.; et al. The Predictive and Prognostic Potential of Plasma Telomerase Reverse Transcriptase (TERT) RNA in Rectal Cancer Patients. Br. J. Cancer 2018, 118, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Pucciarelli, S.; Rampazzo, E.; Briarava, M.; Maretto, I.; Agostini, M.; Digito, M.; Keppel, S.; Friso, M.L.; Lonardi, S.; De Paoli, A.; et al. Telomere-Specific Reverse Transcriptase (hTERT) and Cell-Free RNA in Plasma as Predictors of Pathologic Tumor Response in Rectal Cancer Patients Receiving Neoadjuvant Chemoradiotherapy. Ann. Surg. Oncol. 2012, 19, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, E.; Cecchin, E.; Del Bianco, P.; Menin, C.; Spolverato, G.; Giunco, S.; Lonardi, S.; Malacrida, S.; De Paoli, A.; Toffoli, G.; et al. Genetic Variants of the Gene, Telomere Length, and Circulating as Prognostic Markers in Rectal Cancer Patients. Cancers 2020, 12, 3115. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, M.; Zanussi, S.; Rampazzo, E.; Bidoli, E.; Giunco, S.; Tedeschi, R.; Pratesi, C.; Martorelli, D.; Casarotto, M.; Martellotta, F.; et al. Biological Predictors of Tumors in Solid Organ Transplanted Patients During Oncological Surveillance: Potential Role of Circulating mRNA. Front. Oncol. 2021, 11, 772348. [Google Scholar] [CrossRef]
- Lai, T.-P.; Wright, W.E.; Shay, J.W. Comparison of Telomere Length Measurement Methods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindrose, A.R.; McLester-Davis, L.W.Y.; Tristano, R.I.; Kataria, L.; Gadalla, S.M.; Eisenberg, D.T.A.; Verhulst, S.; Drury, S. Method Comparison Studies of Telomere Length Measurement Using qPCR Approaches: A Critical Appraisal of the Literature. PLoS ONE 2021, 16, e0245582. [Google Scholar] [CrossRef]
- Harley, C.B. Telomere Loss: Mitotic Clock or Genetic Time Bomb? Mutat. Res. 1991, 256, 271–282. [Google Scholar] [CrossRef]
- Allshire, R.C.; Dempster, M.; Hastie, N.D. Human Telomeres Contain at Least Three Types of G–rich Repeat Distributed Non-Randomly. Nucleic Acids Research 1989, 17, 4611–4627. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Length Measurement by a Novel Monochrome Multiplex Quantitative PCR Method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [Green Version]
- Baerlocher, G.M.; Vulto, I.; de Jong, G.; Lansdorp, P.M. Flow Cytometry and FISH to Measure the Average Length of Telomeres (flow FISH). Nat. Protoc. 2006, 1, 2365–2376. [Google Scholar] [CrossRef]
- Rufer, N.; Dragowska, W.; Thornbury, G.; Roosnek, E.; Lansdorp, P.M. Telomere Length Dynamics in Human Lymphocyte Subpopulations Measured by Flow Cytometry. Nat. Biotechnol. 1998, 16, 743–747. [Google Scholar] [CrossRef]
- Baird, D. New Developments in Telomere Length Analysis. Exp. Gerontol. 2005, 40, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-P.; Zhang, N.; Noh, J.; Mender, I.; Tedone, E.; Huang, E.; Wright, W.E.; Danuser, G.; Shay, J.W. A Method for Measuring the Distribution of the Shortest Telomeres in Cells and Tissues. Nat. Commun. 2017, 8, 1356. [Google Scholar] [CrossRef] [Green Version]
- Castle, J.C.; Biery, M.; Bouzek, H.; Xie, T.; Chen, R.; Misura, K.; Jackson, S.; Armour, C.D.; Johnson, J.M.; Rohl, C.A.; et al. DNA Copy Number, Including Telomeres and Mitochondria, Assayed Using next-Generation Sequencing. BMC Genom. 2010, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conomos, D.; Stutz, M.D.; Hills, M.; Neumann, A.A.; Bryan, T.M.; Reddel, R.R.; Pickett, H.A. Variant Repeats Are Interspersed throughout the Telomeres and Recruit Nuclear Receptors in ALT Cells. J. Cell Biol. 2012, 199, 893–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Mangino, M.; Aviv, A.; Spector, T.; Durbin, R. UK10K Consortium Estimating Telomere Length from Whole Genome Sequence Data. Nucleic Acids Res. 2014, 42, e75. [Google Scholar] [CrossRef] [Green Version]
- Nersisyan, L.; Arakelyan, A. Computel: Computation of Mean Telomere Length from Whole-Genome next-Generation Sequencing Data. PLoS ONE 2015, 10, e0125201. [Google Scholar] [CrossRef] [Green Version]
- Farmery, J.H.R.; Smith, M.L.; NIHR BioResource-Rare Diseases; Lynch, A.G. Telomerecat: A Ploidy-Agnostic Method for Estimating Telomere Length from Whole Genome Sequencing Data. Sci. Rep. 2018, 8, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerbach, L.; Sieverling, L.; Deeg, K.I.; Ginsbach, P.; Hutter, B.; Buchhalter, I.; Northcott, P.A.; Mughal, S.S.; Chudasama, P.; Glimm, H.; et al. TelomereHunter - in Silico Estimation of Telomere Content and Composition from Cancer Genomes. BMC Bioinform. 2019, 20, 272. [Google Scholar] [CrossRef] [Green Version]
- Holmes, O.; Nones, K.; Tang, Y.H.; Loffler, K.A.; Lee, M.; Patch, A.-M.; Dagg, R.A.; Lau, L.M.S.; Leonard, C.; Wood, S.; et al. Qmotif: Determination of Telomere Content from Whole-Genome Sequence Data. Bioinform Adv 2022, 2, vbac005. [Google Scholar] [CrossRef]
- Marx, V. Method of the Year: Long-Read Sequencing. Nat. Methods 2023, 20, 6–11. [Google Scholar] [CrossRef]
- Tan, K.-T.; Slevin, M.K.; Meyerson, M.; Li, H. Identifying and Correcting Repeat-Calling Errors in Nanopore Sequencing of Telomeres. Genome Biol. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The Complete Sequence of a Human Genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, K.; Foox, J.; Bezdan, D.; Butler, D.; Luxton, J.J.; Reed, J.; McKenna, M.J.; Taylor, L.; George, K.A.; Meydan, C.; et al. Haplotype Diversity and Sequence Heterogeneity of Human Telomeres. Genome Res. 2021, 31, 1269–1279. [Google Scholar] [CrossRef] [PubMed]


| Type of Cancer/Condition | Material 3 | Method | No. of Studies/ Source | No. of Patients/Controls | Alt. 4 | Ref. |
|---|---|---|---|---|---|---|
| breast | blood, tissue | dot blot, FISH, qPCR, Southern blot, STELA | 36 | 6311 cases | ↓ | [118] |
| facial skin aging, NMSC, skin melanoma | blood | qPCR | UK biobank data | 451,444 cases/ 372,016 controls | ↓ | [116] |
| APML, Barrett carcinoma, breast, CLL, CRC, Ewing sarcoma, esophageal, gastric, gastroenteropancreatic, glioblastoma, glioma, HCC, head and neck, HL, low-risk B-cell precursor ALL, neuroblastoma, NSCLC, ovarian, prostate, renal, urothelial | blood, tissue | dot blot, FISH, MMqPCR, qPCR, Southern blot, STELA | 61 | 14,720 cases | ↑ | [115] |
| bladder, breast, CRC, endometrial, esophageal, gastric, glioma, head and neck, hematological, liver, lung, melanoma, multiple myeloma, NMSC, non-HL, ovarian, pancreatic, prostate, renal, soft tissue sarcoma, thyroid | blood | qPCR | 112 | 64,184 cases/ 278,641 controls | ↑ | [122] |
| bladder, breast, CLL, CRC, esophageal, gastric, glioma, head and neck, HCC, HL, lung, ovarian, prostate, renal, skin basal cell carcinoma, skin melanoma, other conditions 2 | blood, tissue | NA | 21 meta- analyses | 153,451 cases/ 180,130 controls | ↓ | [123] |
| melanoma | blood | qPCR | 1 | 970 cases/ 733 controls | ↓ | [124] |
| prostate | blood, tissue | FISH, qPCR | 12 | 2130 cases/ 2131 controls | ↓ | [125] |
| bladder, brain, breast, CRC, endometrial, hematological, liver, lung, melanoma, pancreatic, prostate, renal, skin-basal cell carcinoma, skin-squamous cell carcinoma | blood | qPCR | 25 | 13,894 cases/ 71,672 controls | ↑ | [109] |
| NSCLC, SCLC | blood, tissue | qPCR, Southern blot, TRF | 14 | 1503 cases | ↑↓ | [121] |
| CRC | blood | qPCR | 7 | 4951 cases/ 7993 controls | ↑↓ | [126] |
| soft tissue sarcomas (ALT+/ALT−) | tissue | APB evaluation, FISH, TRF | 8 | 551 cases | ↑ 5 | [91] |
| CRC | blood, tissue | qPCR | 7 | 956 cases | ↑↓ | [120] |
| adrenocortical, ALL, bladder, breast, cervical, cholangiocarcinoma, CRC, diffuse large B-cell lymphoma, endometrial, esophageal, gastric, glioblastoma, glioma, HCC, head and neck, kidney chromophobe, lung, lymphoid neoplasm, ovarian, pancreatic adenocarcinoma, paraganglioma, pheochromocytoma, prostate, renal, sarcoma, skin melanoma, testicular germ cell, thymus, thyroid carcinoma, uterine carcinosarcoma, uveal melanoma | blood, tissue | WGS, low-pass WGS, WES, TelSeq | TCGA data | 6835 cases/ 11,595 controls | ↑↓ | [29] |
| NMSC, skin melanoma | blood | LTL (qPCR) | 8 | 3068 cases | ↓ | [127] |
| ALL, AML, APML, astrocytoma, brain stem tumor, breast, clear cell sarcoma, CLL, CML, CNS tumors, CRC, esophageal, Ewing sarcoma, gastric, germ cell, HCC, head and neck, hepatoblastoma, HL, lung, lymphoma, multiple myeloma, neuroblastoma, non-HL, ovarian, prostate, rhabdomyosarcoma, severe aplastic anemia, thyroid, Wilms’ tumor | blood, tissue | Flow-FISH, Q-FISH, qPCR, TRF | 25 | 2261 cases | ↑↓ | [119] |
| B-cell lymphoma, basal cell carcinoma, bladder, breast, CRC, endometrial, esophageal, glioma, HCC, head and neck, gastric, lung, melanoma, myeloma, non-HL, oral cavity, oropharyngeal, ovarian, pancreatic, prostate, renal, skin melanoma, squamous cell carcinoma | blood, tissue | Flow-FISH, Q-FISH, qPCR | 51 | 23,379 cases/ 68,792 controls | ↓ | [69] |
| AML, bladder, breast, CLL, CRC, esophageal, gastric, glioblastoma, glioma, HCC, head and neck, myeloproliferative neoplasms, neuroblastoma, NSCLC, ovarian, prostate, renal | blood, tissue | FISH, qPCR, Southern blot | 33 | 15,722 cases | ↓ | [66] |
| lung | blood, sputum, tissue | Q-FISH, qPCR | 9 | 2925 cases/ 2931 controls | ↓ | [128] |
| bladder, breast, CRC, esophageal, gastric, lung, non-HL, ovarian, prostate, renal, skin | blood, sputum, tissue | Q-FISH, qPCR, Southern blot | 21 | 11,255 cases/ 13,101 controls | ↓ | [93] |
| bladder, breast, CRC, esophageal, Ewing sarcoma, gastric, glioblastoma, glioma, head and neck, leukemia, liver, lung, lymphoma, myelodysplastic syndromes, neuroblastoma, oral cavity, ovarian, prostate, renal | blood, tissue | dot blot, flow-FISH, qPCR, Southern blot, STELA, Telo assay | 51 | 14,464 cases | ↓ | [70] |
| bladder, breast, CRC, endometrial, esophageal, glioma, head and neck, lung, neuroblastoma, ovarian, pancreatic, prostate, renal, skin, testicular germ cell, other conditions 2 | blood, tissue | NA | NHGRI-EBI GWAS catalog | 420,081 cases/ 1093,105 controls | ↑ | [117] |
| basal cell, bladder, bone, brain, breast, cervical, CLL, CML, CRC, endometrial, esophageal, eye and/or adnexal, gastric, HL, larynx, leukemia, lung, lymphoma, malignant melanoma, multiple myeloma, NMSC, non-HL, oral cavity, ovarian, prostate, renal, sarcoma/fibrosarcoma, skin, small intestine, squamous cell, testicular, throat, tongue, thyroid | blood | NA | UK biobank data | 78,582 cases | ↑ | [94] |
| Cancer Type | Body Fluid | Method | Telomere Alteration 1 | No. of Patients/Controls | Ref. |
|---|---|---|---|---|---|
| ovarian | plasma | TRF | ↓ | 32 + 10 3/45 | [133] |
| gastric | serum | qPCR | ↓ | 86/86 | [134] |
| endometrial | serum | qPCR | ↓ | 40/31 | [135] |
| hepatocellular 4 | serum | qPCR | ↑ | 140/280 | [136] |
| hepatocellular 4 | serum | qPCR | ↑ | 37/286 | [137] |
| breast (16), colon (16), stomach (3), lung (3), and rectum (2) | serum | qPCR | ↑ | 40/20 | [138] |
| breast | plasma | qPCR | ↓ 2 | 28/28 47/42 | [139] [140] |
| glioblastoma | serum | qPCR | ↑ 2 | 40/9 5 | [141] |
| hepatocellular | plasma | BLESSING/ Telecon 6 | ↓ 2 | 60/40 7 63/50 8 | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holesova, Z.; Krasnicanova, L.; Saade, R.; Pös, O.; Budis, J.; Gazdarica, J.; Repiska, V.; Szemes, T. Telomere Length Changes in Cancer: Insights on Carcinogenesis and Potential for Non-Invasive Diagnostic Strategies. Genes 2023, 14, 715. https://doi.org/10.3390/genes14030715
Holesova Z, Krasnicanova L, Saade R, Pös O, Budis J, Gazdarica J, Repiska V, Szemes T. Telomere Length Changes in Cancer: Insights on Carcinogenesis and Potential for Non-Invasive Diagnostic Strategies. Genes. 2023; 14(3):715. https://doi.org/10.3390/genes14030715
Chicago/Turabian StyleHolesova, Zuzana, Lucia Krasnicanova, Rami Saade, Ondrej Pös, Jaroslav Budis, Juraj Gazdarica, Vanda Repiska, and Tomas Szemes. 2023. "Telomere Length Changes in Cancer: Insights on Carcinogenesis and Potential for Non-Invasive Diagnostic Strategies" Genes 14, no. 3: 715. https://doi.org/10.3390/genes14030715
APA StyleHolesova, Z., Krasnicanova, L., Saade, R., Pös, O., Budis, J., Gazdarica, J., Repiska, V., & Szemes, T. (2023). Telomere Length Changes in Cancer: Insights on Carcinogenesis and Potential for Non-Invasive Diagnostic Strategies. Genes, 14(3), 715. https://doi.org/10.3390/genes14030715